Abstract
Smoking has significantly impacted American mortality and remains a major cause of morbidity and mortality. No previous study has systematically examined the contribution of smoking-attributable deaths to mortality trends among blacks or to black-white mortality differences at older ages over time in the United States. In this article, we employ multiple methods and data sources to provide a comprehensive assessment of this contribution. We find that smoking has contributed to the black-white gap in life expectancy at age 50 for males, accounting for 20 % to 48 % of the gap between 1980 and 2005, but not for females. The fraction of deaths attributable to smoking at ages above 50 is greater for black males than for white males; and among men, current smoking status explains about 20 % of the black excess relative risk in all-cause mortality at ages above 50 without adjustment for socioeconomic characteristics. These findings advance our understanding of the contribution of smoking to contemporary mortality trends and differences and reinforce the need for interventions that better address the needs of all groups.
Keywords: Mortality, Smoking, Race
Introduction
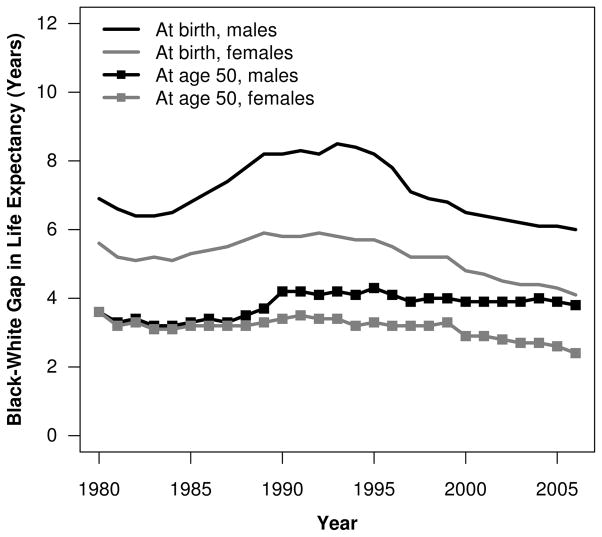
Black-white (B-W) mortality differences in the United States are sizable and persistent, posing a significant, longstanding public health concern. The B-W gap in life expectancy at birth has fluctuated over time, reaching a recent peak of 8.5 years for men and 5.8 years for women in 1993 before declining to 5.9 and 3.9 years, respectively, in 2007 (Arias 2011; National Center for Health Statistics (NCHS) 1997). In contrast, after 1990, the B-W gap in life expectancy at age 50 stagnated for males and declined slightly for females (Fig. 1). Given that 88 % to 96 % of black and white males and females survived to age 50 in 2007 (Arias 2011), an improved understanding of the factors contributing to B-W mortality disparities at older ages is needed.
Fig. 1.
Black-white gap in life expectancy at birth and at age 50 by sex, 1980–2006. Source: NCHS life tables 1980–2006.
Despite the extensive literature on B-W health disparities, reasons for their persistence are not fully resolved (e.g., Smedley et al. 2003; Williams et al. 2010). In this article, we focus on the contribution of smoking to B-W differences in mortality above age 50. Smoking is the leading preventable cause of premature morbidity and mortality in the United States and is strongly linked to chronic diseases prevalent at older ages (U.S. Department of Health and Human Services (DHHS) 2000). For example, 92 % to 96 % of lung cancer deaths occurred above age 50 among blacks and whites in 2005 (NCHS 2010a). Cohort and period data on smoking suggest that the magnitude of smoking-attributable mortality may differ between blacks and whites, especially among males. Consistent with this speculation, blacks have higher death rates from lung cancer and other smoking-related diseases than do whites (Haiman et al. 2006; Harper et al. 2007).
We integrate two approaches to explore the contribution of smoking to B-W mortality differences. No previous study has systematically examined the contribution of smoking-attributable deaths to mortality trends among blacks over time or to black-white mortality differences at older ages in the United States. Smoking is a potentially modifiable behavior, and its contribution to excess mortality among blacks and whites is not well understood.
Background
Black-White Differences in Smoking-Related Mortality
Smoking increases mortality from cardiovascular diseases (including hypertension, ischemic heart disease, cerebrovascular disease, and atherosclerosis) and respiratory diseases (such as pneumonia, influenza, bronchitis, emphysema, and chronic airway obstruction) (DHHS 1989, 2001). Smoking is also a risk factor for 15 cancers (Doll et al. 2005; International Agency for Research on Cancer (IARC) 2004).1 Estimates based on the Cancer Prevention Study II (CPS-II), a U.S.-based prospective cohort study, suggest that at least 25 % of deaths from nine cancers (bladder, esophagus, kidney, larynx, lip, lung, oral cavity, pancreas, and pharynx) are attributable to smoking (DHHS 1989; Preston et al. 2010). Recent estimates propose that at least 40 % of the decline in male cancer mortality between 1991 and 2003 resulted from smoking cessation (DeLancey et al. 2008).
As noted earlier, blacks in the United States suffer disproportionately from smoking-related diseases, with the exception of chronic obstructive pulmonary disease (COPD) (Burns et al. 1997; DHHS 1998; Haiman et al. 2006; Novotny et al. 1988; Williams and Collins 1995). Many smoking-related diseases have also been implicated in the B-W life expectancy gap. For example, Harper et al. (2007) identified cardiovascular diseases as the leading causes of B-W differences in life expectancy at birth in 2003, accounting for 1.9 years of the 6.3-year male life expectancy gap and 1.9 years of the 4.5-year female life expectancy gap. Cancer-related mortality explained nearly an additional year of the B-W gap for men and slightly more than half a year for women (Harper et al. 2007). According to the Surveillance Epidemiology and End Results (SEER) cancer registry, blacks had higher age-adjusted death rates than whites from most smoking-related cancers in 2007, except for kidney and bladder cancer among males and lung and bronchial cancer among females (SEER 2010). Blacks also had higher incidence and lower survival rates from smoking-related cancers than whites (Clegg and Ries 2007; Edwards et al. 2010; Wong et al. 2009). Blacks also appear to experience greater lung cancer risk than whites at lower levels of cigarette consumption (Haiman et al. 2006).
