Abstract
Although current HBV vaccines have an outstanding record of safety and efficacy, reduced immunogenicity is a problem in those of older age, or having renal impairment or diabetes mellitus. In this study, we tested the ability of Advax™ adjuvant, a novel polysaccharide adjuvant based on delta inulin, to enhance the immunogenicity of hepatitis B surface antigen (HBs) in mice and guinea pigs by comparison to the traditional alum adjuvant. Advax™ provided antigen-sparing and significantly enhanced both anti-HBs antibody titers and anti-HBs CD4 and CD8 T-cells, with increases in Th1, Th2 and Th17 cytokine responses. Unlike alum, the adjuvant effect of Advax™ was seen even when injected 24 hours before the HBs antigen. Advax™ adjuvant similarly enhanced humoral and cellular immune responses in guinea pigs to a third generation preS-HBs antigen. Inclusion Advax™ adjuvant when combined with HBs antigen could provide enhanced protection over current generation HBV vaccines for immunization of low responder populations.
Keywords: vaccine, adjuvant, Advax™, inulin, hepatitis B, alum, therapeutic
Introduction
Approximately 50 million people worldwide are infected with hepatitis B virus (HBV) each year. Current prophylactic HBV vaccines based on recombinant hepatitis B surface antigen (HBs) have been highly successful in preventing infection and transmission [1, 2]. HBV envelope is composed of the small protein (HBs), the middle protein comprising HBs protein plus the preS2 region and the large protein comprising the middle protein plus the preS1 region. Third-generation HBV vaccines include the preS1 and preS2 regions and thereby provide additional immune epitopes, and have been reported to induce more rapid seroprotection, provide higher antibody levels and circumvent HBs non-responsiveness [3–6]. HBs has poor immunogenicity and hence requires an adjuvant, typically alum (aluminum hydroxide), to be effective [7, 8]. However, alum-based vaccines have reduced efficacy in older subjects and are not effective in a therapeutic setting [9–11]. New adjuvants including AS04 [12] and CpG oligonucleotides [13] have been used to improve HBs immunogenicity albeit at the expense of increased reactogenicity [12] or uncertain safety [13] which must be factored into the risk-benefit equation of alum substitution in HBV vaccines [13–17].
Advax™ is a novel adjuvant based on delta β-D-(2-1)polyfructofuranosyl-α-D-glucose (inulin) that was developed through the Adjuvant Development Program of the National Institutes of Health. Advax™ has previously been shown to enhance Japanese encephalitis vaccine in mice and horses [18], HIV (in mice) [19], H1N1 influenza in mice [20], avian influenza in ferrets [21], African Horse Sickness in camels [22] and H1N1/2009pdm influenza in humans [23].
This study asked whether Advax™ adjuvant could beneficially substitute for alum in second or third generation HBV vaccines and thereby improve their immunogenicity without compromising tolerability or safety. The results confirm that substitution of alum with Advax™ enhances HBs-specific antibody and T-cell responses in the absence of adverse reactions, identifying it as a promising HBV vaccine adjuvant for use in low responder populations.
Materials and Methods
Animals and blood collection
Female BALB/c and C57BL/6 mice (5–10 weeks of age) bred under specific pathogen-free conditions were supplied by the Flinders University animal facility. Female guinea pigs were purchased from IMVS (Adelaide, Australia). All procedures were performed in accordance with the Animal Experimentation Guidelines of the National Health and Medical Research Council of Australia and approved by the Flinders Animal Welfare Committee (Permit AWC 726/09).
Vaccines and adjuvants
Yeast-derived HBs (Butantan Institute, Brazil), CHO cell recombinant preS (SciGen, Singapore) [24], Advax™ (Vaxine, Australia), and Alhydrogel (Brenntag, Denmark) were used as indicated. Engerix-B, a commercial HBV vaccine manufactured by GlaxoSmithKline (Rixensart, Belgium) that contains 20 mcg of HBsAg adsorbed on 0.5 mg elemental aluminum (Al3+) as aluminum hydroxide together with sodium chloride (9 mg/mL), disodium phosphate dihydrate, 0.98 mg/mL, sodium dihydrogen phosphate dihydrate, 0.71 mg/mL as excipients in 1ml volume, was used as a comparator. For formulation studies, Butantan HBs was adsorbed to the Alhydrogel in a saline buffer for 1 hour at room temperature prior to injection, with confirmation these conditions provided almost complete absorption. The dose of alum is expressed as the content of elemental aluminium (Al3+). Advax™ has not been shown to bind HBs and hence HBs was simply mixed with Advax™ suspension in normal saline immediately prior to injection. All antigens and adjuvants were endotoxin free by commercial LAL assay.
B-cell assays
Serum anti-HBs titers were determined a commercial assay (AxSYM AUSAB, Abbott, USA). Mouse total IgG, IgG1, IgG2a/c and guinea pig IgG1 and IgG2 were determined by ELISA, as previously described [20]. Briefly, HBs (1µg/ml) was absorbed to 96 well plates, blocked with 1% BSA then 100µl of 1:200 dilution of sera for IgG2a/c and 1:1000 dilution for IgG and IgG1 was incubated for 2 hours at RT followed by HRP-conjugated anti-mouse IgG, IgG1 or IgG2a/c for 1 hour then TMB substrate for 10 minutes before stopping with 1M phosphoric acid and measurement of optical density at 450nm (OD450nm). Guinea pig preS IgG1 and IgG2 titers were determined on pooled serum samples using an end-point serial dilution (2-fold) ELISA titration. End-point titers were defined as the reciprocal of the highest serum dilution that resulted in an absorbance value greater than the mean optical density (OD) + 3 standard deviations (SD) of non-immune control sera. Anti-HBs IgG avidity was determined on guinea pig sera using the urea denaturation procedure, as described previously [25]. HBs-specific IgG1 or IgG2 antibody-secreting cells (ASC) were quantitated by ELISPOT. Briefly, bone marrow or spleen cells were incubated in 96-well multiscreen filtration plates coated with 5µg/ml preS antigen. Single-cell suspensions in RPMI/10% FCS were incubated in coated ELISPOT plates for 12 hours at 37°C in 5% CO2, then spots developed with anti-guinea pig IgG1 or IgG2 antibody, rabbit anti-goat IgG HRP (1:4000) and AEC kit then counted using ImmunoSpot S6 Reader.
