Abstract
Molecular control of the pluripotent state is thought to reside in a core circuitry of master transcription factors including the homeodomain-containing protein Nanog1–2, which plays an essential role in establishing ground state pluripotency during somatic cell reprogramming3–4. While the genomic occupancy of Nanog has been extensively investigated, comparatively little is known about Nanog-associated proteins5 and their contribution to the Nanog-mediated reprogramming process. Using enhanced purification techniques and a stringent computational algorithm, we identified 27 high-confidence protein interaction partners of Nanog in mouse ES cells. These consist of 19 novel partners of Nanog that have not been reported before including the Ten eleven translocation (Tet) family methylcytosine hydroxylase Tet1. We confirmed physical association of Nanog with Tet1, and demonstrated that Tet1, in synergy with Nanog, enhances the efficiency of reprogramming. We also found physical association and reprogramming synergy of Tet2 with Nanog, and demonstrated that knockdown of Tet2 abolishes the reprogramming synergy of Nanog with a catalytically deficient mutant of Tet1 (Tet1Mut). These results indicate that the physical interaction between Nanog and Tet1/2 proteins facilitates reprogramming in a manner that is dependent on Tet1/2's catalytic activity. Tet1 and Nanog co-occupy genomic loci of genes associated with both maintenance of pluripotency and lineage commitment in ES cells, and Tet1 binding is reduced upon Nanog depletion. Co-expression of Nanog and Tet1 results in expression priming of and increased 5hmC levels at top ranked common targets Esrrb and Oct4 before reprogramming to naïve pluripotency. We propose that Tet1 is recruited by Nanog to enhance the expression of a subset of key reprogramming target genes. These results provide an insight into the reprogramming mechanism of Nanog and uncover a novel role for 5mC hydroxylases in the establishment of naïve pluripotency.
Keywords: Nanog, Tet1, Tet2, pluripotency, reprogramming, self-renewal, epigenetics
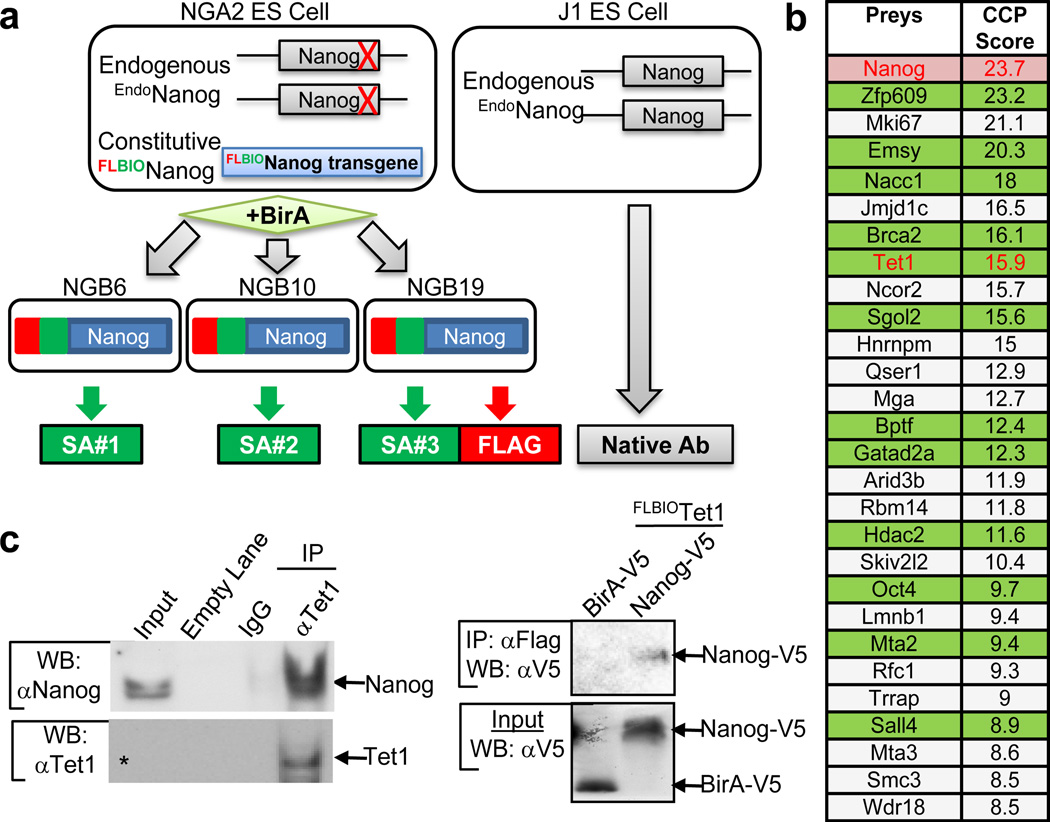
