Abstract
Background
Propofol (2,6-diisopropylphenol) is an IV anesthetic used for general anesthesia. Recent evidence suggests that propofol-anesthetized patients experience less postoperative pain, and that propofol has analgesic properties when applied topically. We presently investigated the antinociceptive effects of topical propofol using behavioral and single-unit electrophysiological methods in rats.
Methods
In behavioral experiments with rats, we assessed the effect of topical hindpaw application of propofol (1–25%) on heat and mechanically evoked paw withdrawals. In electrophysiology experiments we recorded from lumbar dorsal horn wide dynamic range (WDR)-type neurons in pentobarbital-anesthetized rats. We assessed the effect of topical application of propofol to the ipsilateral hindpaw on neuronal responses elicited by noxious heat, cold and mechanical stimuli. We additionally tested if propofol blocks heat sensitization of paw withdrawals and WDR neuronal responses induced by topical application of allyl isothiocyanate (AITC; mustard oil).
Results
Topical application of propofol (1–25%) significantly increased the mean latency of the thermally evoked hindpaw withdrawal reflex on the treated (but not opposite) side in a concentration-dependent manner, with no effect on mechanically evoked hindpaw withdrawal thresholds. Propofol also prevented shortening of paw withdrawal latency induced by AITC. In electrophysiological experiments, topical application of 10 and 25% propofol, but not 1% propofol or vehicle (10% intralipid), to the ipsilateral hindpaw significantly attenuated the magnitude of responses of WDR neurons to noxious heating of glabrous hindpaw skin with no significant change in thermal thresholds. Maximal suppression of noxious heat-evoked responses was achieved 15-min after application followed by recovery to the pre-propofol baseline by 30 min. Responses to skin cooling or graded mechanical stimuli were not significantly affected by any concentration of propofol. Topical application of AITC enhanced the noxious heat-evoked response of dorsal horn neurons. This enhancement of heat-evoked responses was attenuated when 10% propofol was applied topically after application of AITC.
Conclusions
The results indicate that topical propofol inhibits responses of WDR neurons to noxious heat consistent with analgesia, and reduced AITC sensitization of WDR neurons consistent with an antihyperalgesic effect. These results are consistent with clinical studies demonstrating reduced postoperative pain in surgical patients anesthetized with propofol. The mechanism of analgesic action of topical propofol is not clear, but may involve desensitization of TRPV1 or TRPA1 receptors expressed in peripheral nociceptive nerve endings, engagement of endocannabinoids, or activation of peripheral gamma-aminobutyric acid A receptors.
Introduction
Propofol (2,6-diisopropylphenol) is an IV anesthetic that is widely used for the induction and maintenance of general anesthesia. The enhancement of GABAergic and glycinergic neurotransmission by propofol is thought to be responsible for its general anesthetic properties.1 It has been debated whether propofol at subhypnotic concentrations has analgesic properties.2 Animal studies have reported systemic propofol to have either no effect 3–5 or antinociceptive and/or antihyperalgesic effects.6–8 Systemic administration of propofol depressed noxious stimulus-evoked responses of neurons in the spinal cord dorsal9–13 and ventral horns14,15 and either reduced16 or had no effect 5 on formalin-evoked spinal neuronal expression of c-fos, a marker of neuronal activity. Several studies of experimental pain in humans reported analgesic effects of subhypnotic doses of propofol.17–20 Moreover, some recent clinical studies have reported that surgical patients receiving propofol anesthesia experienced reduced postoperative pain21,22 (see also23).
Based on three-compartment models of drug pharmacokinetics,24,25 it would be expected that propofol, which is highly lipid-soluble, would be cleared more slowly from peripheral tissues when the infusion is stopped. If propofol exerted a peripheral antinociceptive effect, this might account for its postsurgical analgesic effect. Peripheral application of propofol has been reported to have antinociceptive effects in the formalin26 and bee venom tests.12 In the latter study, peripherally injected propofol also inhibited the responses of nociceptive dorsal horn neurons. In the present study, we hypothesized that propofol in peripheral tissue such as skin exerts an antinociceptive effect. To mimic the action of residual propofol in peripheral tissues after the cessation of propofol infusion, we applied propofol topically to the skin and investigated potential antinociceptive and antihyperalgesic actions using behavioral and electrophysiological approaches in rats.
Methods
All experiments were conducted using adult male Sprague-Dawley rats under protocols approved by the University of California Davis Institutional Animal Care and Use Committee. Rats were housed individually and had unrestricted access to food and water.
Behavioral Testing
These studies used 8 rats (body weight: 510–696 g). Thermal and mechanical paw withdrawal tests were conducted as previously described.27 For thermal paw withdrawal (Hargreaves28) testing, rats were first habituated over 3 successive daily sessions to stand on a glass surface heated to 30±1°C within a ventilated Plexiglas enclosure. Paw withdrawal latencies were measured by directing a light beam (Plantar Test 390, IITC, Woodland Hills, CA) onto the plantar surface of the hindpaw through the glass plate from below, and the latency from onset of the light to brisk withdrawal of the stimulated paw was measured. To prevent potential tissue damage, a cutoff of 18 seconds was imposed. For formal testing, either vehicle (10% intralipid; Fresenius Kabi, Uppsala, Sweden) or propofol (Sigma-Aldrich Co., St Louis MO) at a concentration of 1%, 10% or 25% (dissolved in 10% intralipid) was applied to the ventral surface of one hindpaw using a cotton swab and allowed to dry for 2 min, after which the rat was placed onto the glass surface. Withdrawal latencies for both the treated and the untreated paw were measured at 5, 15, 30, 45, 60 and 120 min after application.