T-cell assays
Spleens were rinsed in sterile PBS/0.1% BSA and isolated spleen cells were pelleted and incubated in RBC lysis solution. After washing with PBS/0.1% BSA, cells were incubated for 10 minutes at RT with 1µM CFSE (Life Technologies), staining quenched and splenocytes cultured at 106 cells/well in 24-well plates for 5 days at 37°C in 5% CO2 with HBs and then stained with anti-mouse CD4-PE-Cy5 and anti-CD8-PE-Cy7 and analyzed on a FACScanto II (Becton Dickinson, USA). T-cell proliferation was expressed as the ratio of divided daughter cells to total T cells, expressed as a percentage, in analogy to calculation of a stimulation index in thymidine proliferation assays. As many daughter cells are generated from each proliferating antigen-specific T cell, this percentage response is proportional to but not equal to the actual antigen-specific precursor T-cell frequency. HBs-stimulated interferon (IFN)-γ or IL-17 secreting T cells were enumerated by ELISPOT.
Cytometric bead array (CBA)
Splenocytes were re-stimulated for 3 days with HBs antigen in vitro, the supernatants harvested and cytokines measured by mouse Th1/Th2 10plex (Bender MedSystems) and analyzed by FlowCytomix Pro Software (eBioscience, CA, USA).
Statistical analysis
GraphPad Prism 5.0 for Windows was used for drawing graphs and statistical analysis (GraphPad Software, San Diego, CA, USA). Significant differences between experimental and control groups were analyzed by t test or by one-way ANOVA, using Dunnett’s post-test. Differences were considered statistically significant when p < 0.05.
Results
Advax™ adjuvant enhances anti-HBs antibody responses
The ability of Advax™ to induce anti-HBs antibody was assessed 3 weeks after a single intramuscular (i.m.) injection of 0.5ug HBs together with Advax™ in doses ranging from 0.1 to 2mg/mouse. Advax™ significantly increased the anti-HBs titer compared to HBs alone (Fig 1A). There was an adjuvant dose response up to 0.5mg Advax™ after which the adjuvant effect appeared to largely plateau. A dose of 0.5–1mg Advax™/mouse was used for all further murine experiments.
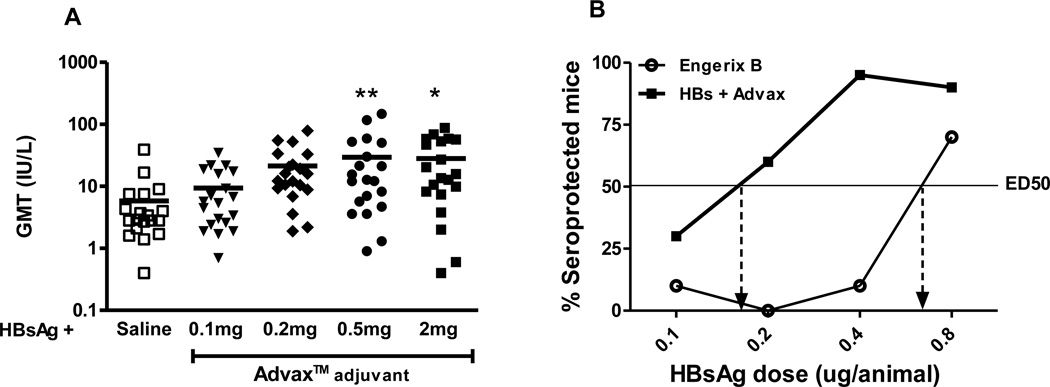
Fig. 1. Advax™ provides HBs antigen-sparing.
(A) The dose response of Advax™ was evaluated in 5-week-old female BALB/c mice (n=20) three weeks following a single i.m. immunization with 0.5µg of HBs formulated with indicated dose of Advax™ in 0.1ml normal saline. Anti-HBs antibody titers are expressed in IU/L and line symbol represents the geometric mean titer. Asterisks designate significant differences (*p < 0.05, **p < 0.01). (B) BALB/c mice (n=20/group) received a single i.p. immunization with HBsAg ranging in dose from 0.1 – 0.8µg plus a constant dose of 1mg Advax™ or 0.1mg alum in saline buffer. The Effective Dose 50% (ED50) for each group was calculated based on the proportion of mice that 28 days post-immunization had anti-HBs antibody ≥ 10 IU/L.
To assess the effect of Advax™ on HBs antigen-sparing, an adaption of the WHO HBs immune-potency test [26] was used where female 35 day old BALB/c mice in groups of 20 were given a single immunization i.p. with HBs (0.1, 0.2, 0.4 or 0.8µg) in normal saline plus a constant dose of Advax™ adjuvant (1mg) or alum (0.1mg). HBs was diluted with normal saline and Alhydrogel or Advax was added keeping the adjuvant dose/mouse constant. Mice were bled at day 28 and sera analyzed for anti-HBs by AxSYM AUSAB assay. The dose of HBs antigen required for 50% of the mice in a group to achieve anti-HBs levels > 10 IU/L, i.e. 50% Effective Dose (ED50), was calculated from the plotted results. The ED50 for the Advax™-adjuvated group was 0.17ug HBs compared to 0.7ug HBs for the alum-adjuvanted group, consistent with Advax™ providing 4-fold antigen sparing (Fig. 1B).