We expanded the Nanog interactome in mouse ES cells using an improved affinity purification and mass spectrometry (AP-MS) strategy6–8 (see Supplementary Information). This analysis identified 27 high-confidence interaction partners of Nanog (Fig. 1a-b, Supplementary Figs. 1–4 and Supplementary Tables 1–2). Notable among the 19 novel interaction partners of Nanog was the methylcytosine hydroxylase Tet19–10 (Fig. 1b). Specific association of Tet1 with Nanog was detected in all five affinity purification runs of three independent APs (Supplementary Fig. 5b and Supplementary Table 2), and the interaction between Nanog and Tet1 was further confirmed by immunoprecipitation and co-immunoprecipitation (IP/coIP) (Fig. 1c and Supplementary Fig. 5c-d). While Nanog clearly associates with Tet1 in ES cells, there also exists Tet1-free Nanog protein as shown by immunodepleting Tet1 in ES cells (Supplementary Fig. 5e). Notably, among the 27 high-confidence interaction partners of Nanog, at least 5 (Nacc1, Sgol2, Qser1, Hdac2, and Oct4) were also associated with Tet1 by coIP and/or IP-MS experiments (Supplementary Fig. 5f-h). Expression of Tet1, like that of Nanog, is up-regulated during reprogramming to pluripotency (Supplementary Fig. 6a). Since Nanog is a critical determinant during establishment of pluripotency3, 11, we investigated whether Tet1 may also be required for efficient nuclear reprogramming. Indeed, RNAi-mediated inhibition of Tet1 during reprogramming reduced generation of induced pluripotent stem (iPS) cells from MEFs (Supplementary Fig. 6b-g and Supplementary Fig. 7). The requirement of Tet1 for efficient reprogramming was confirmed using an independent, heterokaryon-based reprogramming system12 (Supplementary Fig. 8).
Fig. 1. Identification of Tet1 as a novel partner of Nanog.
a, Schematic depiction of ES cells expressing Nanog with Flag (FL) and Biotin (BIO) tags (left), and Nanog antibody (Ab) based affinity purification (right). b, List of 27 preys identified as true interactors ordered by combined cumulative probability (CCP) score. Candidates shaded in green are the ones whose interaction with Nanog has been validated previously5, 25–26 and in this study by IP/coIP. Two previously identified Nanog partners, Dax1 and Zfp2815, were identified by MS (Supplementary Fig. 5a), but not selected as high-confidence interactors using our stringent criteria. c, Validation of Nanog-Tet1 interaction by IP/coIP in ES (left) and HEK293T (right) cells. The asterisk indicates the presence of Tet1 in input that can be visualized under longer exposure (Supplementary Fig. 5c).