We also tested the effect of propofol on allyl isothiocyanate (AITC)-induced hyperalgesia. AITC (mustard oil, 50%, in mineral oil; Sigma) was applied topically to one hindpaw in three treatment groups: (a) AITC alone (no pretreatment), (b) AITC, followed 10 min later by intralipid or (c) AITC, followed 10 min later by topical 10% propofol. All chemicals were applied topically to the hindpaw, and withdrawal latencies for the treated and untreated (contralateral) paws were measured as described above.
For mechanical paw withdrawal threshold measurements, rats were habituated over 3 successive days to standing on a wire mesh screen surface. Baseline mechanical withdrawal thresholds were assessed using an electronic Von Frey filament (1601C, IITC Life Sciences, Woodland Hills, CA) pressed against the plantar surface of one hindpaw. This device registered the force (g) at the moment that the hindpaw was withdrawn away from the filament. Vehicle or propofol was then applied to one hindpaw, and mechanical paw withdrawal thresholds were measured at the same post-application time points as noted above. Topical application of propofol was used because of its permeation through rat skin29,30 and to avoid potential damage from an intraplantar injection.
Thermal withdrawal latencies and mechanical paw withdrawal thresholds were normalized to baseline averages. Treatment groups were compared using two-way repeated measures ANOVA followed by Tukey post hoc comparison tests.
Electrophysiology
Thirty-eight rats (body weight: 400–510 g) were used. Anesthesia was induced with sodium pentobarbital (65 mg/kg ip) and maintained by constant infusion of pentobarbital via a jugular vein catheter at a rate sufficient to maintain areflexia for the duration of the experiment (10–20 mg/kg/hr). Oxygen was delivered via a tracheal cannula. Core body temperature was monitored and maintained by heating pad. A laminectomy exposed the lumbar spinal cord for single-unit recording as detailed previously.31 Briefly, a tungsten microelectrode (FHC, Bowdoin, ME; 10MΏΩ) was driven into the dorsal horn to record extracellular single-unit activity. We specifically searched for mechanoresponsive units that additionally responded to noxious skin heating. Thermal sensitivity was assessed using a Peltier thermode (0.5 in. diameter; NTE-2A, Physitemp, Clifton, NJ) programmed to deliver heat and cold stimuli to the cutaneous mechanical receptive field. The skin-thermode interface temperature was monitored using a thermocouple (IT-21, Physitemp) connected to a BAT-12 (Physitemp) thermometer and was displayed along with single-unit activity and electrocardiogram using Powerlab (ADInstruments, Colorado Springs, CO) and Spike2 (Cambridge Electronic Design, Cambridge UK) interfaces. The heat stimulus was increased from an adapting temperature of 34°C to 52°C over 15 sec, followed by re-cooling to 34°C. A cooling stimulus followed 2 min later (34–0°C over 45 s). Units were additionally tested for mechanical sensitivity using a series of von Frey monofilaments (bending force: 0.68–1258.9 mN) applied in ascending order, cotton wisp, touch, and pinch applied to the receptive field. All of the present units were classified as wide dynamic range (WDR), based on their thermal sensitivity and incrementally increasing responses to graded mechanical stimuli.
To assess effects of propofol on thermally evoked responses, the following protocol was followed. The heat and cold stimulus sequence was first delivered before any chemical stimulation. Two min later the Peltier thermode was withdrawn from the skin and 10% propofol (30 μl) or vehicle (30 μl) was applied topically to the hindpaw receptive field by pipette. The thermode was replaced at the same location 3 min after the chemical application, and the heat and cold stimulus sequence was delivered again 5, 10, 15 and 30 min after 10% propofol or vehicle. The thermode was mounted on a micromanipulator (World Precision Instruments, Sarasota, FL) to allow precise repositioning of the thermode surface at the same skin site for postchemical thermal testing. Additional concentrations of propofol (1%, 25%) were tested in the identical manner out to 15 min after application.
We additionally assessed the effect of topical propofol on sensitization of heat-evoked responses induced by topical application of AITC. The heat and cold stimulus sequence was applied first as above. Two min later AITC (10 μl, 75% in mineral oil; Sigma) was applied topically to the ventral hindpaw. Five min after AITC application either 10% propofol or vehicle was applied as above. The heat and cold stimulus sequence was applied 5, 10, 15, and 30 min after propofol or vehicle application. In many experiments, a second unit was subsequently recorded on the opposite side of the spinal cord and one of the protocols described above was followed on the opposite hindpaw. As previously reported, 31 responses recorded from the second unit did not exhibit any indication of sensitization. At the conclusion of each unit recording session, the recording site was marked by electrolytic lesion, and the spinal cord was post-fixed in 10% buffered formalin. Sections of the spinal cord were cut on a freezing microtome and examined under the light microscope.