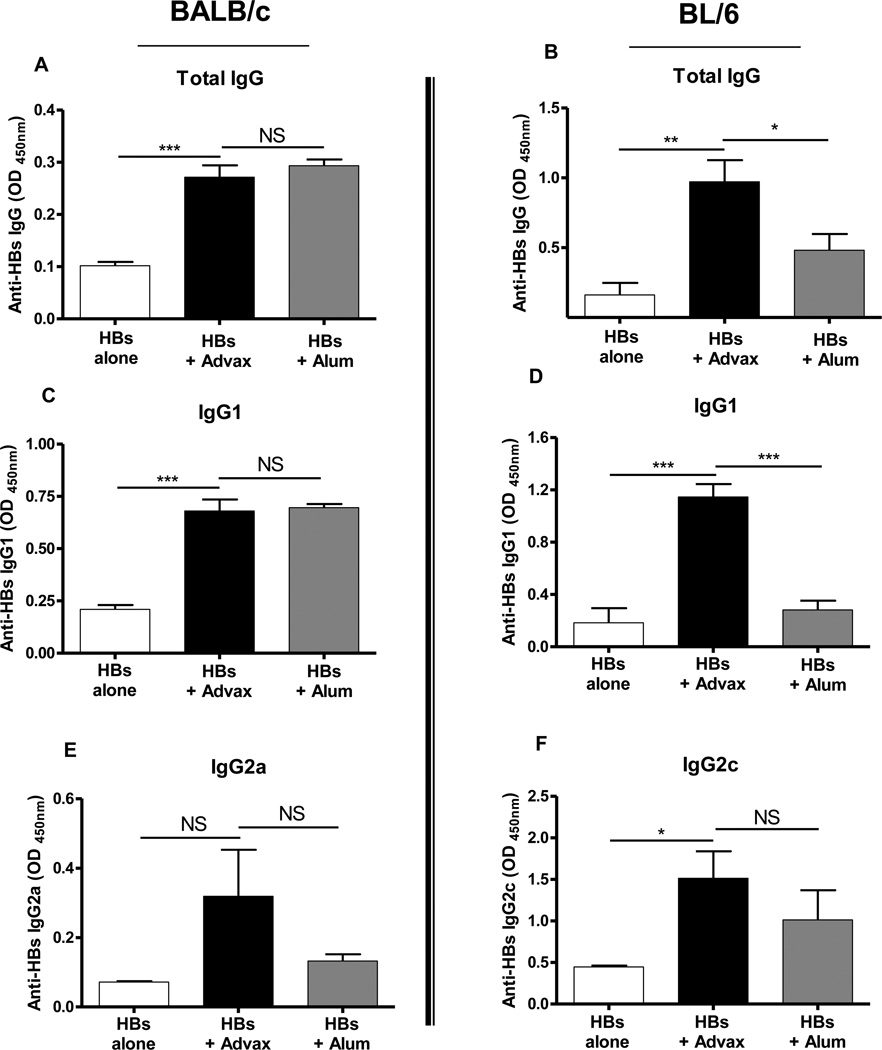
To investigate the effect of Advax™ on IgG subtype production, mice received two immunizations i.m. 2 weeks apart with HBs alone or formulated with Advax™ 1mg or alum 0.1mg. In BALB/c mice, which have a known T helper-2 (Th2) bias [27], Advax™ increased anti-HBs total IgG levels primarily through elevation of IgG1, a T helper 2 (Th2) antibody isotype, with a smaller contribution from IgG2a, a Th1 isotype. In C57BL/6 mice which have a known Th1 bias [28], Advax™ increased anti-HBs total IgG levels by elevation of both IgG1 (Th2 isotype) and IgG2c (Th1 isotype) (Figure 2). Notably, Advax™ achieved equivalent IgG titers to alum in the Th2-biased Balb/c mice but higher titers than alum in the Th1-biased BL/6 mice (Figure 2), consistent with Advax™ providing a more balanced Th1 and Th2 antibody response.
Fig. 2. Advax™ enhances anti-HBs antibody titers.
Adult female BALB/c (A, C, E) or C57BL/6 (B, D, F) mice were immunized twice i.m. at a 2-week interval with HBs 1µg alone (white bars) or together with Advax™ 1mg (black bars) or alum 100µg (grey bars), in 0.1ml normal saline. Blood samples were collected 2 weeks after the second immunization and anti-HBs total IgG (A, B), IgG1 (C, D), IgG2a (E) or IgG2c (F) measured by ELISA. (NS: Not significant, *p < 0.05, **p < 0.01, ***p < 0.001).
Advax™ adjuvant increases anti-HBs T-cell responses
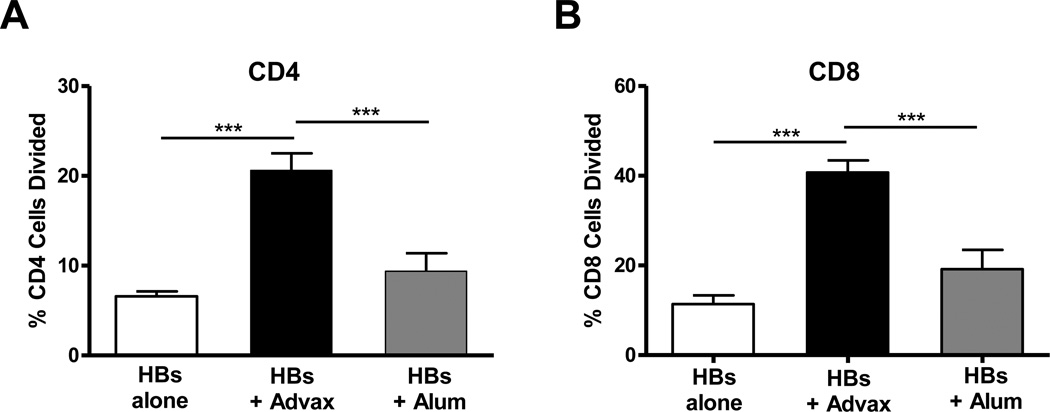
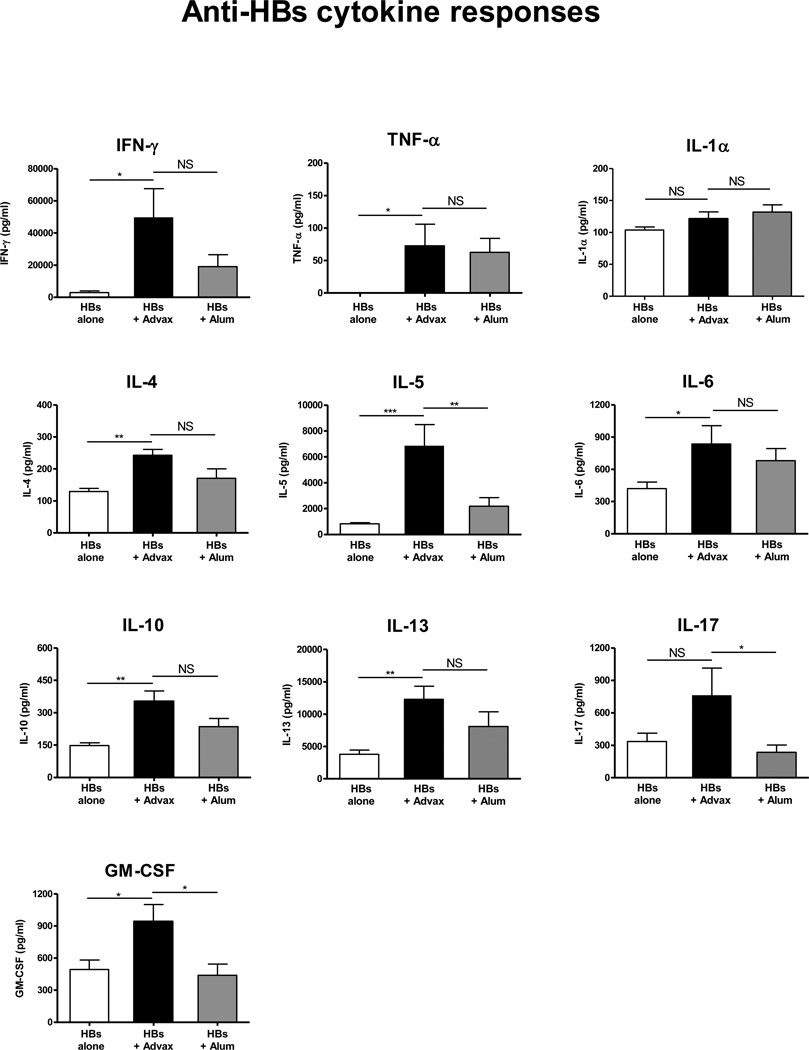
To assess whether Advax™ adjuvant similarly enhances anti-HBs T-cell responses, splenocytes were isolated from mice 3 weeks post-HBs immunization then labeled with CFSE and cultured with HBs for 5 days, in a standard CFSE proliferation assay (ref). Mice receiving HBs with Advax™ had significantly higher HBs-specific CD4+ and CD8+ T-cell proliferation compared to mice immunized with HBs alone (p<0.001) or HBs adjuvated with alum (p<0.001) (Fig. 3). To more fully characterize the cytokine profile induced by immunization of HBs with Advax™, splenocytes from immunized animals were re-stimulated with HBs in vitro for 3 days and supernatants measured for cytokines. Mice immunized with HBs plus Advax™ produced significantly higher IFN-γ, TNF-α, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17 and GM-CSF, compared to mice immunized with HBs alone, and significantly higher IL-5, IL-17 and GM-CSF when compared to mice immunized with HBs plus alum (Fig. 4).