Black-White Differences in Smoking Behavior
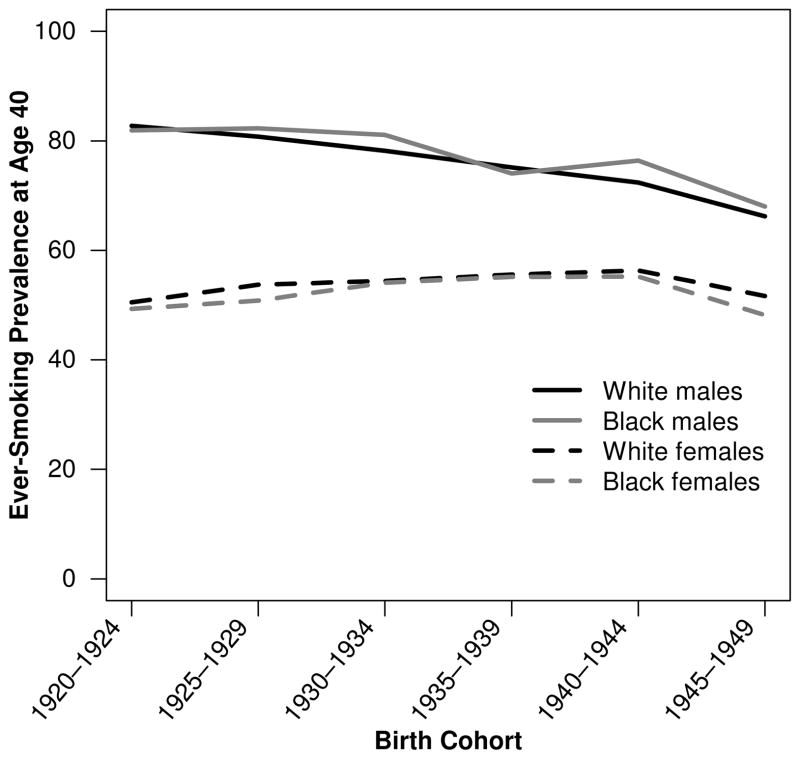
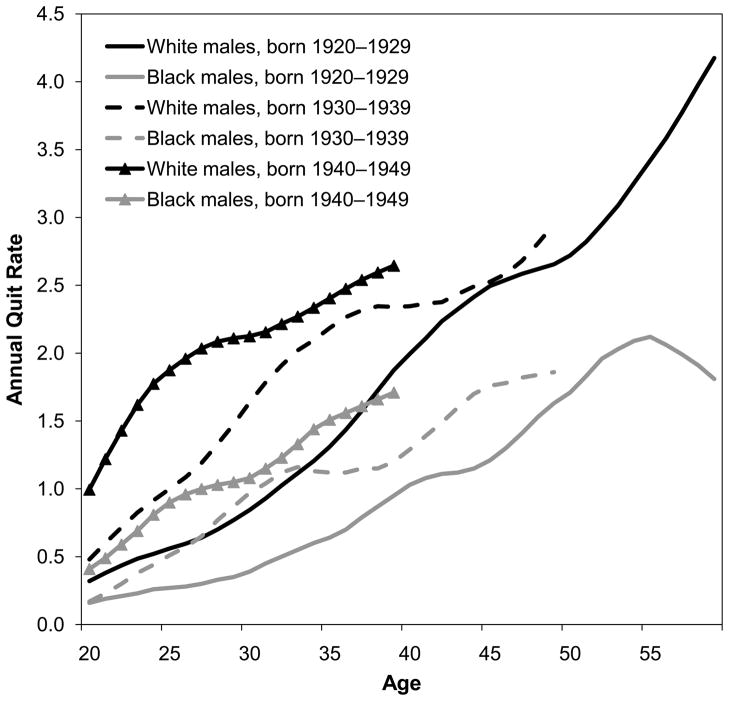
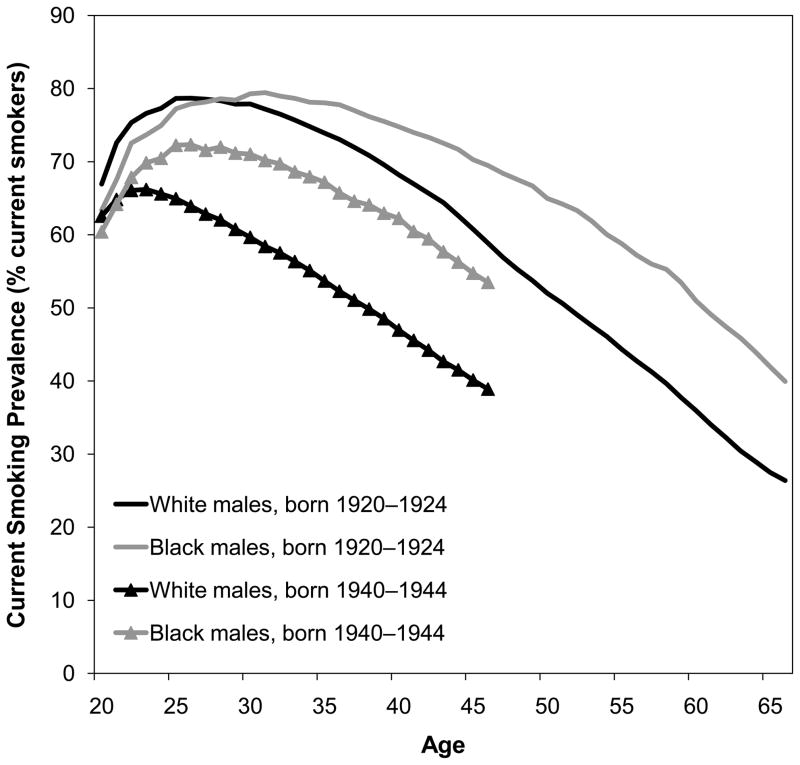
Burns et al. (1997) have made the best reconstructions of cohort smoking histories using National Health Interview Survey (NHIS) data, including adjustment for differential mortality by smoking status. For the 1920–1945 birth cohorts, black men were slightly more likely to be ever-smokers than white men. In contrast, white women from these same birth cohorts were more likely than black women to be ever-smokers (Fig. 2) (Burns et al. 1997). Burns et al. (1997) further noted that black men had lower smoking cessation rates than white men, and smoking cessation began 10 to 20 years later among blacks than whites. Figure 3 shows quit rates among three 10-year cohorts of black and white men born 1920–1949, which overlap with the cohorts under analysis (those aged 50 and older from 1980–2005 or aged 50–84 from 1997–2003).2 For each birth cohort, quit rates were lower among black men than white men. The same pattern is true for women (results not shown) (Burns et al. 1997). These differences are unlikely to be due to differences in the desire to quit because surveys show that blacks appear at least as likely as whites to want to quit smoking (DHHS 1998). Figure 4 shows current smoking prevalence by age for two 5-year cohorts of black men and white men. In the 1920–1924 birth cohorts, white men were slightly more likely to be current smokers until age 28. Thereafter, black men were more likely to be current smokers at every age, and the B-W difference widened with age, reaching a peak of 16 % when the men were in their late 50s through early 60s. In the 1940–1944 cohorts, the crossover occurred at an earlier age, and the B-W gap widened more rapidly and stayed above 10 % for 16 years (at ages 30–46). The same general pattern was observed for all male cohorts born in 1920–1949. In contrast, B-W differences in current smoking prevalence were much smaller among the corresponding female birth cohorts (results not shown) (Burns et al. 1997).
Fig. 2.
Ever-smoking prevalence at age 40 by sex and birth cohort. Source: Burns et al. (1997)
Fig. 3.
Annual quite rates by age, race, and birth cohort, males. Source: Burns et al. (1997)
Fig. 4.
Current smoking prevalence by age, race, and birth cohort, males. Source: Burns et al. (1997)
These observations are consistent with period data on smoking prevalence, cessation, and duration, with whites more likely to be ever-smokers and blacks more likely to be current smokers (DHHS 1998; Fiore et al. 1989; NCHS 2010b; Siahpush et al. 2010). For example, Giovino et al. (1994) showed that in the NHIS between 1965 and 1991, the percentage of adult ever-smokers who were former smokers was higher for whites than for blacks by 9.7 % to 16.8 % (both sexes combined). This racial disparity persisted through 2000 (King et al. 2004). Recent data also show longer