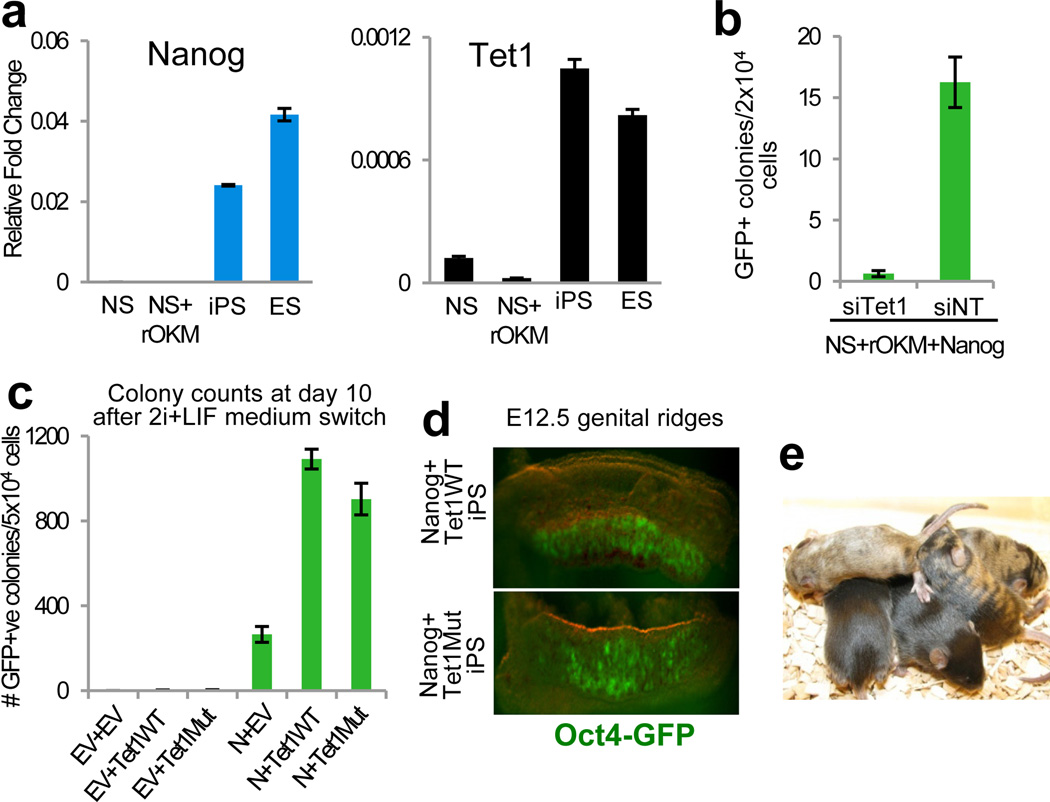
The physical association of Tet1 with Nanog prompted us to consider whether Tet1 may modulate Nanog function in establishing pluripotency. Nanog and Tet1 are only minimally expressed in reprogramming intermediates resulting from retroviral infection of neural stem (NS) cells with the reprogramming factors Oct4, Klf4 and c-Myc (rOKM) (Fig. 2a). We addressed whether Nanog-mediated reprogramming of these cells requires functional contribution of Tet1. A clonal line of reprogramming intermediates was transfected with a PiggyBac (PB) Nanog transgene followed by addition of siRNA against Tet1 (Supplementary Fig. 9a-b). Down-regulation of Tet1 reduced Nanog reprogramming efficiency by 26-fold compared with the non-targeting control (Fig. 2b and Supplementary Fig. 9c), suggesting that Tet1 and/or its associated catalytic activity may be a limiting factor for reprogramming by Nanog.
Fig. 2. Synergy between Nanog and Tet1 during reprogramming.
a, Nanog and Tet1 are specifically expressed in pluripotent cells. b, Knockdown of Tet1 compromises reprogramming activity of a constitutive Nanog transgene in reprogramming intermediates. c, Both wild-type and mutant Tet1 enhance Nanog-dependent reprogramming. Quantification of the number of iPS colonies at day 10 of 2i/LIF treatment in Supplementary Fig. 10b is shown. d-e, Contribution of iPS cells generated with Nanog and Tet1WT (top) or Tet1Mut (bottom) transgenes to the germline at E12.5 (d) and live-born chimeras (e). siTet1, siRNA against Tet1; siNT, non-targeting siRNA control; +rOKM, adult NS cells transduced with retroviral Oct4, Klf4, and c-Myc transgenes. Error bars indicate standard deviation (n=3).