Fig. 3. Advax™ enhances anti-HBs T-cell responses.
Mice (n=8) were immunized twice i.m. at a 2-week interval with HBs 1µg alone (white bars) or together with Advax™ 1mg (black bars) or alum 100µg (grey bars) in 0.1mls normal saline. HBs-stimulated (A) CD4 and (B) CD8 T-cell proliferation was measured by culturing CFSE-labeled splenocytes with HBs antigen for 5 days. Results are presented as mean + SEM. (***p < 0.001).
Fig. 4. Advax™ enhances Th1, Th2 and Th17 cytokine secretion.
Spleens were collected from mice (n=6–8) immunized with HBs 1µg alone (white bars) or with Advax™ 1mg (black bars) or alum 100µg (grey bars), and cultured with HBs antigen for 3 days. Cytokines and chemokines in the supernatant were quantitated by cytokine bead array. Mean + SEM (NS: Not significant, *p < 0.05, **p < 0.01, **p < 0.001).
Advax™ adjuvanticity doesn’t require antigen adsorption
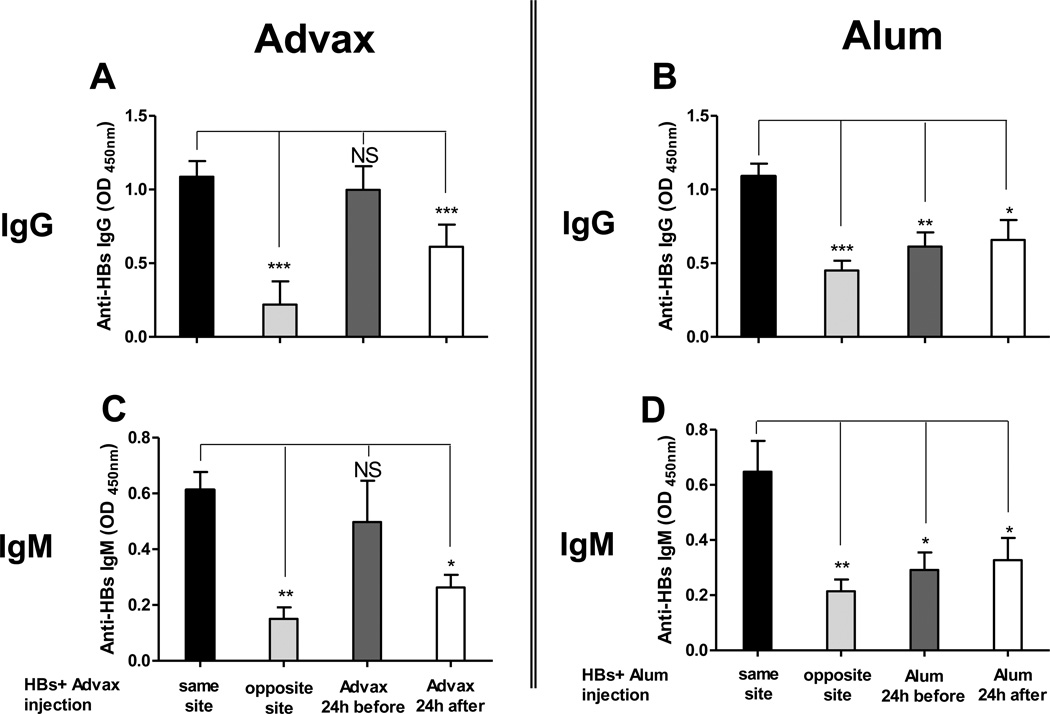
An important characteristic of alum is its ability to absorb antigens. When HBs was co-incubated with Advax™ no absorption was detected (data not shown) suggesting that its mechanism of action is not dependent on antigen binding. To test whether Advax™ needed to be associated with antigen to be effective, BALB/c mice were immunized i.m. with HBs together with Advax™ or alum, or alternatively the Advax™ or alum were injected at a different time or location to the HBs antigen. Advax™ enhanced the anti-HBs IgG and IgM antibody response even if injected 24 hours prior to, but not if injected 24 hours after, the HBs antigen (Fig. 5A & 5C). If Advax™ was injected at the same time as the HBs antigen but into the opposite limb, unexpectedly the anti-HBs response was reduced. By contrast, alum was only effective if it was pre-absorbed and co-injected with HBs antigen, with no adjuvant effect if alum was injected before, after, or at a different site to the antigen (Fig. 5B & 5D).