smoking durations among blacks than whites. Based on the 2003, 2006, and 2007 Current Population Survey Tobacco Use Supplements, Siahpush et al. (2010) estimated the median durations of smoking to be 30 and 28 years among non-Hispanic blacks and non-Hispanic whites, respectively (both sexes combined, among respondents aged 18 and older who reported ever being daily smokers). Longer smoking durations and lower smoking cessation rates among blacks than whites may help explain blacks’ higher mortality from lung cancer and other smoking-related diseases. In some studies, duration of smoking was a stronger predictor of lung cancer risk than the number of cigarettes smoked per day (Flanders et al. 2003; International Agency for Research on Cancer (IARC) 2004; Lubin and Caporaso 2006). Several studies have also found smoking cessation to be beneficial at any age, with the reduction in lung cancer mortality risk being particularly large for smokers who quit before middle age (Halpern et al. 1993; Oza et al. 2011; Peto et al. 2000).
Black-White Differences in Nicotine Metabolism
Compared with whites, blacks smoke fewer cigarettes but inhale more deeply, are more likely to smoke menthol cigarettes and cigarettes with higher tar yields, achieve higher net indexes of smoke exposure, and may be at risk of greater physical dependence and exposure to smoke toxins (Chen 1993; Sellers 1998). These differences may be due to differential environmental and occupational exposures and their interactions with biological mechanisms in how nicotine and other substances in tobacco smoke are metabolized (Williams et al. 2010). Pharmacogenetic differences in nicotine metabolism can affect the risk of becoming a smoker; the amount smoked; the degree of physical dependence; and the absorption, distribution, and excretion of carcinogens in tobacco smoke (Sellers 1998).
The measurement of cotinine, a nicotine metabolite, is a specific and sensitive test for exposure to tobacco smoke and is used to distinguish active and passive smokers from nonsmokers (IARC 2004). It is also a marker of exposure to environmental tobacco smoke (ETS) because it has a longer half-life than nicotine (Caraballo et al. 1998). Based on data from the Third National Health and Nutrition Examination Survey, Caraballo et al. (1998) found that black smokers had significantly higher serum cotinine levels than white or Mexican American smokers at all smoking intensities even after adjustment for other sources of nicotine, such as the number of cigarettes smoked per day and exposure to ETS at home and at work, and age, sex, and body weight. It has been suggested that racial differences in serum cotinine levels may be attributable to racial differences in the accuracy of self-reported smoking. Caraballo et al. (1998) found that black and white self-reported smokers had serum cotinine levels consistent with their reported smoking levels, suggesting that results were not biased by reporting differences.