Action potentials were recorded, stored and analyzed using Spike2 (Cambridge Electronic Design, Cambridge, UK). In a few instances two action potentials were recorded simultaneously and were sorted by spike size and waveform. The number of thermally evoked action potentials was summed over 30 sec for heat stimuli and 45 sec for cold stimuli and baseline-corrected by subtracting the number of spontaneous action potentials counted over the same time period before the thermal stimulus. Thermal thresholds were defined as the temperature at which the unit activity changed by > 2 SD from the pre-stimulus firing rate. For mechanical testing, the firing rate 15 sec immediately before each stimulus was summed and subtracted from the firing rate 15 sec after application of the mechanical stimulus. In most cases unit responses were normalized to the pre-propofol or pre-AITC baseline, and were analyzed using one-way repeated measures ANOVA with Tukey post hoc comparison tests between time points. Statistical analyses were conducted using Graph pad prism (Graph Pad, La Jolla, CA, USA) or SPSS (SPSS 9.0, SPSS, Chicago, IL) software, where p < 0.05 was considered significant. Error reported is the standard error of the mean (SEM).
Results
Behavioral experiments
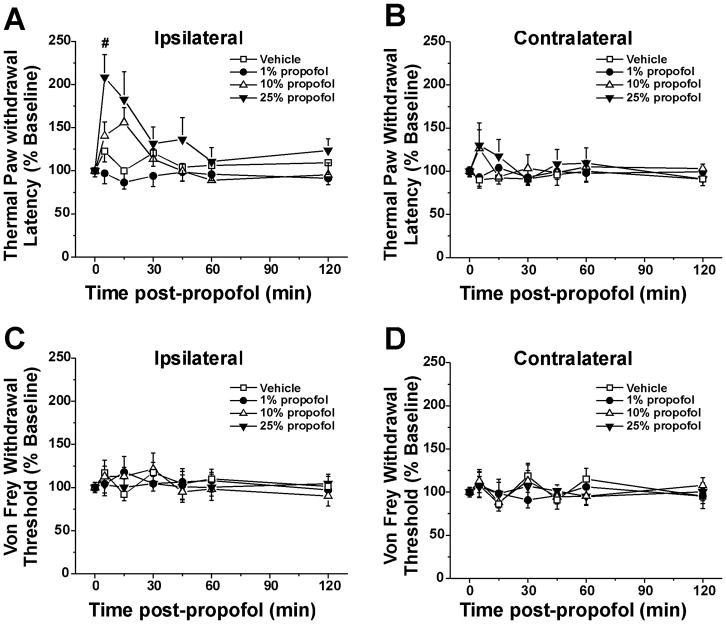
The hindpaw receiving topical propofol (ipsilateral) exhibited a concentration-dependent increase in withdrawal latency (Fig. 1A). The group tested with 25% propofol was significantly different from vehicles and 1% propofol. (Fig. 1A, p < 0.05, two-way repeated measures ANOVA, Tukey post hoc), with a significant increase in latency at 5 min after propofol (p< 0.05, one-way ANOVA with Tukey post hoc). Withdrawal latencies for the hindpaw contralateral to the side of propofol application were not affected at any concentration of propofol (Fig. 1B, two-way repeated measures ANOVA, p>0.1). There was no significant effect of propofol at any concentration on von Frey mechanically evoked withdrawal thresholds for the ipsilateral (Fig. 1C) or contralateral hindpaw (Fig. 1D).
Figure 1.
Topical propofol suppression of thermal but not mechanical paw withdrawal. A. Thermal paw withdrawal (Hargreaves) test: ipsilateral hindpaw. The hindpaw receiving topical propofol exhibited a concentration-dependent increase in withdrawal latency. (F(3,28)=6.877, P=0.001, two-way repeated-measures ANOVA) with 25% significantly different from vehicle and 1%. #: p<0.05 for pre versus 5 min, Tukey post hoc test. Error bars: SEM; n=7–8/group. B. As in A for contralateral hindpaw. No significant between-group differences. C. Von Frey paw withdrawal threshold: ipsilateral hindpaw. No significant between-group differences. D. As in C for contralateral hindpaw. No significant between group differences.
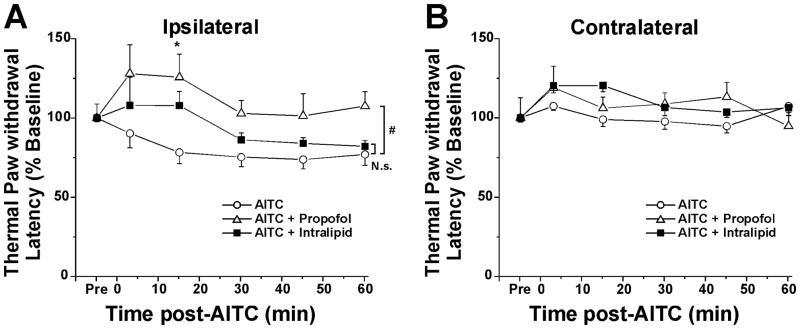
Topical hindpaw application of AITC resulted in a significant decrease in paw withdrawal latency that was not significantly affected by topical post-application of intralipid (Fig. 2A). However, topical application of 10% propofol 10 min after AITC resulted in increased paw withdrawal latency that was significant at 15 min (Fig. 2A; p<0.05, Tukey post hoc) and prevented hyperalgesia as evidenced by a significant difference between the AITC-only versus propofol + AITC groups (p<0.05, 2-way repeated measures ANOVA). None of the treatments significantly affected contralateral paw withdrawal latency (Fig. 2B).
Figure 2.
Antihyperalgesic effect of propofol. A: Withdrawal latency versus time after hindpaw treatment with allyl isothiocyanate (AITC)-only (○), AITC+ propofol (Δ) or AITC + intralipid vehicle (■). #: significant between-group difference (p<0.05, 2-way repeated-measures ANOVA). *: p<0.05, Tukey post hoc test. No significant difference between AITC-only and AITC + intralipid (p>0.05, 2-way repeated measures ANOVA). B: As in A for contralateral hindpaw. No significant differences.
