Abstract
Purpose
Parallel MRI methods are typically associated with a degradation of the signal-to-noise ratio (SNR). High scan time reduction factors are therefore restricted to applications with high intrinsic SNR. One possibility to increase the intrinsic SNR is to simultaneously excite several slices by means of multiband radio-frequency (RF) pulses and subsequently separate the slices by parallel MRI reconstruction algorithms. However, the separation of closely spaced slices may suffer from severe noise amplification when there is insufficient coil sensitivity variation along the slice direction. The purpose of this work is to apply a phase-constrained reconstruction for multiband experiments in order to minimize the noise amplification.
Methods
Pre-defined phase differences between neighboring slices are induced and slice separation is performed by a phase-constrained parallel MRI reconstruction. Phase differences between neighboring slices are tailored to achieve optimal slice separation with minimized noise amplification. The potential of the method is demonstrated through multiband in-vivo experiments.
Results
Noise amplification in multiband phase-constrained reconstructions is significantly reduced in comparison to standard multiband reconstruction when the phase difference between neighboring slices (distance = 12 mm) is 90°.
Conclusions
Multiband phase constrained parallel MRI has the potential for accelerated multi-slice imaging with an improved SNR performance.
Keywords: Parallel MRI, radio-frequency pulses, multi-slice imaging, GRAPPA, SENSE
INTRODUCTION
Scan time reductions in MRI can be achieved by applying parallel imaging methods. Typically, this is accomplished by regularly undersampling the k-space, which leads to well-known aliasing artifacts. Specialized reconstruction algorithms, such as Sensitivity Encoding (SENSE) (1) or Generalized Auto-calibrating Partially Parallel Acquisitions (GRAPPA) (2), generate non-aliased images from the undersampled data by incorporating knowledge about spatial sensitivity variations within a multi-coil receiver array.
In clinical applications, the use of parallel MRI allows for significant scan time reductions on the order of R=2 or R=3. Higher acceleration factors are typically not achieved because parallel imaging methods are associated with a degradation of the signal-to-noise ratio (SNR). There are basically two reasons for the SNR loss, namely (1) the reduced amount of acquired data and (2) the spatially varying noise amplification due to the reconstruction process (1). The noise amplification can be quantified by the so-called geometry factor (g-factor) (1,3) and depends on several factors such as the number of receiver coils, coil geometry, image plane orientation and acceleration factor.
The SNR loss is a major drawback because it restricts the use of high acceleration factors to applications with high intrinsic SNR. One possibility to increase the intrinsic SNR is to simultaneously excite several slices by means of multiband radio-frequency (RF) pulses. The signals from the individual slices are subsequently separated with standard parallel imaging algorithms (4). Due to the SNR advantage, multiband parallel MRI approaches have gained renewed interest in recent years (5,6,7).
The standard multiband parallel MRI approach requires sufficient coil sensitivity variations along the slice direction and hence works only for relatively widely spaced slices. Otherwise, the reconstruction problem becomes ill-conditioned and the image quality may suffer from severe noise amplification due to large g-factors. To overcome this problem, the individual slices can be shifted with respect to each other using the controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) approach (8). In CAIPIRINHA, the noise amplification (i.e. g-factor) is reduced because coil sensitivity variations along both slice and phase encoding direction are utilized. In order to shift the slices with respect to each other, a separate RF phase cycle is applied to each slice. However, for some sequences the implementation of such a phase cycle is not straightforward. For example, balanced steady state free precession (bSSFP, also known as TrueFISP) sequences require dedicated RF phase cycles to meet the steady state condition. Therefore, specialized RF cycles have to be applied to meet the requirements for both the steady-state condition and CAIPIRINHA (9). Additionally, single-shot sequences such as EPI or HASTE work with a single excitation pulse and thus do not permit RF phase cycling. For the EPI sequence, the individual slices may be shifted by applying specialized slice select gradient blips simultaneously with the phase encode blips (6).