We then asked if ectopic Tet1 expression could enhance Nanog reprogramming activity. NS+rOKM cells were transfected with PB vectors expressing Nanog, Tet1, or Tet1 bearing two mutations in the catalytic domain (Tet1H1671Y, D1673A or Tet1Mut)10 (Supplementary Fig. 10a). Individual expression of Tet1WT or Tet1Mut did not have a significant effect on generation of Oct4-GFP positive colonies (Fig. 2c and Supplementary Fig. 10b-c). In contrast, Nanog expression enhanced the generation of iPS cell colonies by more than 10-fold (Fig. 2c and Supplementary Fig. 10b-c), in accordance with previous studies11, 13. Importantly, Nanog-mediated reprogramming efficiency was further augmented by up to 4-fold in the presence of Tet1WT transgene, a synergistic effect that is highly reproducible (Fig. 2c and Supplementary Fig. 10b-e). A similar reprogramming synergy was also observed for the combination of Nanog with Tet1Mut (Fig. 2c and Supplementary Fig. 10b-e). iPS cells derived with Nanog and either Tet1WT or Tet1Mut transgenes contributed to the germ lineage and live-born chimeras following blastocyst injection (Fig. 2d-e). Together, our data show that Nanog and Tet1 enhance the efficiency of somatic cell reprogramming in a cooperative manner. This conclusion was corroborated in MEFs, where the combined action of Nanog and Tet1WT increased reprogramming efficiency by up to 16-fold (Supplementary Fig. 11).
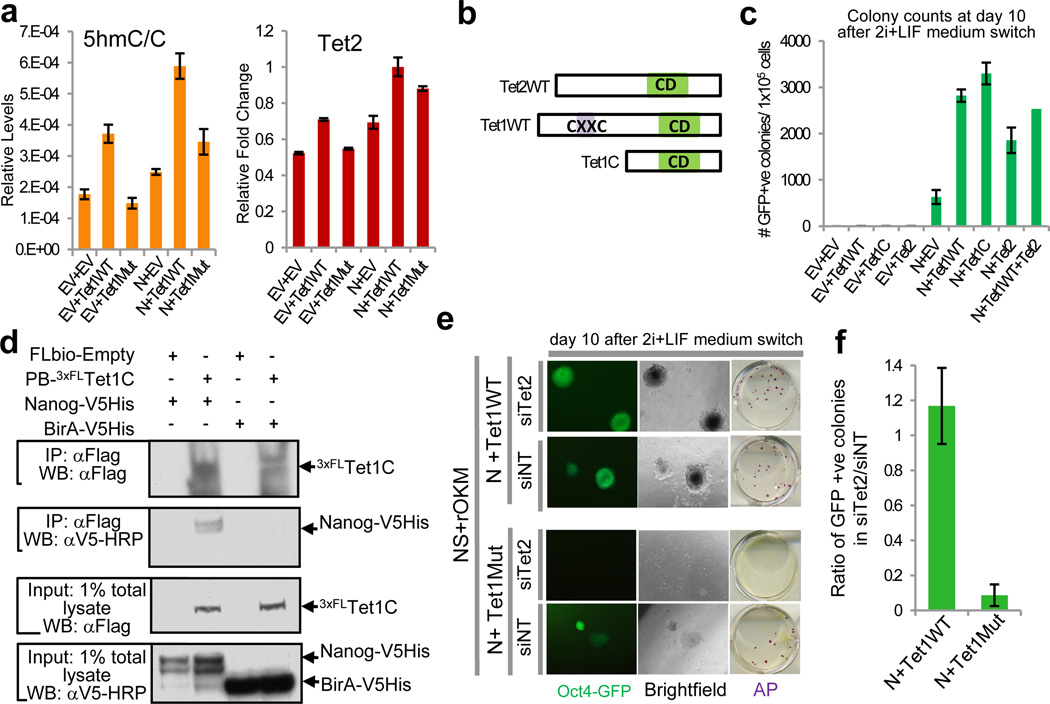
To explore the molecular mechanism underlying the Nanog-Tet1 partnership during reprogramming, we quantified global 5hmC levels14. As expected, 5hmC levels were increased upon Tet1WT but not Tet1Mut expression in NS+rOKM cells (Fig. 3a, left). Unexpectedly, co-expression of Nanog and either Tet1WT or Tet1Mut resulted in increased 5hmC levels (Fig. 3a, left). These results suggest that Nanog can potentiate 5hmC modifications by its association with Tet1, and that transcriptional activation of endogenous Tet1 and/or its paralog Tet2 may compensate for the lack of catalytic activity of Tet1Mut during reprogramming with Nanog. Indeed, Tet2 was upregulated by Nanog and Tet1WT or Tet1Mut, and its expression levels follow a very similar trend to that of 5hmC/C levels (Fig. 3a and Supplementary Fig. 10f-g). Tet2 was identified in two out of three independent APs in our Nanog interactomics study (Supplementary Fig. 12a-b), and physical association of Tet2 with Nanog was confirmed by IP/coIP (Supplementary Fig. 12c-d). Tet2 was recently found to contribute to an epigenetic program that directs subsequent transcriptional induction at the