Electrophysiological experiments
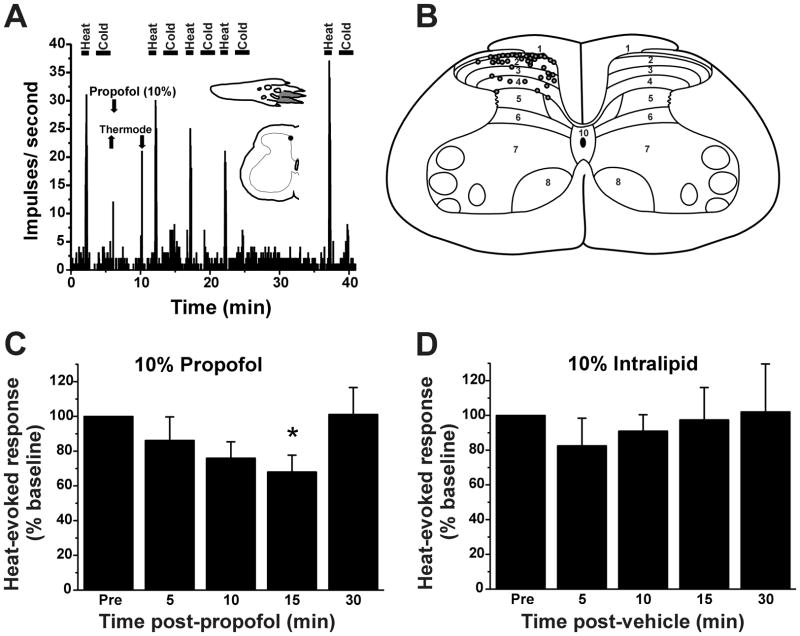
A total of 62 heat-responsive dorsal horn units were recorded. All units were of the WDR-type, responding to innocuous mechanical stimulation as well as noxious heat. An example is shown in Fig. 3A. Units were mainly located in lamina I, with some in the deeper dorsal horn (Fig. 3B), at mean depth of 214 ± 37 [SEM] μm below the surface.
Figure 3.
Propofol suppresses dorsal horn neuronal responses to heat. A. Peristimulus-time histogram (PSTH, bins: 1 sec) of typical dorsal horn unit’s responses to noxious heat and cold, before and after 10% propofol (arrow). Lower arrows: placement of Peltier thermode. Insets: hindpaw receptive field (upper) and spinal recording site (lower). B. Recording sites on L4 spinal cord (adapted from Paxinos and Watson46 but significantly changed for this publication). C. Mean normalized responses to heat relative to 10% propofol application. *: p<0.05 (vs. pre), one way repeated measures ANOVA with Tukey post hoc (n=11). Error bars: SEM. D. Graph as in C for vehicle (10% intralipid). No significant effect (n=6).
Heat-evoked responses of dorsal horn units were significantly reduced by topical application of 10% and 25% (but not 1%) propofol. Fig. 3A shows an example of a unit for which topical application of 10% propofol resulted in a time-dependent decline in the magnitude of the noxious heat-evoked response over the first 10–15 min, followed by recovery 30 min later. Fig. 3C plots the mean normalized heat-evoked responses of 11 units at various times relative to topical application of propofol, which resulted in a significant reduction at 15 min (p <0.05, one-way repeated measures ANOVA with Tukey post hoc test). Topical application of vehicle (10% intralipid) did not affect heat-evoked responses at any time point (Fig. 3D). No unit was directly excited by application of 10% propofol. Fig. 4 summarizes the data, showing that 1% propofol was ineffective while both 10% and 25% propofol concentrations significantly attenuated heat-evoked responses at the 15-min time points after propofol, with significant inhibition also at 10 min after 25% propofol (p <0.05, one-way repeated measures ANOVA with Tukey post hoc test). Neither 10% nor 25% propofol significantly affected thermal thresholds (10% propofol: pretreatment 42.1 +/− 0.3 [SEM] °C; posttreatment 42.6 +/− 0.4 °C; 25% propofol: pretreatment 41.4 +/− 0.4 °C, posttreatment: 42.5 +/− 0.8 °C).
Figure 4.
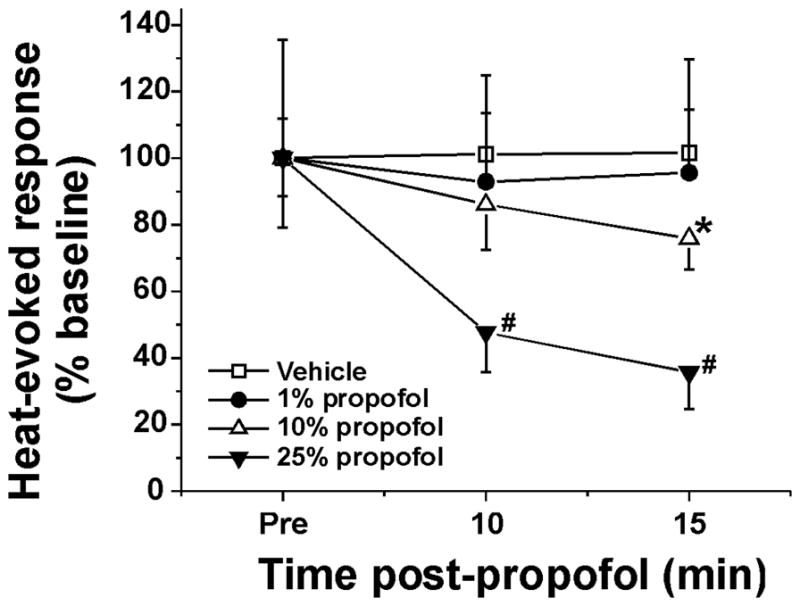
Concentration-dependent suppression of heat-evoked responses of dorsal horn neurons by propofol. Mean normalized heat-evoked responses versus time after propofol. *, #: different from pre (p< 0.05, one way repeated measures ANOVA with Tukey post hoc) for 10% (*; n=22) and 25% propofol (#; n=9). Error bars: SEM.