Pérez-Stable et al. (1998) examined the potential mechanism underlying higher cotinine levels in blacks in their hospital-based study of 40 black and 39 white smokers. The participants received infusions of deuterium-labeled nicotine and cotinine, allowing for the accurate determination of daily nicotine intake from smoking. Black smokers in this study smoked fewer cigarettes per day but had higher overall levels of serum cotinine than did white smokers. The authors attributed this difference to slower clearance of cotinine and higher intake of nicotine per cigarette smoked among blacks. A greater intake of nicotine and tobacco smoke carcinogens per cigarette may thus be related to blacks’ elevated burden of smoking-related diseases (Caraballo et al. 1998; Haiman et al. 2006).
Socioeconomic Status, Residential Context, Health Care, and Black-White Differences in Smoking-Attributable Mortality
Although racial identity is often conceptualized as an individual determinant of health and mortality, race captures important macro-level influences that condition individuals’ life chances in the United States (e.g., Massey and Denton 1993; Smelser et al. 2001). Furthermore, U.S. racial classifications reflect prevailing political, ideological, and social forces rather than meaningful biological differences (Omi and Winant 1994; Zuberi 2001). These stratification processes shape B-W differences in socioeconomic status (SES), residential context, and access to health care, all of which are prominent explanations for B-W differences in health and mortality (Hayward et al. 2000; Howard et al. 2000; Smedley et al. 2003; Williams et al. 2010). Thus, they are also likely to contribute to B-W differences in smoking-attributable mortality.
In large part, racial/ethnic differences in SES explain racial/ethnic variation in smoking behavior (Flint and Novotny 1998; Kiefe et al. 2001; King 1997; King et al. 2004). It has been hypothesized that the benefits of smoking cessation are viewed as marginal by low SES individuals who have greater exposure to many health-eroding circumstances that cumulate over the life course (Hayward et al. 2000; Link and Phelan 1995; Williams et al. 2010; Yao and Robert 2008). Smoking may serve as a “self-medicating mechanism” and a “form of relaxation” among low-income groups facing high levels of stress (Cutler and Lleras-Muney 2010; Lawlor et al. 2003; Lutfey and Freese 2005; Pampel et al. 2010). Furthermore, evidence suggests that cigarette companies target advertising in black communities (Altman et al. 1991; Landrine et al. 2005) and that the treatment effects of smoking cessation interventions are weaker for blacks than for whites (Murray et al. 2001). In addition, success in quitting smoking is greater among higher-SES individuals of most racial/ethnic groups (Barbeau et al. 2004). Differential access to and quality of health care may further contribute to differential impacts of smoking on mortality by SES and/or by race/ethnicity. Smoking is a risk factor for many forms of heart disease and cancer, which may be more effectively managed by timely and high-quality health care (Smedley et al. 2003; Williams et al. 2010). Some authors have further speculated that smoking may be more harmful for low-SES individuals because of their already poorer health status, whereas others propose the opposite—that the marginal impact of unhealthy behaviors is smaller for low-SES than high-SES groups (Blaxter 1990; Pampel and Rogers 2004). For example, Pampel and Rogers (2004) found that smoking was more predictive of morbidity, but not mortality, among low-SES than high-SES individuals, but they did not find a significant interaction between smoking and morbidity or mortality by race/ethnicity after controlling for SES.
Aims of This Study
Using complementary methods and data sources, we estimate (1) the magnitude of smoking-attributable mortality among blacks and whites in the United States, by sex, between 1980 and 2005; (2) the contribution of smoking-attributable mortality to the B-W gaps in life expectancy at age 50 and to the B-W gaps in expected years lived between ages 50 and 85; (3) the extent to which differences in smoking behavior explain B-W differences in mortality; and (4) whether the association between smoking and mortality differs between blacks and whites. We hypothesize that B-W differences in smoking behavior explain part of the B-W mortality differences among men albeit to a lesser extent among women. We further hypothesize that the association between smoking and mortality varies between blacks and whites.
Data and Methods
Indirect Estimation of Smoking-Attributable Mortality, 1980–2005
Data
For the indirect estimates of smoking-attributable mortality, we use vital statistics mortality data and population estimates available from the National Cancer Institute’s SEER database (http://seer.cancer.gov/) and calculate age-specific death rates from lung cancer and from all other causes combined by race, sex, and 5-year age group (50–54 to 85 and older). These data include all blacks and whites regardless of whether individuals identified as Hispanic because of the uneven quality of Hispanic ethnicity reporting in vital statistics and census data over the study period (Arias et al. 2008). We test the sensitivity of our results to the inclusion of Hispanics using the NHIS.
Methodology
Indirect estimation of smoking-attributable mortality is based on a method developed by Preston et al. (2010), which assumes that lung cancer death rates can be used as a proxy for the impact of smoking on mortality from all other causes of death. Lung cancer is one of the most accurately reported cancers on the death certificate, and reporting quality has been high since at least the 1970s (Percy et al. 1990, 1981). Furthermore, approximately 90 % of lung cancer deaths are attributable to smoking, and thus lung cancer mortality is thought to accurately proxy the impact of smoking on mortality from other causes (Preston et al. 2010).
Age-specific lung cancer death rates are used to predict age-specific smoking-related mortality from all other causes. The model, based on negative binomial regression, produces age- and sex-specific coefficients that, along with lung cancer death rates among nonsmokers, are used to estimate the fraction of deaths from all other causes that is attributable to smoking. Preston et al. (2011) estimated this model using data from 21 developed countries for the period 1950–2007. Fenelon and Preston (2012) applied the same model to U.S. state-level data for the period 1996–2004. We performed all analyses using the age- and sex-specific coefficients from both studies and found that the results were not sensitive to the choice of coefficients. We report results based on the coefficients published in Fenelon and Preston (2012). Both Preston et al. (2011) and Fenelon and Preston (2012) demonstrated that this method produces results very similar to those obtained from an older, widely used method developed by Peto et al. (1992).