In this work, an alternative approach for improving the imaging quality in multiband parallel MRI experiments is presented. The basic idea is to apply a pre-defined phase difference between the excited slices and perform a phase-constrained parallel MRI reconstruction (10,11,12,13,14). Unlike conventional parallel imaging reconstruction methods, a phase-constrained reconstruction utilizes background phase variations in addition to coil sensitivity variations. In other words, the background phase contributes to the encoding power and thereby improves the conditioning of the reconstruction problem. For example, it has been demonstrated that two aliased signals can be perfectly separated when a phase difference of 90° exists between the two signals, even when there is no difference in the coil sensitivities (14). In the presented approach, the phase difference between the excited slices can be tailored to achieve an optimal image reconstruction (i.e. reconstruction with minimized g-factor). Another advantage of this approach is that it does not require an RF phase cycle during the acquisition. Therefore it is ideally suited for multi-band single-shot experiments.
METHODS
Background
When only small tip angles are required, multiband RF pulses for simultaneous excitation may be generated by the summation of several individual single-band pulses (15). An offset along the slice direction and a phase factor can be attributed to each individual slice prior to the summation. For example, for a dual band pulse the phase of slice 1 may be φ1=0° and the phase of slice 2 may be φ2=90°. Assuming an experiment with such a pulse, full k-space sampling (i.e. no partial Fourier acquisition) and a receive coil with uniform sensitivity (i.e. same magnitude and zero phase at all locations), the information about slice 1 is contained in the real part of the signal and the information about slice 2 is contained in the imaginary part. Thus, the two slices could be perfectly separated even without applying a parallel imaging algorithm. Under typical imaging conditions the separation process is more complicated particularly when the number of slices is increased and when multi-coil receiver arrays are used for signal detection.
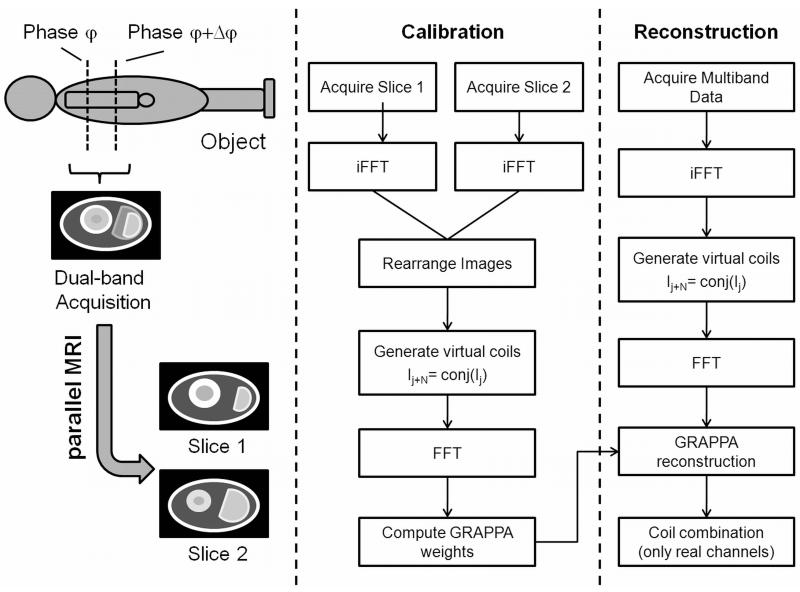
Typically, parallel MRI algorithms do not take advantage of additional phase differences between the slices; only magnitude and phase variations arising from the sensitivity patterns of the receiver coils are considered for image reconstruction. Therefore, we propose to use phase-constrained parallel MRI algorithms that additionally include background phase information during the reconstruction process. When there is no intrinsic phase difference between the individual slices (e.g. as a result of field inhomogeneities), then the background phase is defined by the phase factor that has been attributed to each individual slice before generating the multiband RF pulse. A schematic of the proposed approach is shown in Figure 1, left.