Twenty-nine heat-responsive units also responded to the cold stimulus. Responses elicited by the cold stimulus were not significantly affected by topical hindpaw application of 1%, 10% or 25% propofol. Likewise, none of the propofol concentrations significantly affected mechanically evoked responses of 29 WDR units tested (Fig. 5).
Figure 5.
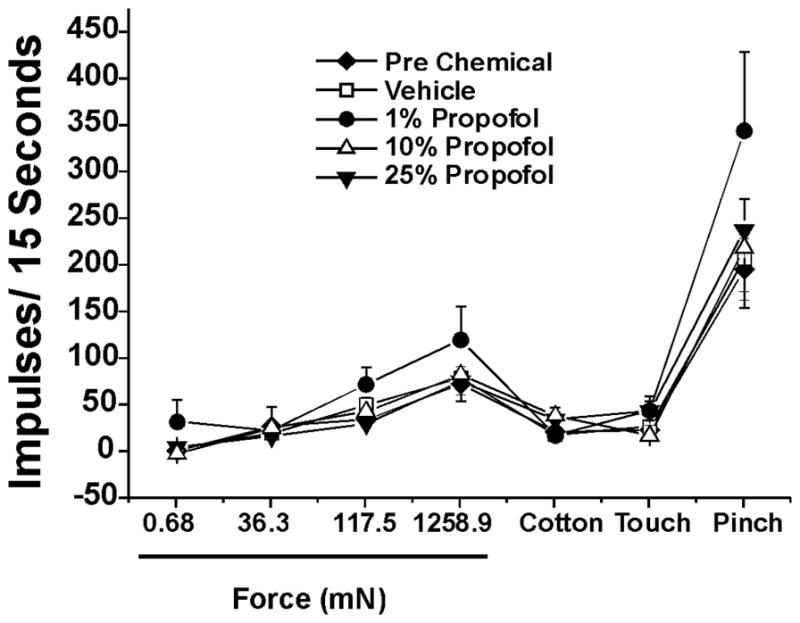
Lack of effect of propofol on responses of lumber spinal neurons to mechanical stimuli. Graph plots mean responses to graded von Frey filament stimuli, cotton touch and pinch, before (◆, pre) and after topical application of vehicle (□) or propofol at the indicated concentrations. There were no significant differences among pre-chemical, vehicle and propofol groups at any propofol concentration. (p> 0.05, one-way ANOVA; pretreatment n=28, after vehicle n=28, 1% propofol n=8, 10% propofol n=11, 25% propofol n=9).
We additionally tested if propofol affects AITC enhancement of heat-evoked responses. When AITC was applied topically to the hindpaw followed by topical application of vehicle, there was a subsequent significant increase in the magnitude of the heat-evoked response (Fig. 6A), consistent with our previous report.31 However, when AITC was applied topically followed by topical application of 10% propofol, there was no change in the magnitude of heat-evoked responses compared to the pre-AITC + propofol response (Fig. 6B), indicating that topical propofol reduced the hyperalgesic effect of AITC.
Figure 6.
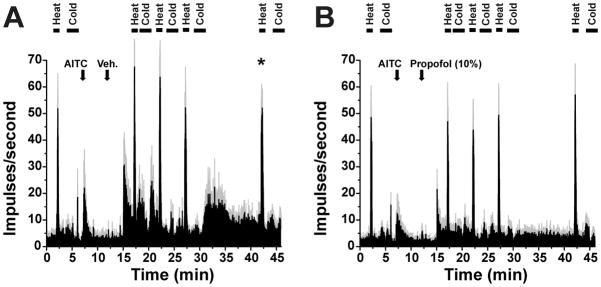
Propofol blocks allyl isothiocyanate (AITC) enhancement of heat-evoked responses. A: Averaged peristimulus-time histogram (PSTH) (error bars: SEM) to heat and cold stimuli (bars), before and after AITC followed by vehicle (10% intralipid)(at arrows). *; p<0.05, repeated-measures ANOVA with Tukey post hoc, n=6). B: As in A for separate units receiving AITC followed by 10% propofol. No significant change in heat-evoked responses (p> 0.5, repeated-measures ANOVA with Tukey post hoc, n=10).