We estimate age-specific death rates from which smoking-attributable mortality is removed, , as follows:
| (1) |
where mi is the age-specific death rate from all causes combined, Ai is the fraction of deaths in each age group that are attributable to smoking, and i = 50–54, …, 80–84, 85 and older (see Preston et al. 2010). We then use standard life table procedures to calculate life expectancy at age 50 by race and sex with and without the inclusion of smoking-attributable deaths to determine the extent to which smoking contributes to the B-W gap in life expectancy at age 50 and whether this contribution has changed over time (Preston et al. 2001). We take the race-age-sex-specific nax and ∞m85 values from annual U.S. life tables constructed by the NCHS because estimates of old-age mortality based on vital statistics and census data without adjustment are likely to be flawed (CDC/NCHS 2010; Elo 2001; Preston and Elo 2006). Our estimates of life expectancy at age 50 are very close to the published life table values.
Estimates of Smoking-Attributable Mortality Based on the NHIS, 1997–2003
Data
We use seven waves of the NHIS (1997–2003), including the adult supplement that collects information on smoking behavior for individuals aged 18 and older, that have been linked to the National Death Index (NDI) through 2006. The NHIS is the most comprehensive nationally representative data source for studying socioeconomic and behavioral determinants of mortality in the United States (e.g., Hummer et al. 1999; Pampel and Rogers 2004; Rogers et al. 2005). The NHIS has several strengths: it has a large sample size; oversamples blacks, Hispanics, and Asians; provides high-quality information on respondents’ health and sociodemographic characteristics; is representative of the U.S. civilian noninstitutionalized population; and has achieved a lengthy mortality follow-up while maintaining a close correspondence between the survival experience of NHIS cohorts and the U.S. population (CDC/NCHS 2012; Ingram et al. 2008).
We restrict our sample to individuals aged 50–84 at the time of the survey, an age range similar to that used in the indirect estimation of smoking-attributable mortality. These ages accounted for more than 98 % of the B-W life expectancy difference at age 50 in 1980 and 2005 (calculations by the authors). We further limit the sample to non-Hispanic whites and non-Hispanic blacks and test the sensitivity of our results to the inclusion of all whites and blacks without excluding Hispanics. We focus the analysis on males because smoking-attributable mortality makes a large contribution to B-W mortality differences among men but not among women (see upcoming Fig. 6).
Fig. 6.
Black-white (B-W) gap in life expectancy at age 50 with and without smoking-attributable deaths, United States 1980–2005. Source: Calculations by the authors
The pooled 1997–2003 NHIS adult sample consists of 30,422 non-Hispanic white and non-Hispanic black men aged 50–84. We drop individuals ineligible for mortality follow-up because of a lack of information necessary for the linkage to the NDI and missing quarter of birth (N = 1,677). We also exclude 1,087 men with missing information on smoking behavior and 494 men with missing information on other explanatory variables. The final sample consists of 23,701 non-Hispanic white men and 3,463 non-Hispanic black men, of whom 4,831 died during the follow-up period.
Explanatory Variables
Our smoking variable distinguishes among never-smokers,3 current smokers, and former smokers, taking into account smoking intensity (number of cigarettes smoked per day) among current smokers and time since quitting among former smokers. Time since quitting is calculated from respondents’ reported number of years since quitting. Thus, our smoking variable is coded as follows: never-smoker; former smoker who quit 30 or more, 20–29, 10–19, 5–9, or 0–4 years ago; and current smoker who smoked less than one or one or more packs of cigarettes per day. Individuals who smoked two or more packs of cigarettes per day were rare in this sample of older adults.
Most smokers (86.1 %) began smoking by age 20, and nearly all (98.9 %) had begun by age 30. Most current smokers were long-time smokers, with 93 % having smoked for more than 30 years (tabulations by the authors). Thus, being a current smoker captures both current smoking intensity and long smoking duration. Furthermore, recent quitters (those who quit less than 10 years ago) were also long-time smokers, with 99 % having smoked for more than 20 years. Because smoking duration and quit rates vary between blacks and whites, our smoking variable captures these black-white differences.
We also control for other individual-level attributes which vary by smoking status and race and which can confound the relationship between smoking and mortality. These include body mass index (BMI), coded as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obese I (30.0–34.9 kg/m2), and obese II/III (≥35.0 kg/m2); marital status (never married, currently married, and widowed/divorced/separated); educational attainment (<12 years, 12 years, 1–3 years of college, and 4 or more years of college); and family income. We use the imputed family income variable available from the Integrated Health Interview Series (http://www.ihis.us/ihis/). We convert the categorical income variable into a linear variable by taking the midpoint of each category and dividing by 10,000. We assign the value of the open-ended category, which begins at $75,000, by estimating a median value for this category (Denny et al. 2010; Parker and Fenwick 1983; Rogers et al. 2005).4 We then take the natural logarithm of the linear income variable in our models to capture its nonlinear association with mortality. We also control for region of residence to capture regional residential differences between blacks and whites and differential mortality by region.
Methods and Analytic Strategy
We use Cox proportional hazards regression to estimate all-cause mortality. We focus on all-cause mortality because smoking contributes to mortality from multiple causes and because our indirect estimation of smoking-attributable mortality captures mortality from all smoking-related causes. Our Cox proportional hazards model is in the form of
| (2) |
where h(t) is the unspecified baseline hazard function; t measures age; i refers to the individual; S to smoking behavior; X to other explanatory variables, including baseline age and race/ethnicity; and Y refers to the survey year. Individuals who were alive on December 31, 2006 are censored on this date (Allison 1995). Age is used as the analysis time, which allows for unrestricted nonlinearity in its effects and, in the construction of the partial likelihood, makes comparisons only among people of the same age. Hazard ratios are the exponentiated coefficients from the regression models (HR = eβ). We use t tests to assess the significance of individual coefficients. We take into account the complex survey design of the NHIS to obtain corrected standard errors. All models are weighted and estimated with Stata 11.