Figure 1.
Schematic of the multiband phase-constrained parallel MRI approach (left). In this example, a dual-band acquisition is performed. A slice selective RF pulse is applied for simultaneous excitation of two slices with a pre-defined phase difference Δφ. The resulting image is the complex sum of the corresponding individual slices. A phase-constrained parallel MRI reconstruction is subsequently performed to separate the signals from the individual slices (left). The flowcharts illustrate the calibration (middle) and reconstruction (right) steps for separating two simultaneously excited slices using a phase-constrained SENSE/GRAPPA combination.
Image reconstruction
In principle, any phase-constrained parallel MRI algorithm can be used. Here, the virtual coil concept (14) has been applied because it allows one to realize phase-constrained reconstructions using standard parallel MRI algorithms including SENSE and GRAPPA.
Aliased slices were separated using a SENSE/GRAPPA-combination (16). Detailed flowcharts of the calibration and reconstruction steps of a dual-band experiment are presented in Figure 1. For calibration of the reconstruction algorithm, reference images of the target slices are rearranged in an extended multi-coil image matrix with 2 × FOV in the phase encoding direction (16). Additional virtual coil images are generated by computing the complex conjugate of the real coil images. GRAPPA reconstruction weights are computed after Fourier-transform of the extended image matrix. For separation of the aliased slices, the dual-band data are Fourier-transformed into image space and virtual coil images are generated by computing the complex conjugate of the real coil images. The images are Fourier-transformed into k-space and a GRAPPA reconstruction is performed to separate the slices. In a final step, the real channels of the GRAPPA reconstructed images are combined using a sum-of-squares combination.
In addition to the SENSE/GRAPPA-combination a regularized phase-constrained SENSE reconstruction (11) was used to demonstrate the application of alternative reconstruction algorithms.
Image reconstruction algorithms were programmed in Matlab (The Mathworks, Natick, MA, USA). All image reconstructions were done offline using a standard personal computer.
Experiments
Brain imaging experiments were performed on normal volunteers using a 1.5 T clinical system (Avanto, Siemens Healthcare, Erlangen, Germany) equipped with a 12-channel head coil array (Siemens Healthcare, Erlangen, Germany) and a 3.0 T clinical MRI system (Skyra, Siemens Healthcare, Erlangen, Germany) equipped with a 16-channel head coil array (Siemens Healthcare, Erlangen, Germany). In accordance with the institutional regulations, the volunteers were informed about the study and written consents were obtained prior to the imaging sessions.
To investigate the impact of the phase difference Δφ on the reconstruction quality, TrueFISP images from two slices of the brain were acquired using the 3 T system. The sequence parameters were: TR = 4.28 ms, TE = 2.14 ms, flip angle = 15°, FOV = 200 mm × 245 mm, matrix size = 208 × 256, slice thickness = 4 mm, slice distance = 12 mm. Dual-band acquisitions were simulated by the summation of the raw data of both slices. Prior to the summation, a phase difference between the two slices was introduced by multiplying the signals from the second slice by a pre-defined phase factor Δφ. Conventional and phase-constrained reconstructions using different phase factors (Δφ = 0°, 10°, 20°, …, 350°, 360°) were performed using the SENSE/GRAPPA-combination. As a quality metric, the noise amplification (GRAPPA g-factor) was calculated from the reconstruction parameters (3).
A multiband RF pulse (number of simultaneously excited slices nSlice=4) with a pre-defined phase-difference of Δφ = 90° between neighboring slices was implemented in a TrueFISP sequence. Using this sequence, multiband experiments were performed on the human brain using the 3 T system with the following parameters: TR = 4.78 ms, TE = 2.39 ms, flip angle = 25°, FOV = 230 mm × 230 mm, matrix size = 192 × 192, slice thickness = 5 mm, slice distance = 15 mm. Four reference images of the corresponding slices were acquired using a single-band TrueFISP sequence with the same parameters. From each reference slice, 32 central k-space lines were used to generate the auto-calibration signals (ACS) for the parallel MRI reconstruction. Both conventional and phase-constrained parallel MRI reconstructions of the multiband data set were performed with the SENSE/GRAPPA-combination to separate the aliased slices.