Fig. 5. Advax™ activity is not dependent on antigen absorption.
Female BALB/c mice (n=6) were immunized i.m. in the hindlimb twice at 3 week interval with HBs 2µg alone or with Advax™ 0.5 mg or alum 0.1 mg. Adjuvants were injected 24 hours before or after HBs injection or at the same time but in the opposite limb site. Anti-HBs IgG and IgM from HBs and Advax™ (A, C respectively) or alum groups (B, D respectively) presented as mean + SEM. (NS: Not significant, *p < 0.05, ** p < 0.01, *** p < 0.001).
Advax™ enhances immunogenicity of a third generation HBs antigen
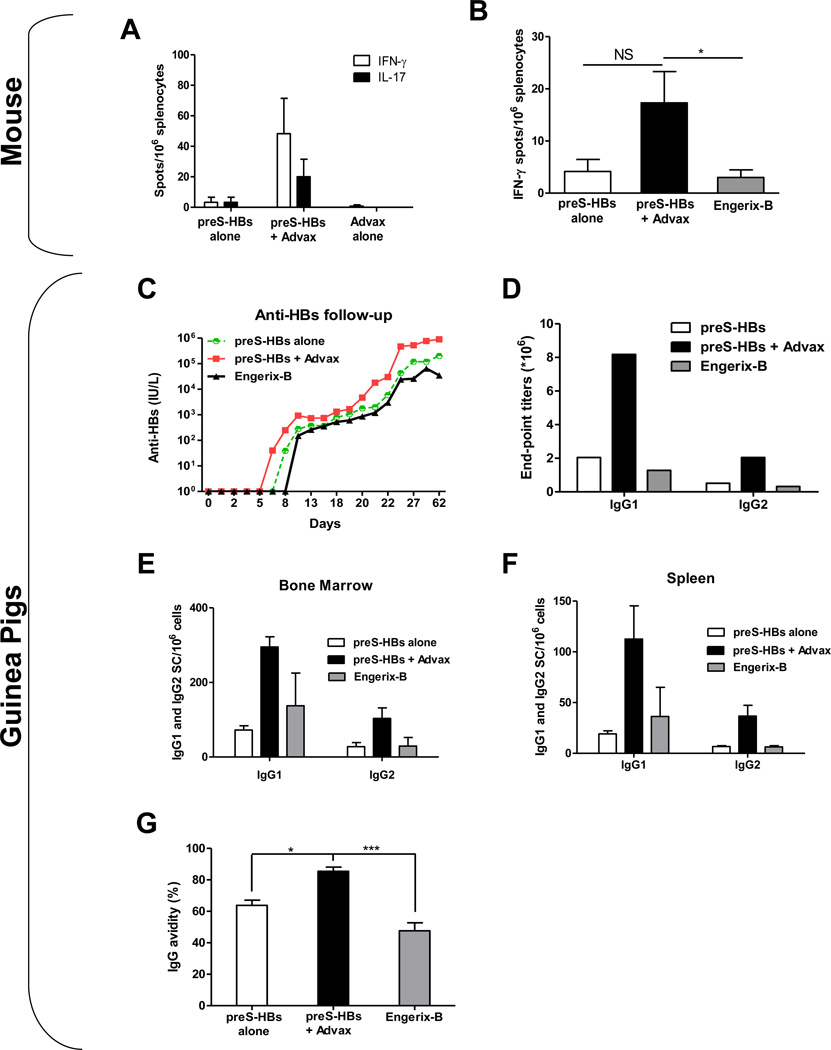
We next asked whether Advax™ similarly enhances responses to a third-generation CHO cell-derived preS antigen. As seen with second generation HBs antigens, Advax™ enhanced the anti-preS antibody response when compared to the effect of preS antigen alone (data not shown). Splenocytes of animals immunised with preS plus Advax™ demonstrated a higher frequency of HBs-specific T cells secreting IFN-γ or IL-17 compared to preS alone (Fig. 6A). Given potential development of preS-Advax™ as a therapeutic HBV vaccine, the ability of the preS-Advax™ formulation to induce anti-HBs IFN-γ-secreting T cells was directly compared to a commercial alum-based HBs vaccine (Engerix-B). Mice that received preS-Advax™ had an approximately 4-fold higher frequency of IFN-γ-secreting T cells (p<0.05) when compared to Engerix-B (Fig. 6B).
Fig. 6. Advax™ induces maximal antibody responses in mice and guinea pigs to a third generation preS HBV vaccine.