We specify three models: Model 1 estimates the magnitude of excess mortality among non-Hispanic black males relative to non-Hispanic white males, controlling for baseline age, survey year, and BMI. In Model 2, we add smoking behavior to assess whether controlling for smoking helps explain the black-white difference. In Model 3, we introduce marital status, education, family income, and region to assess whether they modify the black and smoking coefficients. We then interact race with smoking status to test whether smoking has a differential impact on mortality among non-Hispanic whites and non-Hispanic blacks.
We use the hazard ratios from Model 3 to calculate population attributable risk fractions (PAFs) due to smoking and compare them with those obtained from indirect estimation methods. We use the method recommended for calculating PAFs with a multicategory exposure variable in the presence of confounding (Rockhill et al. 1998):
| (3) |
where i refers to smoking category, and pdi is the fraction of total deaths occurring in the ith smoking category. RRi is the hazard ratio from Model 3 for the ith smoking category. The PAF estimates the proportion of deaths that could be avoided if smoking were eliminated (Flegal et al. 2005; Mehta and Chang 2009). In practice, it estimates the proportion of deaths that could be avoided if all current and former smokers experienced the mortality risks of never-smokers.
To estimate the percentage of the black-white gap in expected years lived between ages 50 and 85 that is attributable to smoking from the NHIS, we estimated a discrete-time hazard model with the same covariates included in Model 3 and five-year age group as a time-varying covariate. The coefficients from this model are nearly identical to those obtained from the Cox regression. We use the coefficients from this model to calculate death rates for age groups 50–54 through 80–84 using the following equation (Rogers et al. 2005):
| (4) |
where z is the sum of the coefficients for a given age group evaluated at the race-specific means and proportions shown in Table 1. Because the estimates based on the NHIS do not cover the entire age range (e.g., the oldest respondent would have been aged 93 by the end of follow-up), we calculate the expected number of years lived between ages 50 and 85 using standard life table techniques with the assumption that respondents dying in a particular age interval do so halfway through the interval. We estimate expected number of years lived between ages 50 and 85 in the absence of smoking by setting the proportions of never-smokers equal to 1 and all other smoking categories to 0.
Table 1.
Descriptive statistics, means, and standard deviations or percentages for individual-level characteristics, National Health Interview Survey, males, 1997–2003
| Variable | Total | Non-Hispanic Whites (91 %) | Non-Hispanic Blacks (9 %) | p Value |
|---|---|---|---|---|
|
| ||||
| N = 27,164 | N = 23,701 | N = 3,463 | ||
| Smoking Behavior | ||||
| Never-smoker | 33.1 | 32.8 | 35.7 | .0000 |
| Former smoker, quit 0–4 years ago | 5.6 | 5.5 | 5.7 | |
| Former smoker, quit 5–9 years ago | 4.4 | 4.4 | 4.5 | |
| Former smoker, quit 10–19 years ago | 12.0 | 12.2 | 10.1 | |
| Former smoker, quit 20–29 years ago | 11.7 | 12.0 | 8.8 | |
| Former smoker, quit 30+ years ago | 13.8 | 14.5 | 7.1 | |
| Current smoker, <1 pack per day | 7.3 | 6.2 | 18.1 | |
| Current smoker, 1+ packs per day | 12.2 | 12.4 | 10.1 | |
| Body Mass Index | ||||
| Underweight, BMI <18.5 | 0.7 | 0.7 | 1.0 | .0000 |
| Normal, BMI 18.5–24.9 | 27.8 | 27.6 | 30.0 | |
| Overweight, BMI 25–29.9 | 46.7 | 47.1 | 42.8 | |
| Obese I, BMI 30–34.9 | 18.5 | 18.5 | 18.5 | |
| Obese II and III, BMI 35+ | 6.3 | 6.1 | 7.8 | |
| Sociodemographic Characteristics | ||||
| Mean age at baseline (SD) | 62.776 (0.069) | 62.894 (0.074) | 61.610 (0.190) | .0000 |
| Marital status | ||||
| Never married | 5.0 | 4.5 | 9.5 | .0000 |
| Currently married | 76.3 | 78.1 | 58.6 | |
| Widowed/divorced/separated | 18.7 | 17.3 | 32.0 | |
| Educational attainment | ||||
| Less than high school (<12 years) | 17.4 | 15.6 | 35.7 | .0000 |
| High school graduate (12 years) | 31.4 | 31.5 | 30.0 | |
| 1–3 years of college | 23.7 | 24.0 | 20.5 | |
| 4+ years of college | 27.5 | 28.9 | 13.9 | |
| Mean family income (10,000s) (SD) | 5.510 (0.035) | 5.653 (0.037) | 4.087 (0.105) | .0000 |
| Region | ||||
| Northeast | 20.2 | 20.6 | 16.1 | .0000 |
| North Central | 26.6 | 27.4 | 18.4 | |
| South | 37.2 | 35.2 | 57.4 | |
| West | 16.0 | 16.8 | 8.1 | |
| Mean Years of Follow-up | ||||
| Alive (censored) | 6.130 | 6.136 | 6.071 | |
| Dead | 3.807 | 3.817 | 3.732 | |
Source: Authors’ calculations based on 1997–2003 NHIS data linked to the National Death Index through 2006; p values correspond to t tests and chi-square tests for the equality of means/distributions of variables between non-Hispanic black and white males.