Additionally, the reference slices were used to simulate CAIPIRINHA acquisitions. To that end, the four slices were shifted by FOV/4 with respect to each other and then added by a complex summation. Conventional and phase-constrained CAIPIRINHA reconstructions were performed using the SENSE/GRAPPA combination. For calculation of the reconstruction parameters, the four reference images were rearranged equidistantly in an extended matrix with FOVACS = 5 × FOV. Neighboring reference images were thereby concatenated by “zero-images” of size FOV/4 to account for the FOV shift generated by the CAIPIRINHA acquisition (see Discussion for more details). After Fourier-transform of the extended matrix, the parameters for an R=5 reconstruction were obtained. Using these reconstruction parameters, the noise amplification (GRAPPA g-factor) was estimated (3).
To investigate the applicability of the phase-constrained approach for single shot EPI, a dual-band RF pulse with a pre-defined phase-difference of Δφ = 90° between the two bands was implemented in a EPI sequence. Single-shot EPI imaging was performed on the 1.5 T system using the following parameters: TR = 2000 ms, TE = 30 ms, FOV = 256 × 256 mm2, Matrix 68 × 96, slice thickness = 4 mm, slice distance = 12 mm. Standard phase correction was done using three pre-scan echoes without phase-encoding. Reference single-slice images were acquired at the corresponding slice positions using the same sequence parameters. The reference images were used to determine the coil sensitivity maps and the object phase using array correlation statistics (17). Conventional and phase-constrained SENSE reconstructions (11) were performed for slice separation. For display, the images were Hanning filtered and zero-filled to a matrix size of 192 × 192. G-factor maps were calculated as described in (14).
RESULTS
Figure 2 (top) shows phase-constrained parallel MRI reconstructions and corresponding g-factor maps of simulated dual-band acquisitions. Strong noise amplification can be seen in the reconstructions with Δφ=150° due to large g-factor values. In contrast, the reconstruction quality with the phase difference Δφ=70° is significantly improved. In Figure 2 (bottom), the mean g-factor from a small region-of-interest (5×5 pixels) within the center of slice 1 is plotted against the phase difference Δφ. The g-factor is minimal for Δφ=70° (gmin=1.16) and maximal for Δφ=150° (gmax=4.76). The shape of the g-factor plot resembles the 1/sin(Δφ) function that describes the g-factor in case of a single homogenous coil and acceleration factor R=2 (see Equation 11 from Ref. (14)). Due to an intrinsic phase difference of about 20° between both slices, the minimal g-factor appears at Δφ=70° and not as expected at Δφ=90°.
Figure 2.
Results from simulated dual-band acquisitions with phase difference Δφ = 150° and Δφ = 70° between the individual slices. Reconstructed images of both slices and corresponding geometry factor maps are displayed. The distance between both slices was 12 mm. In addition, the geometry factor (g-Factor) is plotted versus the phase difference Δφ between two simultaneously excited slices. The g-factor was obtained from a central region-of-interest (5×5 pixels) of slice 1.