Balb/C mice (n=3) were immunized either (A) once with a high dose of preS (5µg) alone or with Advax™ adjuvant, with a control group administered Advax™ alone or (B) twice with a prime-boost regimen of preS (1µg) alone or with Advax™ adjuvant, with a control group administered Engerix-B. (A) PreS-stimulated IFN-γ (white bar) or IL-17 (black bar) responses of splenocytes collected 18 days after immunization with a single dose of preS alone or with Advax™, or Advax™ alone, demonstrating immunization with a combination of preS plus Advax™ induced the highest antigen-stimulated cytokine secretion after a single priming immunisation. (B) PreS-stimulated IFN-γ response by splenocytes collected 4 weeks after a second immunization of preS alone (white bar) or with Advax™ (back bar) or Engerix-B control (grey bar), demonstrating the highest recall IFN-γ response in animals receing prime-boost immunization with preS plus Advax™ (C–F) Guinea pigs (GP) (3 animals/ group) were immunized 3 times i.m. at 17 day intervals with either Engerix-B 20µg, preS 20µg alone or preS 20µg plus Advax™ 10mg. (C) Anti-HBs titers in pooled sera determined by AUSAB kit, demonstrating immunization with preS plus Advax™ induced ~10 fold higher titers than the other groups. (D) GP anti-preS IgG1 and IgG2 titers determined on pooled serum samples by end-point dilution ELISA, with the highest IgG1 and IgG2 titers in the group receiving preS plus Advax™. (E–F) Pre-S specific IgG1 or IgG2 ASC in bone marrow (E) or spleen (F) 8 months after immunization with Engerix-B (grey bars), preS alone (white bars) or with Advax™ (black bars). (G) Antibody avidity by urea elution for individual sera 4 weeks after immunization. (Asterisks designate significant differences (*p < 0.05, **p < 0.01, *** p < 0.001))
To assess whether the adjuvant effect of Advax™ translated to a larger species, guinea pigs (n=3/group) were immunized 3 times i.m. with either preS 20µg, preS-Advax™ 20µg/10mg or Engerix-B 20µg as the current commercial comparator. The preS-Advax™ induced the fastest antibody kinetics and the highest overall anti-HBs titers (Fig. 6C) with final anti-HBs titers for preS-Advax™ being ~10 fold higher than for Engerix-B (Fig 6D). After the first immunization, anti-HBs titers were first detectable on day 6 in the preS-Advax™ group, on day 8 in the preS group and on day 11 in the Engerix-B group, consistent with Advax™ accelerating the development of the anti-HBs IgG response (Fig 6C). The preS-Advax™ enhanced both IgG1 and IgG2 titers when compared to preS alone or Engerix-B (Fig. 6D). To test whether higher serum antibody titers reflected an increased frequency of antibody secreting cells (ASC) in the bone marrow or spleen of Advax™-immunized animals, the guinea pigs were sacrificed 8 months after the last immunization and assayed for anti-HBs ASC by ELISPOT. Animals that received preS-Advax™ had 2–4 fold higher IgG1 and IgG2 ASC in bone marrow and spleen when compared to animals immunized with preS alone or Engerix-B (Fig 6E & F), with these differences being similar to the differences in serum antibody titers between groups (Fig 6D). Animals that received preS alone or preS+Advax™ had ~2 fold higher ratio of IgG2 to IgG1 ASC compared to animals immunized with Engerix-B (Table 1). Since antibody quality may play an important role in vaccine protection alongside antibody quantity, the avidity of anti-HBs antibodies was compared using the urea denaturation procedure, as previously described [25]. Animals receiving preS-Advax™ had a larger amount of high avidity anti-HBs IgG when compared to preS alone (p<0.05) or Engerix-B (p<0.001) (Fig. 6G).
Table 1.
Guinea Pig IgG1/IgG2 ratio for Antibody Secreting Cells (ASC) in bone marrow and spleen, as compared to the IgG1/IgG2 ratio of serum antibody.
| preS alone IgG1/IgG2 |
preS + Advax IgG1/IgG2 |
Engerix-B IgG1/IgG2 |
|
|---|---|---|---|
| Bone marrow ASC | 3.2 | 3.2 | 6.1 |
| Spleen ASC | 2.9 | 3.6 | 5.1 |
| Serum antibody | 4 | 4 | 4 |
IgG1/IgG2 ratio for relative frequency of anti-HBs IgG1 and IgG2 ASC were measured by ELISPOT in bone marrow and spleen from guinea pigs 8 months post- the third immunization with preS alone, preS plus Advax™ or Engerix B. Anti-HBs IgG1 and IgG2 titers were measured in pooled guinea pig sera 2 months after the final immunization.
Advax™-formulated HBV vaccine has low reactogenicity
Enhancement of HBs vaccine potency should not be at the expense of safety or tolerability. To address this issue, safety and tolerability assessments were performed in guinea pigs immunized intramuscularly with a prospective human vaccine dose of preS + Advax™, namely 20µg and 10mg, respectively. Vaccine tolerability and safety were assessed by regular visual and manual inspection of the injection site, daily rectal temperatures and tracking of animal weights and clinical condition. Adavax™ was well tolerated with animals that received preS-Advax™ exhibiting no significant adverse reactions, fever, weight loss or change in clinical condition, post-immunization (data not shown).
Discussion
There is a need for more potent immunogenicity of vaccines for low responder populations and for potential therapeutic use. The results confirm that Advax™, a novel adjuvant derived from delta inulin, can successfully substitute for alum in recombinant HBV vaccines with the combination of Advax™ adjuvant with either a second-generation HBs vaccine or a third-generation preS vaccine resulting in superior B and T-cell responses in mice and guinea pigs. In particular, the anti-HBs T-cell responses induced by Advax™ were markedly superior to the alum comparator. In BALB/c mice that have a known Th2 bias [28], Advax™ predominantly increased total IgG via a stimulatory effect on IgG1 (Th2 isotype) together with a lesser effect on IgG2a (Th1 isotype) whereas in C57BL/6 mice Advax™ enhanced both IgG1 (Th2) and IgG2c (Th1) isotypes. Similarly, in guinea pigs where IgG1 also corresponds to a Th2 isotype and IgG2 to a Th1 isotype [29] Advax™ enhanced both IgG1 and IgG2 responses equaly, consistent with it being a non-polarizing adjuvant [20] compared to alum which causes a marked Th2 skew in immune response. Advax™ induced a high frequency of anti-HBs IgG and IgM ASC in spleen and bone marrow of both mice and guinea pigs, when compared to HBs alone or with alum, thereby explaining the higher serum antibody titers seen in Advax™ immunized animals. This suggests the mechanism whereby Advax™ enhances serum antibody titers is via an increase in total ASC number rather than by increasing the amount of antibody secreted per ASC. This could suggest that Advax™ directly or indirectly assists additional rounds of antigen-specific B-cell proliferation within secondary lymphoid tissues, although another possibility is that Advax™ confers a survival advantage to antigen-specific B cells such that more antigen-specific plasma cells live sufficiently long to find a survival niche within the bone marrow and spleen [30]. Interestingly, unlike other adjuvants [31, 32], no increase was found in the number of germinal centers in the draining lymph nodes of Advax™-immunized animals (unpublished data). The longevity of vaccine protection is likely to be predicted by the vaccine-induced frequency of ASC residing in the bone marrow and the spleen, organs that provide a long-term survival niche for plasma cells. This would explain the maintenance of high serum anti-HBs levels 8 months post-immunization in the guinea pigs receiving preS +Advax™ and similar observations in other studies that mice receiving for example Advax™ adjuvanted influenza vaccine maintained high serum antibody levels even one year post-immunization [20].
The ability to induce faster vaccine protection is a useful attribute, e.g. in travellers or pandemic vaccines where there may only be a short window between vaccination and exposure. As shown in the guinea pig study, Advax™ accelerated the kinetics of anti-HBs antibody appearance after primary immunization, with anti-HBs antibody detectable by day 6 after preS + Advax™ immunization as compared to day 11 for Engerix-B. The mechanism whereby Advax™ accelerated the antibody response is currently not known but will be an interesting area for future study.