pluripotency loci Nanog and Esrrb during the early stage of somatic cell reprogramming15. Tet1 and Tet2 share the common C-terminal catalytic domain but are divergent in their N termini for a CXXC DNA-binding domain, which renders Tet2 functionally similar to a truncated form of Tet1, Tet1C (Fig. 3b). We investigated whether the catalytic activity of Tet1 is sufficient to enhance Nanog-mediated reprogramming. Indeed, Tet1C acts together with Nanog to enhance reprogramming (Fig. 3c) and retains its physical association with Nanog (Fig. 3d). Not surprisingly, we also observed reprogramming synergy between Nanog and Tet2 (Fig. 3c and Supplementary Fig. 13). Tet1 and Tet2 function is redundant in the context of Nanog-induced reprogramming, as exogenously expressing both Tet enzymes together with Nanog does not enhance somatic cell reprogramming beyond expressing Nanog with either individual enzyme (Fig. 3c).
Fig. 3. Synergy between Nanog and Tet1/2 during reprogramming is dependent upon catalytic activity of Tet1/2.
a, Measurement of global levels of 5hmC (left) and Tet2 expression (right) in reprogramming intermediates transfected with PB transgenes. b, Schematic depiction of wild-type (WT) Tet1, Tet2, and the truncated Tet1 mutant (Tet1C). Note the absence of a CXXC DNA binding domain in Tet2 and Tet1C proteins. c, Quantification of GFP+ iPS colonies. d, Physical association of Nanog with Tet1C. CoIP was performed in HEK293T cells. e, Tet2 knockdown (siTet2) reduces reprogramming efficiency in intermediate cells transgenic for Nanog+Tet1Mut compared to Nanog+Tet1WT. Non-targeting siRNA (siNT) serves as a control. f, Quantification of the number of iPS colonies in (e). Error bars indicate standard deviation (n=3). CD, catalytic domain.
Given that endogenous Tet2 was up-regulated in the presence of Nanog and Tet1Mut (Fig. 3a right and Supplementary Fig. 10f), and Tet2 synergizes with Nanog during reprogramming (Fig. 3c), we tested whether knockdown of Tet2 could abrogate the reprogramming synergy of Nanog and Tet1. Indeed, siRNAs directed against Tet2 diminished the reprogramming synergy of Nanog and Tet1Mut, but did not affect that of Nanog and Tet1WT (Fig. 3e-f). This result confirms that Tet2 activation compensates for the lack of catalytic activity of Tet1Mut during reprogramming with Nanog. Together, our results demonstrate that neither Tet1 nor Tet2 is sufficient for the induction of pluripotency (Fig. 2c, Supplementary Fig. 10–11, and Fig. 3c), but either enzyme can partner with Nanog to enhance reprogramming of somatic cells to naïve pluripotency.