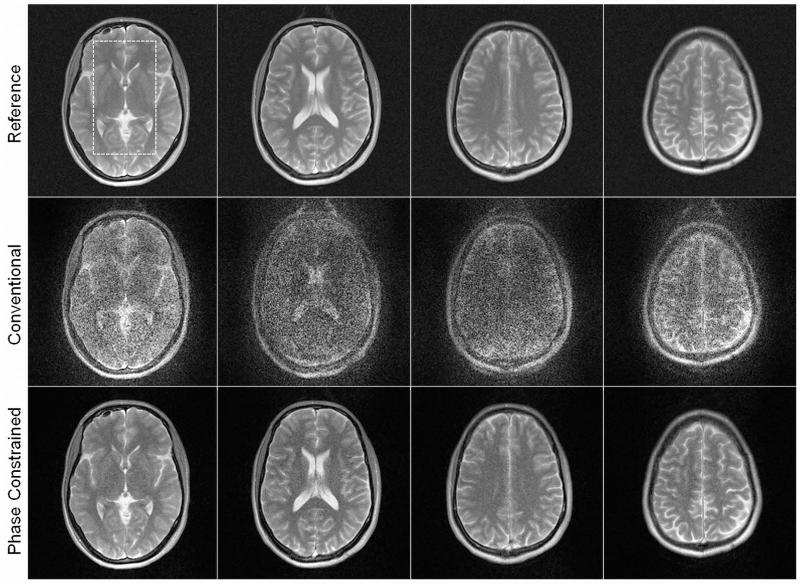
Conventional and phase-constrained parallel MRI reconstructions from the multiband experiment (nSlice=4) are presented in Figure 3. The image quality of the conventional reconstruction is severely degraded by the amplified noise. Insufficient coil sensitivity variations along the slice direction result in relatively high g-factors (gmean=8.7 and gmax=12.0; calculated from the region-of-interest shown in Figure 3, top, left). However, the noise level is significantly reduced when the phase-constrained reconstruction is used (gmean=2.1 and gmax=2.5).
Figure 3.
Results from a multiband parallel MRI experiment. Four slices were excited simultaneously and subsequently separated with a conventional parallel MRI algorithm (middle row) and a phase constrained parallel MRI algorithm (bottom row). Reference images obtained from four single-band experiments are illustrated for comparison (top row). The distance between the individual slices was 15 mm. A phase-difference of 90° between neighboring slices was applied in the multiband RF pulse. Geometry factors were obtained from a region-of-interest as indicated by the dashed rectangular box in the top left image. The resulting geometry factors are reported in the text.
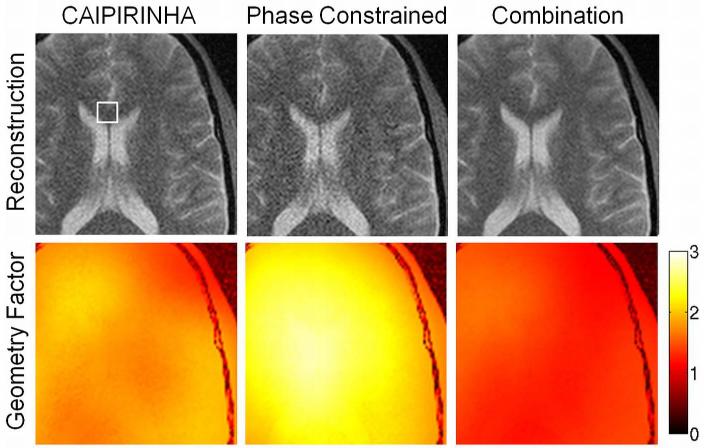
Figure 4 illustrates the combination of CAIPIRINHA with a phase-constrained reconstruction. It shows magnified views of an exemplary slice obtained with a phase-constrained reconstruction (no CAIPIRINHA acquisition) (left), a conventional reconstruction using the CAIPIRINHA acquisition (middle) and a phase-constrained reconstruction using the CAIPIRINHA acquisition (right). Overall, the combination of CAIPIRINHA and the phase-constrained reconstruction yields the best image quality. This is reflected in the reduced g-factors (Figure 4, bottom). The mean g-factors obtained from a small region-of-interest (Figure 4, top, left) are g=2.61 for the standard phase-constrained approach, g=1.90 for the standard CAIPIRINHA approach and g=1.47 for the combination of the phase-constrained approach and CAIPIRINHA.