Discussion
In the present study, topical application of propofol dose-dependently suppressed thermal paw withdrawals without affecting mechanical sensitivity, indicating that topical propofol acts as an analgesic but not as a local anesthetic. Propofol also suppressed noxious heat-evoked responses of WDR dorsal horn neurons, without affecting their responses to mechanical or cold stimuli. WDR neurons encode the intensity of noxious stimuli32 and appear to be sufficient for pain sensation.33 The correspondence between the inhibitory effect of propofol on nocifensive behavior and neuronal responses supports the argument that WDR neurons are involved in signaling heat pain. We additionally observed that topical propofol reduced or prevented AITC enhancement of thermal paw withdrawals and heat-evoked responses of WDR neurons, implying an antihyperalgesic effect. We believe that the observed analgesic and antihyperalgesic effects are mediated locally in skin tissue. Although we did not presently measure tissue or plasma propofol concentrations, it was previously reported that transdermal application of propofol at concentrations comparable to the highest concentration used presently (25%) resulted in plasma concentrations in the 50–300 ng/ml range,30 which is well below the range of plasma propofol concentrations for sedation (3–8 μg/ml). These findings are discussed below in relation to potential mechanisms underlying the antinociceptive and antihyperalgesic effects of topical propofol.
Our finding that topical application of propofol-induced antinociception and inhibited spinal WDR neurons is consistent with a previous report that peripherally applied propofol reduced nocifensive behavior and spinal neuronal activity elicited by subcutaneous injection of bee venom.12 However, these findings are in marked contrast to the well-known algesic effects of propofol, which induces pain upon IV34 or intradermal35 injection in humans and elicits nocifensive behavior in mice in a TRPA1-dependent manner.36 Propofol directly activates TRPV1 and TRPA136–38 with a greater effect on TRPA1.35 Propofol also reversed desensitization of TRPV1 as manifested by resensitization of capsicin-evoked responses in dorsal root ganglion cells and prolongation of capsaicin-evoked nocifensive behavior38 in a TRPA1-dependent manner.39 It is thus difficult to reconcile these pronociceptive actions of propofol with the presently observed antinociceptive effects. Repeated or continuous application of propofol led to desensitization of TRPV1 expressed in human embryonic kidney cells, and high concentrations of propofol blocked TRPA1 activation.35 Thus, it is possible that high concentrations of topically applied propofol may desensitize TRPV1 and/or TRPA1 expressed in cutaneous nociceptive nerve endings, thereby reducing their sensitivity to noxious heat, consistent with the presently observed heat analgesia and inhibition of spinal neuronal responses to noxious heat. TRPA1 is also expressed by skin cells including keratinocytes,40 and it is conceivable that propofol may act at TRPA1 or TRPV1 expressed in non-neural cells to indirectly inhibit nociceptive nerve endings in the skin.
There are several additional potential explanations for the local antinociceptive effect of propofol. Propofol inhibits fatty acid amide hydrolase, the enzyme that degrades the endocannabinoid anandamide.41 The antinociceptive effect of hindpaw injection of propofol in the formalin test was attenuated by antagonists of cannabinoid type 1 and 2 receptors,26 suggesting that propofol antinoception involved an increase in local skin levels of anandamide, which in turn inhibited nociceptors via a variety of possible actions (see 42 for recent review). The antinoceptive effect was considered to be local, since administration of an even larger dose of propofol to the contralateral hindpaw had no effect. In the present study, we did not observe any change in withdrawal latency for the hindpaw contralateral to the one receiving propofol (Fig. 1B), arguing against a systemically mediated antinociceptive effect.
Another possibility is that the antinociceptive effect of peripherally applied propofol is mediated via an interaction with gamma-aminobutyric acid A (GABAA) receptors. Subunits of GABAA receptors are localized to unmyelinated fibers in glabrous skin, and intraplantar injection of the GABAA receptor agonist muscimol was antinociceptive in the formalin test.43 Propofol was shown to excite dorsal root ganglion neurons via GABAA receptors; unlike propofol, intradermal injection of GABA did not elicit pain in humans.35 We therefore speculate that propofol may activate peripheral nerve endings expressing GABAA receptors to induce a local antinocicptive, rather than pronociceptive, effect.
Propofol or its halogenated analog was reported to inhibit voltage-sensitive sodium channels44 and to reduced axonal excitability,45 potentially contributing to antinociception. Finally, the present study cannot exclude a possible central inhibitory mechanism triggered by peripheral propofol. Propofol excitation of nociceptor nerve endings via TRPV1 and TRPA1 could provide a barrage of nociceptive input to the central nervous system to engage antinociceptive mechanisms that might provide descending inhibition of heat-sensitive dorsal horn neurons. However, propofol, even at the highest concentration tested, was not presently observed to directly excite any spinal WDR neurons, arguing against this possibility.
In conclusion, our data show that topical application of propofol has analgesic and potentially antihyperalgesic effects via inhibition of nociceptive spinal WDR neurons. Importantly, propofol reduced responses of WDR neurons to noxious heat, but not to cold or innocuous mechanical stimuli, indicating a selective analgesic effect and not a generalized local anesthesia. These results contrast with previous reports of pronociceptive effects of propofol and might be explained by direct or indirect effects that reduce the excitability of nociceptive nerve endings in the skin. Additional preclinical studies are needed to clarify the mechanism by which peripheral propofol induces its antinociceptive effect. The antinociceptive effect of topical propofol is consistent with clinical reports of postoperative analgesia and reduced opiate requirements in surgical patients emerging from anesthesia induced by propofol infusion.
Acknowledgments
Funding: This work was funded by the National Institutes of Health (NIH) DE013685 and AR057194.
The authors thank Minh Trannguyen for excellent technical assistance.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Kenichi Takechi, MD
Contribution: This author helped design and conduct the study, collected and analyzed data, and wrote the first draft of the manuscript.
Attestation: Kenichi Takechi attests to having approved the final manuscript.
Name: Mirela Iodi Carstens, BA
Contribution: This author helped design and conduct the study, performed histology.