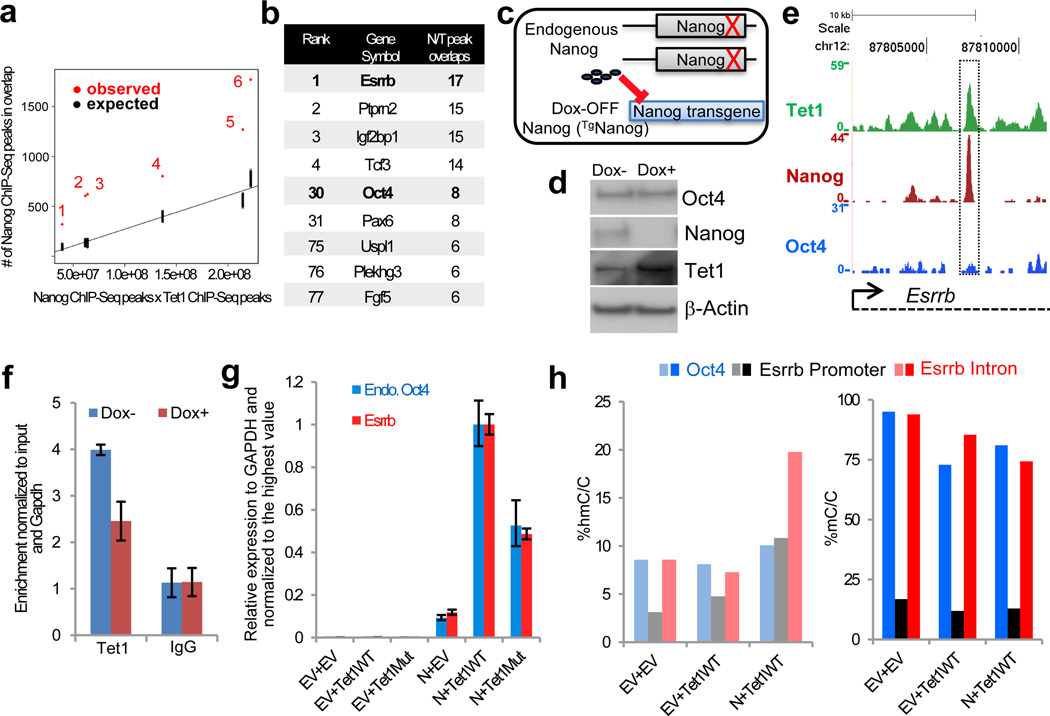
We compared deposited ChIP-Seq data for both Nanog16–17 and Tet118–19 and found a statistically significant overlap between Nanog and Tet1 binding sites in the mouse ES cell genome (p < 2e–4, permutation test) (Fig. 4a and Supplementary Table 3). Gene ontology (GO) analysis revealed that genes with roles in “multicellular organismal development” and “positive regulation of transcription from Pol II promoter” are enriched in the common targets (Supplementary Fig. 14). We ranked the common target genes of Nanog and Tet1 based on the number of overlapping ChIP-Seq peaks in four studies (Fig. 4b and Supplementary Table 4). Among the common targets with the highest number of overlapping Nanog and Tet1 peaks was Esrrb (Fig. 4b). To investigate whether Nanog may be required to direct Tet1 to shared target genes, we used ES cells containing an inducible Nanog transgene in a Nanog−/− background21 (Fig. 4c-d). Loss of Nanog expression reduced Tet1 binding to a number of common targets, including Esrrb (Fig. 4e-f and Supplementary Fig. 15). Nanog-dependent binding of Tet1 to the Esrrb locus appears to be independent of Oct4, as Oct4 is not present at the same genomic location (Fig. 4e). Thus, Nanog is responsible for the recruitment of Tet1 to a subset of shared genomic loci that are implicated in both the regulation of pluripotency (e.g., Esrrb) and lineage commitment (e.g., Pax6). Such Nanog-dependent target binding of Tet1 is highlighted by the fact that the truncated form of Tet1 lacking the CXXC DNA binding domain (i.e., Tet1C) maintains its physical interaction and reprogramming synergy with Nanog (Fig. 3b-d).