Figure 4.
Simulation results of a CAIPIRINHA acquisition. Image reconstructions (top row) and geometry factor maps (bottom row) of an exemplary slice are presented. The figure shows magnified views of a standard reconstruction using the CAIPIRINHA acquisition scheme (left), a phase constrained reconstruction without the CAIPIRINHA acquisition scheme (middle) and the combination of a phase constrained reconstruction with a CAIPIRINHA acquisition scheme (right). Mean geometry factors were obtained from a small region-of-interest (11 × 11 pixels) as indicated by the small rectangular box in the top left image. The resulting geometry factors are reported in the text.
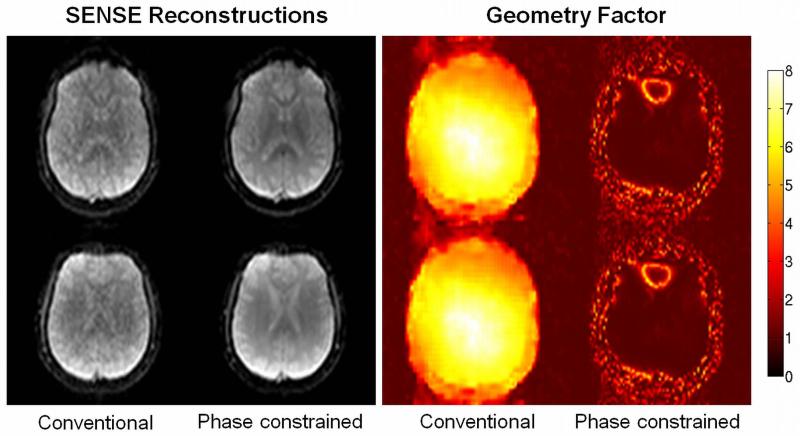
Figure 5 demonstrates the applicability of the phase-constrained multiband approach to single-shot acquisitions. Conventional and phase-constrained SENSE reconstructions of a dual-band single-shot EPI acquisition are presented as well as the corresponding g-factor maps. The g-factors in the phase-constrained reconstructions are close to g=1 except in the frontal region-of the brain where the air-tissue interface causes strong phase variations.
Figure 5.
Conventional and phase-constrained SENSE reconstructions of a dual-band single-shot EPI acquisition (left). The distance between the two simultaneously excited slices was 12 mm. A phase difference of 90° was applied. Corresponding geometry factor maps are presented (right).
DISCUSSION AND CONCLUSIONS
In this work, we propose the use of phase-constrained reconstructions for achieving better image quality in multiband parallel MRI experiments. In this approach, a priori phase information about the individual slices is incorporated in the reconstruction process. It has been demonstrated that high reconstruction quality can be achieved even for closely spaced slices (e.g. 15 mm slice distance, see Figure 3) by applying a phase difference of Δφ=90° between neighboring slices.
This approach can be combined with the CAIPIRINHA acquisition scheme. Simulations results (Figure 4) indicate that a further image quality improvement can be achieved and thus the combination of CAIPIRINHA and a phase-constrained reconstruction seems favorable. Please note that the SNR in the simulated phase-constrained images (Figure 4, middle) is reduced compared with the actual multiband images (Figure 3). This can be explained because the noise of four single-slice acquisitions is summed in the simulation whereas the noise originates from a single acquisition in the multiband experiment. However, the g-factors of the simulated and the actual multiband acquisitions are identical.