Antibody quality as measured by avidity/affinity is equally important as antibody quantity in vaccine protection [33–35]. In the guinea pig study, Advax™ induced a higher amount of high avidity anti-HBs antibody consistent with an increase in not just the quantity, but also the quality, of the B-cell response by Advax™. In association with driving faster kinetics of antibody production, this suggests that Advax™ directly or indirectly drives additional rounds of antigen-specific B-cell proliferation within secondary lymphoid tissues with associated enhanced affinity maturation, a feature that is consistent with the higher anti-HBs ASC frequency seen in the bone marrow and spleen of Advax™-immunized animals.
Another important adjuvant attribute is antigen-sparing [36]. When formulated with Advax™, 4-fold less HBs antigen was required to achieve the same antibody titers as HBs formulated with alum. This is very similar to the levels of antigen-sparing previously seen with Advax™ for other antigens, e.g. influenza [20] or Japanese encephalitis [18] antigen. Antigen-sparing capability is critical for pandemic vaccines, where antigen supply is limiting but could also be useful for vaccines such as rabies, polio or Japanese encephalitis, to make them more affordable for developing world immunization programs [37].
Another important adjuvant attribute, particularly for therapeutic vaccines, is the ability to induce robust T-cell responses. Advax™ strongly increased HBs-specific CD4 and CD8 T-cell proliferative recall responses. Supernatants of HBs-stimulated splenocytes from Advax™-immunized animals demonstrated increases in Th1, Th2 and Th17 cytokines, consistent with a broad enhancement of memory T cell phenotypes. Increases in IFN-γ and IL17 production in culture supernatants of Advax™-immunized animals were paralleled by increases in the frequency of HBs-specific IFN-γ and IL17-secreting T cells by ELISPOT, indicating that increased Advax™-associated cytokine production occurs via an increase in the frequency of antigen-specific T cells rather than by an increase the amount of cytokine production per cell. The ability of Advax™ to induce strong cellular immunity against HBV may be useful for therapeutic approaches, as the inability of alum-based vaccines to induce cellular immunity has been implicated in their failure in previous therapeutic HBV vaccine trials [38]. The induction of memory CD8 T cells by exogenous antigen requires antigen cross-presentation, normally a feature restricted to dendritic cells [39]. The strong anti-HBs CD8 T-cell recall responses seen in Advax™-immunized animals indicate Advax™ enhances antigen cross-presentation possibly by a chemotactic effect that recruits dendritic cells to the site of immunization. Future studies will examine the functional nature of the CD8 T cells generated in the presence of Advax™, to see whether or not they have features of cytotoxic T lymphocytes and are able to kill HBs-labeled targets.
Although the exact mechanism of action of Advax™ remains to be fully characterised, the antigen absorption and temporal immunization studies provide important clues. Unlike alum, Advax™ did not adsorb HBs, and the temporal immunization studies confirmed that Advax™ did not require temporal association with the antigen for its adjuvant effect, as an adjuvant effect was evident even when Advax™ was injected 24 hours prior to injection of the antigen. Similar results showing Advax™ still has adjuvant activity when injected prior to the antigen at the same site have been observed for influenza vaccine (unpublished data), confirming this temporal effect is not restricted to HBs. As previously found by other studies (40), alum had no adjuvant effect unless pre-absorbed and co-injected with the HBs antigen. Interestingly, when Advax™ was injected at the same time as the HBs antigen but into the opposite limb it actually reduced the anti-HBs antibody response, suggesting it might thereby be acting as an immune decoy, e.g. drawing dendritic cells away from the site of antigen injection on the other side of the body. This indicates that Advax™ does not require direct contact with the antigen, but rather might prime a local antigen presenting cell population, e.g. a dendritic cell, that upon later injection of the antigen, has enhanced antigen uptake capability and/or enhanced ability to present the antigen to T or B cells, e.g. via upregulation of antigen presentation molecules such as MHC class I or class II and/or upregulation of co-stimulatory molecules such as CD11c, CD40, CD80 and CD86.
Whilst the mechanism of action of this intriguing new polysaccharide adjuvant will be important to decipher, the critical attributes for prospective human vaccine adjuvants are efficacy, safety and tolerability. Reassuringly, there was no evidence of any local or systemic toxicity from multiple injections of Advax™ adjuvant with HBs antigen. This is consistent with data from a recent human influenza vaccine trial that confirmed that Advax™ was safe and well tolerated [23]. A clinical trial is currently being planned to test the preS + Advax™ vaccine developed in this study, for its effectiveness in a human low responder population.
Highlights.
Advax adjuvant provided HBs dose-sparing when compared to alum
Advax induced strong anti-HBs CD4 and CD8 T cell responses
Advax simultaneously enhanced Th1, Th2 and Th17 responses
Unlike alum, Advax worked even when injected 24 hours before the antigen
Acknowledgements
We thank Bruce Lyons for performing the HBs temporal immunization study as an employee of Vaxine. The Butantan Institute, Brazil is thanked for the gift of the HBs antigen. Stacey Gorski is thanked for critical review of the manuscript and Helene Mauboussin, Sandra Laloi and Anna Derks for their technical assistance with animal experiments. This work was supported by AusIndustry through the Biotechnology Innovation Fund, START, Commercial Ready and IIFSA funding programs. This work was also supported in part by contracts U01 AI061142 and HHSN272200800039C from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and competing interests disclosure
NP, FS, YHO, and ST are directors and/or employees of Vaxine Pty Ltd, the company owning the intellectual property over Advax™ adjuvants.