Fig. 4. Mechanism and genome-wide significance of the Nanog/Tet1 interaction.
a, Scatterplot showing the observed vs. expected overlap in genomic binding sites of Nanog and Tet1 according to comparisons performed in Supplementary Table 3. b, Ranked list of common targets of Nanog and Tet1 based on the comparisons in Supplementary Table 3. c, Schematic representation of ES cells harboring a doxycycline (Dox)-suppressible Nanog transgene in a Nanog−/− genetic background21. d, Western blot analysis of Oct4, Nanog, and Tet1 expression in NgcKO ES cells treated with (+) or without (-) Dox. e, Overlapping peaks of Tet1 and Nanog from ChIP-Seq studies16–18 in the Esrrb locus. f, Relative enrichment of Tet1 in the absence (−) and presence (+) of Dox in the Esrrb genomic locus as shown in (e). g, Transcriptional priming of Esrrb and Oct4 by Nanog and Tet1 in reprogramming intermediates. h, Relative enrichment of 5hmC and 5mC in the Esrrb and Oct4 loci. Error bars indicate standard deviation (n=3).
Since the functional synergy between Nanog and Tet enzymes was dependent on catalytic activity, we examined 5hmC levels at Nanog/Tet1 peaks in mouse ES cells. A recent study reported 5hmC enrichment at promoter-distal NANOG binding sites in human ES cells22 (Supplementary Fig. 16a). In contrast, we observed an inverse correlation between 5hmC and Nanog/Tet1 binding at actively expressed target genes in mouse ES cells (Supplementary Fig. 16). This led us to consider whether 5hmC may be transiently deposited to common Nanog/Tet1 targets prior to the establishment of pluripotency, that is, during in vitro reprogramming when Nanog is required3. We focused on target gene regulation of Esrrb and Oct4, two key pluripotency genes that are among top ranked common targets of Nanog and Tet1 (Fig. 4b). Significantly, we observed expression priming of both Esrrb and Oct4 by combined expression of Nanog with Tet1WT, Tet1Mut, Tet1C or Tet2 in reprogramming intermediates of two independent cellular systems (Fig. 4g, Supplementary Figs. 11g and 13b). More importantly, we detected increased 5hmC and decreased 5mC levels at these loci when Nanog is co-expressed with Tet1 (Fig. 4h). Thus, Nanog and Tet1 act before the transition to naïve pluripotency by inducing local transcriptional changes in shared target genes that are critically involved in the regulation of pluripotency.
In summary, we identified 5mC hydroxylases Tet1 and Tet2 as novel interaction partners of Nanog. Tet1/2 and Nanog synergistically enhance the efficiency of reprogramming and this phenotype is dependent on the hydroxylation of 5mC to 5hmC during somatic cell reprogramming. This study thus provides mechanistic insight into how Nanog establishes pluripotency, demonstrating that interactions between Nanog and epigenetic regulators fine-tune induced pluripotency. Future experimental work is needed to delineate the precise composition of Nanog-Tet1/2 protein complexes, and the contribution of other interaction partners to the reprogramming mechanism described herein. Our work supports an emerging view that Tet proteins can overcome epigenetic roadblocks during reprogramming and transdifferentiation15, 23.
METHODS SUMMARY
Affinity purification coupled with mass spectrometry (AP-MS)
Nuclear extraction and affinity purification of FLBIONanog-associated protein complexes were performed as previously described5, with several modifications as described7. Three biological replicates were performed for SA agarose-based affinity purification and one each for Flag and Nanog antibody-based affinity purifications. Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) was employed by the Taplin Biological Mass Spectrometry Facility at Harvard Medical School to sequence and identify Nanog AP samples.
Reprogramming assays
To investigate the consequences of Nanog and Tet1 co-expression during reprogramming, adult NS cells were infected with pMX-based retroviral reprogramming factors24. Cultures were switched to ES cell medium (serum/LIF) at day 3 post-transduction. A clonal line of proliferative, Oct4-GFP negative cells (reprogramming intermediates) was transfected using nucleofection (Amaxa) with various combinations of Nanog and Tet1 PB transgenes. Selection for stable transgene expression was applied to transfectants for a minimum of 12 days and maintained until medium switch to 2i/LIF. Puromycin selection for an Oct4-GFP-IRES-puro reporter transgene was applied from day 6 of 2i/LIF treatment. GFP-positive colonies were scored at day 10. Similar reprogramming assays were applied to Nanog-GFP-IRES-puro reporter MEFs with modifications described in Supplemental Information.