Attestation: Mirela Iodi Carstens attests to having approved the final manuscript.
Name: Amanda H. Klein, PhD
Contribution: This author helped design and conduct the study and analyze data.
Attestation: Amanda H. Klein attests to having approved the final manuscript.
Name: E. Carstens, PhD
Contribution: This author helped design and conduct the study, assisted with data collection and analysis, and edited the manuscript.
Attestation: E. Carstens is the archival author. E. Carstens attests to having approved the final manuscript.
Contributor Information
Kenichi Takechi, Department of Anesthesiology and Resuscitology, Ehime University Medical School, Matsuyama, Japan.
Mirela Iodi Carstens, Department of Neurobiology, Physiology and Behavior, University of California, Davis, Davis, California.
Amanda H. Klein, Department of Neurobiology, Physiology and Behavior, University of California, Davis, Davis, California.
E. Carstens, Department of Neurobiology, Physiology and Behavior, University of California, Davis, Davis, California.
References
- 1.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Fassoulaki A. Is propofol an analgesic? Eur J Anaesthesiol. 2011;28(7):481–2. doi: 10.1097/EJA.0b013e32834584a5. [DOI] [PubMed] [Google Scholar]
- 3.Gilron I, Coderre TJ. Preemptive analgesic effects of steroid anesthesia with alphaxalone in the rat formalin test. Evidence for differential GABA(A) Receptor modulation in persistent nociception. Anesthesiology. 1996;84(3):572–9. doi: 10.1097/00000542-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Marota JJ, Crosby G. Pentobarbitone, but not propofol, produces pre-emptive analgesia in the rat formalin model. Br J Anaesth. 1994;72(6):662–7. doi: 10.1093/bja/72.6.662. [DOI] [PubMed] [Google Scholar]
- 5.Merrill AW, Barter LS, Rudolph U, Eger EI, 2nd, Antognini JF, Carstens MI, Carstens E. Propofol’s effects on nociceptive behavior and spinal c-fos expression after intraplantar formalin injection in mice with a mutation in the gamma-aminobutyric acid-type(A) receptor beta3 subunit. Anesth Analg. 2006;103(2):478–83. doi: 10.1213/01.ane.0000223847.50233.1b. [DOI] [PubMed] [Google Scholar]
- 6.Anwar MM, Abdel-Rahmen MS. Effect of propofol on perception of pain in mice: mechanisms of action. Comp Biochem Physiol A. 1998;120:249–53. doi: 10.1016/s1095-6433(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 7.Nadeson R, Goodchild CS. Antinociceptive properties of propofol: involvement of spinal cord gamma-aminobutyric acid(A) receptors. J Pharmacol Exp Ther. 1997;282(3):1181–6. [PubMed] [Google Scholar]
- 8.O’Connor TC, Abram SE. Inhibition of nociception-induced spinal sensitization by anesthetic agents. Anesthesiology. 1995;82(1):259–66. doi: 10.1097/00000542-199501000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Antognini JF, Wang XW, Piercy M, Carstens E. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth. 2000;47(3):273–9. doi: 10.1007/BF03018926. [DOI] [PubMed] [Google Scholar]
- 10.Barter LS, Mark LO, Jinks SL, Carstens EE, Antognini JF. Immobilizing doses of halothane, isoflurane or propofol, do not preferentially depress noxious heat-evoked responses of rat lumbar dorsal horn neurons with ascending projections. Anesth Analg. 2008;106(3):985–90. doi: 10.1213/ane.0b013e318163f8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuyo T, Antognini JF, Carstens E. Etomidate depresses lumbar dorsal horn neuronal responses to noxious thermal stimulation in rats. Anesth Analg. 2006;102(4):1169–73. doi: 10.1213/01.ane.0000204764.13637.d4. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y-Y, Li K-C, Chen J. Evidence for peripherally antinociceptive action of propofol in rats: behavioral and spinal neuronal responses to subcutaneous bee venom. Brain Res. 2005;1043(1–2):231–5. doi: 10.1016/j.brainres.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 13.Uchida H, Kishikawa K, Collins JG. Effect of propofol on spinal dorsal horn neurons. Comparison with lack of ketamine effects. Anesthesiology. 1995;83(6):1312–22. doi: 10.1097/00000542-199512000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Yao A, Atherley R, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg. 2007;105(4):1020–6. doi: 10.1213/01.ane.0000280483.17854.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108(5):1531–7. doi: 10.1213/ane.0b013e31819d9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilron I, Quirion R, Coderre TJ. Pre- versus postinjury effects of intravenous GABAergic anesthetics on formalin-induced Fos immunoreactivity in the rat spinal cord. Anesth Analg. 1999;88(2):414–20. doi: 10.1097/00000539-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 17.Anker-Møller E, Spangsberg N, Arendt-Nielsen L, Schultz P, Kristensen MS, Bjerring P. Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth. 1991;66(2):185–8. doi: 10.1093/bja/66.2.185. [DOI] [PubMed] [Google Scholar]
- 18.Bandschapp O, Filitz J, Ihmsen H, Berset A, Urwyler A, Koppert W, Ruppen W. Analgesic and antihyperalgesic properties of propofol in a human pain model. Anesthesiology. 2010;113(2):421–8. doi: 10.1097/ALN.0b013e3181e33ac8. [DOI] [PubMed] [Google Scholar]
- 19.Hand R, Jr, Riley GP, Nick ML, Shott S, Faut-Callahan M. The analgesic effects of subhypnotic doses of propofol in human volunteers with experimentally induced tourniquet pain. AANA J. 2001;69(6):466–70. [PubMed] [Google Scholar]