Images from CAIPIRINHA acquisitions with NSlice simultaneously excited slices can be reconstructed with a standard SENSE algorithm, for example. To that end, the coil sensitivity maps of the corresponding slices have to be shifted along the phase encoding direction according to the FOV shifts generated by the acquisition scheme. The shifted coil sensitivity maps are then rearranged in a matrix of size NSlice × FOV along the phase encoding direction (8). However, this approach produces artifacts when GRAPPA-type reconstructions are applied (6). The GRAPPA algorithm assumes smooth coil profile variations and therefore it cannot handle the discontinuities caused by the FOV shift. In this work, we have used an adapted SENSE/GRAPPA combination where the NSlice references slices are rearranged equidistantly in a matrix of size (NSlice+1) × FOV along the phase encoding dimension. Zero images of size FOV/NSlice are inserted between neighboring reference slices to account for the FOV shifts generated by the CAIPIRINHA acquisition (9,18). In that way, the discontinuities described in reference (6) are avoided and artifact-free images can be obtained.
Phase differences of 90 degrees between neighboring slices were used in this work. However, for experiments with more than two simultaneously excited slices other phase differences may be more optimal. Particularly, non-optimal experimental conditions (e.g. magnetic field inhomogeneities) may lead to intrinsic phase differences between neighboring slices. For this reason, the minimal g-factor in Figure 2 appears at Δφ=70° and not as expected at Δφ=90°. Intrinsic phase differences should be considered when designing the multiband RF pulse. In an ideal experiment the intrinsic phase differences are determined by a pre-scan. Subsequently, an optimized multiband RF pulse with adapted phases is generated on-the-fly during the imaging session, allowing the noise amplification to be further reduced. In this work, phase differences along the slice direction were controlled in a global manner. In-plane phase variations were not considered. However, in principle one may design a multi-dimensional RF pulse that generates optimal phase differences between neighboring slices at each location. Proof-of-principle experiments have been demonstrated for single-slice experiments (19), but could be extended for simultaneous multi-slice imaging.
Phase-constrained reconstructions work very well as long as the background phase varies smoothly. Abrupt phase changes that may occur at air-tissue boundaries or when non-periodic motion or flow occurs are problematic. These abrupt changes are typically not accounted for when low-resolution calibration signals are used and therefore artifacts may appear in the reconstructed images. In this work, abrupt phase variations were minimized by using the flow-compensated TrueFISP sequence and second-order volume shimming. As an alternative, iterative methods may be used to obtain high-resolution estimates of the background phase (13). For the EPI acquisition, a regularized phase constrained algorithm was applied to achieve a trade-off between artifacts and noise reduction. For best image quality the regularization parameter was chosen empirically.
One disadvantage of multiband MRI is the increased RF power deposition. Therefore, the specific absorption rate (SAR) per unit time is increased and the RF peak power limits may be exceeded when large numbers of simultaneously excited slices or high flip angles are used. However, new methods for generating multiband RF pulses with reduced RF power deposition have been recently presented (20,21). It has been demonstrated that the peak RF power of multiband pulses can be minimized when optimized phases are applied to each excitation band (21). These pulses may be beneficial for phase-constrained multiband MRI in terms of peak RF power and image quality. Although further investigations are necessary, phase constrained multiband parallel MRI in combination may become an interesting candidate for efficient data sampling even for high field applications.
In conclusion, multiband phase-constrained parallel MRI allows accelerated multi-slice imaging with an improved SNR performance when compared to standard multiband parallel MRI.
ACKNOWLEDGEMENTS
This work was in part sponsored by the German Research Foundation (DFG JA 827/9-1) and NIH R01HL094557. The authors thank Siemens Healthcare (Erlangen, Germany) for technical support.