Full Methods and any associated references are available in the Supplemental Information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank William Mansfield for blastocyst injections, Aliaksandra Radzisheuskaya for cell culture assistance, and Dr. Rudolf Jaenisch at MIT for Tet1−/− ES cells. This study was supported by a grant from the NIH (1R01-GM095942-01A1), a grant from NY state Dept. of Health (NYSTEM#C026420), and a seed fund from the Black Family Stem Cell Institute to J.W., by the Wellcome Trust Fellowship WT086692MA and the Isaac Newton Trust Grant 11.19(ad) to J.C.R.S., who is a Wellcome Trust Career Development Fellow, and by the Wellcome Trust Fellowship WT079249 to T.W.T.
Footnotes
Author Contributions
J.C.R.S. and J.W. conceived the project, designed the experiments, prepared and approved the manuscript.
T.W.T. designed and performed experiments and wrote the manuscript draft.
Y.C., J.D., F.F., M.F., and A.S. designed and performed experiments and prepared the manuscript.
T.A.H. designed and performed experiments.
P.V.S. performed interactomics data analysis.
M.L. provided technical assistance.
S.D. provided bioinformatic analysis.
S.D., D.N.L., Z.L., and M.X. contributed to the reagents.
W.R. designed experiments and contributed to the reagents.
The authors declare no competing financial interests.
References
- 1.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 2.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 3.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. doi:S0092-8674(09)00969-6 [pii] 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. doi:nature05284 [pii] 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Cantor AB, Orkin SH, Wang J. Use of in vivo biotinylation to study protein-protein and protein-DNA interactions in mouse embryonic stem cells. Nature protocols. 2009;4:506–517. doi: 10.1038/nprot.2009.23. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Xu H, Faiola F, Ma'ayan A, Wang J. Oct4 links multiple epigenetic pathways to the pluripotency network. Cell research. 2011 doi: 10.1038/cr.2011.179. doi:cr2011179 [pii]10.1038/cr.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Cantor AB, Orkin SH. Tandem affinity purification of protein complexes in mouse embryonic stem cells using in vivo biotinylation. Current protocols in stem cell biology. 2009:5. doi: 10.1002/9780470151808.sc01b05s8. Chapter 1, Unit1B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science (New York, N.Y. 2009;324:930–935. doi: 10.1126/science.1170116. doi:1170116 [pii]10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theunissen TW, et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol. 2011;21:65–71. doi: 10.1016/j.cub.2010.11.074. doi:S0960-9822(10)01584-8 [pii]10.1016/j.cub.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira CF, Fisher AG. Heterokaryon-based reprogramming for pluripotency. Current protocols in stem cell biology. 2009:1. doi: 10.1002/9780470151808.sc04b01s9. Chapter 4, Unit 4B. [DOI] [PubMed] [Google Scholar]
- 13.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. doi:nature08592 [pii]10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ficz G, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011 doi: 10.1038/nature10008. doi:nature10008 [pii]10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 15.Doege C, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. doi:10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Marson A, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. doi:S0092-8674(08)00938-0 [pii] 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. doi:nature10066 [pii]10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, et al. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011:389–393. doi: 10.1038/nature09934. doi:nature09934 [pii]10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawlaty MM, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell stem cell. 2011;9:166–175. doi: 10.1016/j.stem.2011.07.010. doi:S1934-5909(11)00340-7 [pii]10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das S, Jena S, Levasseur DN. Alternative Splicing Produces Nanog Protein Variants with Different Capacities for Self-Renewal and Pluripotency in Embryonic Stem Cells. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.290189. doi:M111.290189 [pii]10.1074/jbc.M111.290189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. doi:S0092-8674(12)00534-X [pii]10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallin EM, et al. Tet2 Facilitates the Derepression of Myeloid Target Genes during CEBPalpha-Induced Transdifferentiation of Pre-B Cells. Mol Cell. 2012;48:266–276. doi: 10.1016/j.molcel.2012.08.007. doi:S1097-2765(12)00694-6 [pii]10.1016/j.molcel.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS biology. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Levasseur DN, Orkin SH. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6326–6331. doi: 10.1073/pnas.0802288105. doi:0802288105 [pii] 10.1073/pnas.0802288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang J, et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nature cell biology. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.