Results
Indirect Estimates of Smoking-Attributable Mortality, 1980–2005
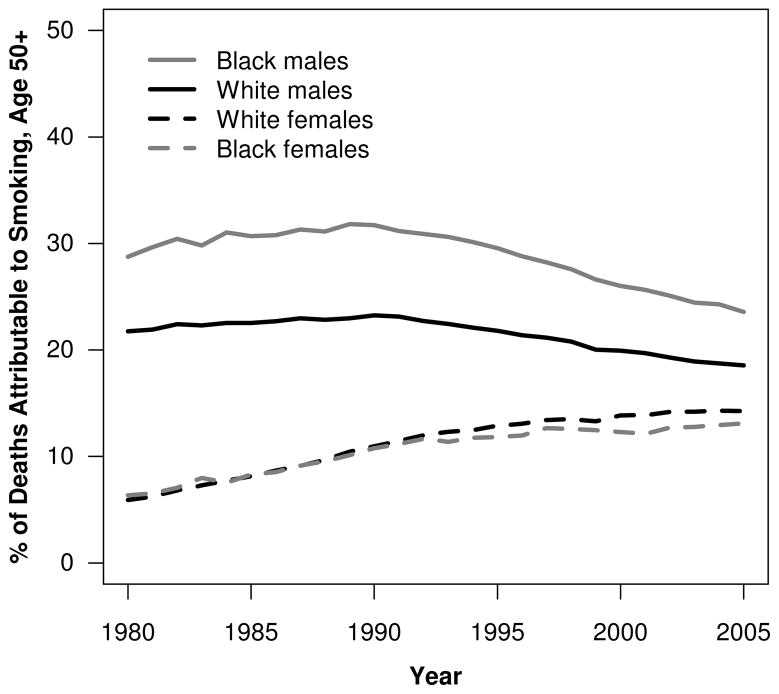
Figure 5 shows the percentage of deaths attributable to smoking by race and sex between 1980 and 2005. Among men, this fraction increased until around 1990 and was higher among black men than white men throughout the period. This B-W difference reached a peak (8.9 %) in 1989, when 31.8 % and 23.0 % of deaths among black and white men older than age 50, respectively, were attributable to smoking. The gap subsequently declined to 5.0 % by 2005, when 23.6 % and 18.5 % of deaths were attributable to smoking among black and white men, respectively.
Fig. 5.
Percentage of deaths attributable to smoking at ages above 50 by sex among blacks and whites, United States 1980–2005. Source: Calculations by the authors
In contrast, smoking-attributable deaths constituted a much smaller percentage of deaths among women, but their contribution rose steadily throughout the period, reflecting women’s later uptake of smoking (Burns et al. 1997; Preston and Wang 2006). Furthermore, smoking-attributable deaths made up a smaller percentage of all deaths among black women than among white women, except in the early 1980s, when this percentage was slightly higher among blacks (Fig. 5). For example, in 1980, the percentage of deaths attributable to smoking was 5.9 % for white females and 6.3 % for black females. By 2005, these percentages had increased to 14.3 % and 13.1 %, respectively.
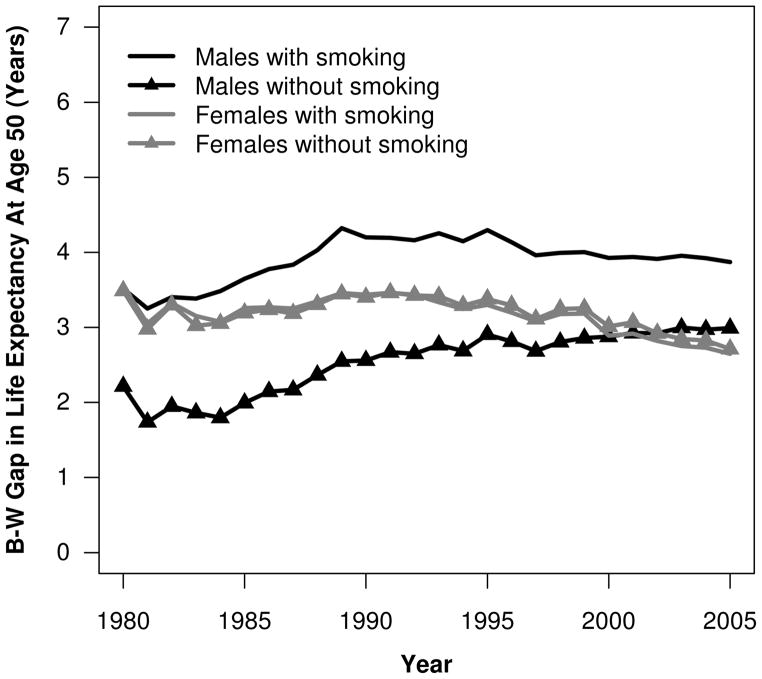
Smoking-attributable mortality reduced life expectancy at age 50 for white males by 2.86 years in 1990 (29.62 vs. 26.76) and by 2.21 years in 2005 (31.18 vs. 28.98). The comparable values for black males were 4.50 years in 1990 (27.06 vs. 22.56) and 3.09 years in 2005 (28.19 vs. 25.11). Figure 6 shows the contribution of smoking-attributable mortality to B-W differences in life expectancy at age 50 over time. In the absence of smoking-attributable mortality, the B-W gap in male life expectancy at age 50 would have been about 1.64 years smaller in 1990 and 0.88 years smaller in 2005. Thus, smoking-attributable mortality accounted for 39 % (1.64/4.20) of the B-W gap in male life expectancy at age 50 in 1990 and almost 23 % (0.88/3.87) in 2005.