References
- 1.Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. The American journal of medicine. 1989 Sep 4;87(3A):14S–20S. doi: 10.1016/0002-9343(89)90525-1. [DOI] [PubMed] [Google Scholar]
- 2.Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63(10):1021–1051. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 3.Milich DR, Thornton GB, Neurath AR, Kent SB, Michel ML, Tiollais P, et al. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- 4.Milich DR, McLachlan A, Chisari FV, Kent SB, Thorton GB. Immune response to the pre-S(1) region of the hepatitis B surface antigen (HBsAg): a pre-S(1)-specific T cell response can bypass nonresponsiveness to the pre-S(2) and S regions of HBsAg. Journal of immunology. 1986 Jul 1;137(1):315–322. [PubMed] [Google Scholar]
- 5.Shapira MY, Zeira E, Adler R, Shouval D. Rapid seroprotection against hepatitis B following the first dose of a Pre-S1/Pre-S2/S vaccine. Journal of hepatology. 2001 Jan;34(1):123–127. doi: 10.1016/s0168-8278(00)00082-9. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman JN, Zuckerman AJ. Recombinant hepatitis B triple antigen vaccine: Hepacare. Expert review of vaccines. 2002 Aug;1(2):141–144. doi: 10.1586/14760584.1.2.141. [DOI] [PubMed] [Google Scholar]
- 7.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001 Feb 28;19(15–16):2055–2060. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 8.Mahboubi A, Fazeli MR, Dinarvand R, Samadi N, Sharifzadeh M, Ilka H, et al. Comparison of the adjuvanticity of aluminum salts and their combination in hepatitis B recombinant protein vaccine in assessed mice. Iranian journal of immunology : IJI. 2008 Sep;5(3):163–170. doi: 10.22034/iji.2008.17162. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert CL, Klopfer SO, Martin JC, Schodel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults >/=50 years. Human vaccines. 2011 Dec;7(12):1336–1342. doi: 10.4161/hv.7.12.18333. [DOI] [PubMed] [Google Scholar]
- 10.Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, et al. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine. 2011 Nov 21;29(50):9316–9320. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis B virus vaccine in chronic kidney disease: improved immunogenicity by adjuvants? A meta-analysis of randomized trials. Vaccine. 2012 Mar 16;30(13):2295–2300. doi: 10.1016/j.vaccine.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 12.Levie K, Gjorup I, Skinhoj P, Stoffel M. A 2-dose regimen of a recombinant hepatitis B vaccine with the immune stimulant AS04 compared with the standard 3-dose regimen of Engerix-B in healthy young adults. Scandinavian journal of infectious diseases. 2002;34(8):610–614. doi: 10.1080/00365540110080881. [DOI] [PubMed] [Google Scholar]
- 13.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. Journal of clinical immunology. 2004 Nov;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 14.Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine. 2012 Mar 28;30(15):2556–2563. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 15.Nevens F, Zuckerman JN, Burroughs AK, Jung MC, Bayas JM, Kallinowski B, et al. Immunogenicity and safety of an experimental adjuvanted hepatitis B candidate vaccine in liver transplant patients. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006 Oct;12(10):1489–1495. doi: 10.1002/lt.20836. [DOI] [PubMed] [Google Scholar]
- 16.Petrovsky N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert review of vaccines. 2008 Feb;7(1):7–10. doi: 10.1586/14760584.7.1.7. [DOI] [PubMed] [Google Scholar]
- 17.Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, et al. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine. 2006 Jan 9;24(1):20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- 18.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, et al. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. The Journal of general virology. 2010 Jun;91(Pt 6):1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, et al. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. The Journal of general virology. 2011 Jan;92(Pt 1):128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda-Okubo Y, Saade F, Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012 Aug 3;30(36):5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011 Aug 26;29(37):6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckersley AM, Petrovsky N, Kinne J, Wernery R, Wernery U. Improving the dromedary antibody response: The hunt for the ideal camel adjuvant. J Camel Pract And Res. 2011;18(1):35–46. [Google Scholar]
- 23.Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012 Aug 3;30(36):5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diminsky D, Schirmbeck R, Reimann J, Barenholz Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997 Apr-May;15(6–7):637–647. doi: 10.1016/s0264-410x(96)00239-3. [DOI] [PubMed] [Google Scholar]
- 25.Hedman K, Rousseau SA. Measurement of avidity of specific IgG for verification of recent primary rubella. Journal of medical virology. 1989 Apr;27(4):288–292. doi: 10.1002/jmv.1890270406. [DOI] [PubMed] [Google Scholar]
- 26.Yuasa T, Shimojo H. Potency test of hepatitis B vaccines by the parallel line assay method in mice. Japanese journal of medical science & biology. 1985 Feb;38(1):9–18. doi: 10.7883/yoken1952.38.9. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- 28.Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Annales de l'Institut Pasteur Immunology. 1987 Sep-Oct;138(5):744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 29.Tayyari F, Sutton TC, Manson HE, Hegele RG. CpG-oligodeoxynucleotides inhibit RSV-enhanced allergic sensitisation in guinea pigs. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005 Feb;25(2):295–302. doi: 10.1183/09031936.05.00016304. [DOI] [PubMed] [Google Scholar]
- 30.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature reviews Immunology. 2006 Oct;6(10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 31.Bemark M, Bergqvist P, Stensson A, Holmberg A, Mattsson J, Lycke NY. A unique role of the cholera toxin A1-DD adjuvant for long-term plasma and memory B cell development. Journal of immunology. 2011 Feb 1;186(3):1399–1410. doi: 10.4049/jimmunol.1002881. [DOI] [PubMed] [Google Scholar]
- 32.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011 Feb 24;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA : the journal of the American Medical Association. 1992 Mar 18;267(11):1489–1494. [PubMed] [Google Scholar]
- 34.Lucas AH, Granoff DM. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. Journal of immunology. 1995 Apr 15;154(8):4195–4202. [PubMed] [Google Scholar]
- 35.Granoff DM, Shackelford PG, Holmes SJ, Lucas AH. Variable region expression in the antibody responses of infants vaccinated with Haemophilus influenzae type b polysaccharide-protein conjugates. Description of a new lambda light chain-associated idiotype and the relation between idiotype expression, avidity, and vaccine formulation. The Collaborative Vaccine Study Group. The Journal of clinical investigation. 1993 Mar;91(3):788–796. doi: 10.1172/JCI116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolai Petrovsky SH, Yoshikazu Honda A. Bruce Lyons. New-Age Vaccine Adjuvants: Friend or Foe? BioPharm International. 2007 Aug [Google Scholar]
- 37.Smith J, Lipsitch M, Almond JW. Vaccine production, distribution, access, and uptake. Lancet. 2011 Jul 30;378(9789):428–438. doi: 10.1016/S0140-6736(11)60478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert review of gastroenterology & hepatology. 2009 Oct;3(5):561–569. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- 39.Heath WR, Carbone FR. Cytotoxic T lymphocyte activation by cross-priming. Current opinion in immunology. 1999 Jun;11(3):314–318. doi: 10.1016/s0952-7915(99)80050-8. [DOI] [PubMed] [Google Scholar]
- 40.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007 Oct;6(5):685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]