LITERATURE
- 1.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999 Nov;42(5):952–62. [PubMed] [Google Scholar]
- 2.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002 Jun;47(6):1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 3.Breuer FA, Kannengiesser SA, Blaimer M, Seiberlich N, Jakob PM, Griswold MA. General formulation for quantitative G-factor calculation in GRAPPA reconstructions. Magn Reson Med. 2009 Sep;62(3):739–46. doi: 10.1002/mrm.22066. [DOI] [PubMed] [Google Scholar]
- 4.Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001 Feb;13(2):313–7. doi: 10.1002/1522-2586(200102)13:2<313::aid-jmri1045>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010 May;63(5):1144–53. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210–24. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010 Dec 20;5(12):e15710. doi: 10.1371/journal.pone.0015710. Erratum in: PLoS One. 2011;6(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005 Mar;53(3):684–91. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]
- 9.Stäb D, Ritter CO, Breuer FA, Weng AM, Hahn D, Köstler H. CAIPIRINHA accelerated SSFP imaging. Magn Reson Med. 2011 Jan;65(1):157–64. doi: 10.1002/mrm.22600. [DOI] [PubMed] [Google Scholar]
- 10.Samsonov AA, Kholmovski EG, Parker DL, Johnson CR. POCSENSE: POCS-based reconstruction for sensitivity encoded magnetic resonance imaging. Magn Reson Med. 2004 Dec;52(6):1397–406. doi: 10.1002/mrm.20285. [DOI] [PubMed] [Google Scholar]
- 11.Bydder M, Robson MD. Partial fourier partially parallel imaging. Magn Reson Med. 2005 Jun;53(6):1393–401. doi: 10.1002/mrm.20492. [DOI] [PubMed] [Google Scholar]
- 12.Willig-Onwuachi JD, Yeh EN, Grant AK, Ohliger MA, McKenzie CA, Sodickson DK. Phase-constrained parallel MR image reconstruction. J Magn Reson. 2005 Oct;176(2):187–98. doi: 10.1016/j.jmr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Lew C, Pineda AR, Clayton D, Spielman D, Chan F, Bammer R. SENSE phase-constrained magnitude reconstruction with iterative phase refinement. Magn Reson Med. 2007 Nov;58(5):910–21. doi: 10.1002/mrm.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaimer M, Gutberlet M, Kellman P, Breuer FA, Köstler H, Griswold MA. Virtual coil concept for improved parallel MRI employing conjugate symmetric signals. Magn Reson Med. 2009 Jan;61(1):93–102. doi: 10.1002/mrm.21652. [DOI] [PubMed] [Google Scholar]
- 15.Müller S. Multifrequency selective rf pulses for multislice MR imaging. Magn Reson Med. 1988 Mar;6(3):364–71. doi: 10.1002/mrm.1910060315. [DOI] [PubMed] [Google Scholar]
- 16.Blaimer M, Breuer FA, Seiberlich N, Mueller MF, Heidemann RM, Jellus V, Wiggins G, Wald LL, Griswold MA, Jakob PM. Accelerated volumetric MRI with a SENSE/GRAPPA combination. J Magn Reson Imaging. 2006 Aug;24(2):444–50. doi: 10.1002/jmri.20632. [DOI] [PubMed] [Google Scholar]
- 17.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000 May;43(5):682–90. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Zhu K, Kerr A, Pauly JM. Autocalibrating CAIPIRINHA: Reformulating CAIPIRINHA as a 3D Problem; Proceedings of the 20th annual meeting of the ISMRM; Melbourne, Australia. 2012.p. 518. [Google Scholar]
- 19.Schneider JT, Blaimer M, Ullmann P. Tailoring the Image Background Phase by Spatially Selective Excitation for Improved Parallel Imaging Reconstruction Performance; Proceedings of the 20th annual meeting of the ISMRM, Melbourne; Australia. 2012.p. 516. [Google Scholar]
- 20.Norris DG, Koopmans PJ, Boyacioğlu R, Barth M. Power Independent of Number of Slices (PINS) radiofrequency pulses for low-power simultaneous multislice excitation. Magn Reson Med. 2011 Nov;66(5):1234–40. doi: 10.1002/mrm.23152. [DOI] [PubMed] [Google Scholar]
- 21.Wong E. Optimized Phase Schedules for Minimizing Peak RF Power in Simultaneous Multi-Slice RF Excitation Pulses; Proceedings of the 20th annual meeting of the ISMRM; Melbourne, Australia. 2012.p. 2209. [Google Scholar]