- 20.Zacny JP, Coalson DW, Young CJ, Klafta JM, Lichtor JL, Rupani G, Thapar P, Apfelbaum JL. Propofol at conscious sedation doses produces mild analgesia to cold pressor-induced pain in healthy volunteers. J Clin Anesth. 1996;8(6):469–74. doi: 10.1016/0952-8180(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg. 2008;106(1):264–269. doi: 10.1213/01.ane.0000287653.77372.d9. [DOI] [PubMed] [Google Scholar]
- 22.Tan T, Bhinder R, Carey M, Briggs L. Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesth Analg. 2010;111(1):83–5. doi: 10.1213/ANE.0b013e3181c0ee9e. [DOI] [PubMed] [Google Scholar]
- 23.Boccara G, Mann C, Pouzeratte Y, Bellavoir A, Rouvier A, Colson P. Improved postoperative analgesia with isoflurane than with propofol anaesthesia. Can J Anaesth. 1998;45(9):839–42. doi: 10.1007/BF03012216. [DOI] [PubMed] [Google Scholar]
- 24.Barr J, Egan TD, Sandoval NF, Zomorodi K, Cohane C, Gambus PL, Shafer SL. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmacodynamic model. Anesthesiology. 2001;95(2):324–33. doi: 10.1097/00000542-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hill SA. Pharmacokinetics of drug infusions. Cont Edu Anaesth Crit Care & Pain. 2004;4:76–80. [Google Scholar]
- 26.Guindon J, LoVerme J, Piomelli D, Beaulieu P. The antinociceptive effects of local injections of propofol in rats are mediated in part by cannabinoid CB1 and CB2 receptors. Anesth Analg. 2007;104(6):1563–9. doi: 10.1213/01.ane.0000263278.05423.a3. [DOI] [PubMed] [Google Scholar]
- 27.Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212(2):179–86. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Schmook FP, Meingassner JG, Billich A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm. 2001;215(1–2):51–6. doi: 10.1016/s0378-5173(00)00665-7. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Yamato K, Akiyama H, Tsuji K, Onishi H, Machida Y. Transdermal absorption of propofol in rats. Biol Pharm Bull. 2005;28(5):870–5. doi: 10.1248/bpb.28.870. [DOI] [PubMed] [Google Scholar]
- 31.Merrill AW, Cuellar JM, Judd JH, Carstens MI, Carstens E. Effects of TRPA1 agonists mustard oil and cinnamaldehyde on lumbar spinal wide-dynamic range neuronal responses to innocuous and noxious cutaneous stimuli in rats. J Neurophysiol. 2008;99(2):415–25. doi: 10.1152/jn.00883.2007. [DOI] [PubMed] [Google Scholar]
- 32.Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr, Oliveras JL. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Res. 1986;374(2):385–8. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- 33.Mayer DJ, Price DD, Becker DP. Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain. 1975;1(1):51–8. doi: 10.1016/0304-3959(75)90004-4. [DOI] [PubMed] [Google Scholar]
- 34.Klement W, Arndt JO. Pain on injection of propofol: effects of concentration and diluent. Br J Anaesth. 1991;67(3):281–4. doi: 10.1093/bja/67.3.281. [DOI] [PubMed] [Google Scholar]
- 35.Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285(45):34781–92. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci US A. 2008;105(25):8784–9. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsutsumi S, Tomioka A, Sudo M, Nakamura A, Shirakura K, Takagishi K, Kohama K. Propofol activates vanilloid receptor channels expressed in human embryonic kidney 293 cells. Neurosci Lett. 2001;312(1):45–9. doi: 10.1016/s0304-3940(01)02185-1. [DOI] [PubMed] [Google Scholar]
- 38.Wickley PJ, Yuge R, Russell MS, Zhang H, Sulak MA, Damron DS. Propofol modulates agonist-induced transient receptor potential vanilloid subtype-1 receptor desensitization via a protein kinase Cepsilon-dependent pathway in mouse dorsal root ganglion sensory neurons. Anesthesiology. 2010;113(4):833–44. doi: 10.1097/ALN.0b013e3181eaa9a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Wickley PJ, Sinha S, Bratz IN, Damron DS. Propofol restores transient receptor potential vanilloid receptor subtype-1 sensitivity via activation of transient receptor potential ankyrin receptor subtype-1 in sensory neurons. Anesthesiology. 2011;114(5):1169–79. doi: 10.1097/ALN.0b013e31820dee67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129(9):2312–5. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- 41.Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, Falck JR, Nithipatikom K, Campbell WB, Hillard CJ. The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139(5):1005–13. doi: 10.1038/sj.bjp.0705334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kress M, Kuner R. Mode of action of cannabinoids on nociceptive nerve endings. Exp Brain Res. 2009;196(1):79–88. doi: 10.1007/s00221-009-1762-0. [DOI] [PubMed] [Google Scholar]
- 43.Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93(2):713–22. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 44.Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, Hecker H, Dengler R, Leuwer M. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155(2):265–75. doi: 10.1038/bjp.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neukom L, Vastani N, Seifert B, Spahn DR, Maurer K. Propofol decreases the axonal excitability in rat primary sensory afferents. Life Sci. 2012;90(9–10):343–50. doi: 10.1016/j.lfs.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; San Diego: 1998. [Google Scholar]