The magnitude of smoking-attributable mortality was much smaller among women. In 1980, it reduced life expectancy at age 50 by 0.74 years for white females and 0.80 years for black females. By 2005, these figures had increased to 1.72 and 1.66 years, respectively. Because the impact of smoking on life expectancy at age 50 was similar for white and black women, the elimination of smoking-attributable deaths had little impact on the B-W gap in female life expectancy at age 50 (Fig. 6). Supplemental tables showing the fraction of deaths attributable to smoking above age 50 and life expectancy at age 50 with and without smoking by race and sex are available online (Online Resource 1).
Smoking Behavior and Mortality Among Non-Hispanic Black and Non-Hispanic White Men: Results From the NHIS
Table 1 provides sample characteristics for the entire sample and for non-Hispanic white and non-Hispanic black males in the NHIS. In this section, from this point forward, “black” refers to non-Hispanic black men, and “white” refers to non-Hispanic white men unless otherwise specified. There were significant differences in smoking status between the two groups. A higher percentage of blacks (35.7 %) than whites (32.8 %) were never-smokers and current smokers (28.2 % and 18.6 %, respectively), whereas a higher percentage of whites (48.6 %) than blacks (36.2 %) were former smokers. Furthermore, 26.5 % of whites had quit smoking more than 20 years ago compared with only 15.9 % of blacks. These smoking patterns are consistent with results from prior studies: black men were more likely to be current smokers and to have stopped smoking more recently than their white counterparts.
Blacks and whites also differed significantly on other characteristics. On average, blacks were slightly younger, less likely to be currently married, and more likely to be widowed/divorced/separated or never married than whites. About 36 % of blacks had less than a high school education, compared with 15.6 % of whites; and blacks were less likely to have attended at least four years of college. Blacks also had significantly lower family incomes and were more likely to live in the South.
Table 2 presents the hazard ratios from multivariate models for black men relative to white men and for former and current smokers relative to nonsmokers. All models control for age at baseline, BMI, and survey year. Model 2 adds smoking status, and Model 3 adjusts for marital status, educational attainment, family income, and region of residence. The hazard of dying is about 50 % higher for blacks than for whites in Model 1. The introduction of smoking status decreases this relative risk by 20 %,5 suggesting that smoking plays a role in the excess mortality of black men relative to white men at ages 50 and older. However, a far greater reduction occurs when we introduce controls for marital status, education, family income, and region. The hazard ratio for black men is reduced from 1.39 to 1.15, or by 62 %, supporting the notion that socioeconomic circumstances are the key explanations for higher mortality among black men.
Table 2.
Hazard ratios from Cox regression models, National Health Interview Survey, non-Hispanic white and non-Hispanic black males aged 50–84, 1997–2003 (reference category in parentheses)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Race (non-Hispanic white) | |||
| Non-Hispanic black | 1.49*** | 1.39*** | 1.15** |
| Smoking Status (ref. = never-smoker) | |||
| Former smoker, quit 30+ years ago | 1.06 | 1.06 | |
| Former smoker, quit 20–29 years ago | 1.20** | 1.18** | |
| Former smoker, quit 10–19 years ago | 1.70*** | 1.66*** | |
| Former smoker, quit 5–9 years ago | 2.30*** | 2.14*** | |
| Former smoker, quit 0–4 years ago | 2.57*** | 2.37*** | |
| Current smoker, <1 pack per day | 2.52*** | 2.28*** | |
| Current smoker, 1+ packs per day | 3.05*** | 2.66*** | |
| N (unweighted) | 27,164 | 27,164 | 27,164 |
Notes: All models control for survey year, age at baseline, and BMI. Model 3 also includes controls for marital status, educational attainment, family income, and region of residence.
p < .01;
p < .001
One of the most striking findings is the variation in mortality by smoking status (Table 2). Compared with never-smokers, current heavy smokers (one or more packs of cigarettes per day) have the highest risk (2.66, Model 3) followed by current light smokers (less than one pack of cigarettes per day) and those who quit less than five years ago (2.28 and 2.37, respectively, Model 3). The elevated risk among recent quitters is consistent with other studies and may be due to individuals quitting smoking because of illness (U.S. DHHS 1990). Among former smokers, hazard ratios decline with time since quitting, and those who quit more than 30 years ago experience risks similar to never-smokers. The introduction of other explanatory variables (Model 3 vs. Model 2) results in small declines in the hazard ratios for the smoking categories, but they remain highly significant (except for those who quit 30 or more years ago, which is not significant in either model).
We hypothesized that the associations between smoking and mortality would vary by race. To test this possibility, we included interaction terms between race and smoking status in Model 3. These interactions were not jointly significant, providing little support for the hypothesis that the effects of smoking differ between blacks and whites.
Table 3 shows the PAFs due to smoking by race and smoking category calculated using the hazard ratios from Model 3, Table 2. Attributable risk fractions are presented as percentages, reflecting the percentage of deaths that could be avoided if smokers had the same mortality risks as never-smokers. Because the association between smoking and mortality did not vary between blacks and whites, variation in the PAFs arises from between-group differences in the distribution of deaths by smoking category (i.e., the pdis from Eq. (3)).
Table 3.
Smoking-attributable mortality (%) by smoking status, National Health Interview Survey, non-Hispanic white and non-Hispanic black males aged 50–84, 1997–2003
| Smoking Status (ref. = never-smoker) | Non-Hispanic White Males | Non-Hispanic Black Males |
|---|---|---|
| Former Smoker, Quit 30+ Years Ago | 0.88 | 0.38 |
| Former Smoker, Quit 20–29 Years Ago | 1.65 | 0.98 |
| Former Smoker, Quit 10–19 Years Ago | 5.53 | 4.31 |
| Former Smoker, Quit 5–9 Years Ago | 3.23 | 2.51 |
| Former Smoker, Quit 0–4 Years Ago | 4.42 | 4.03 |
| Current Smoker, <1 Pack Per Day | 4.08 | 13.03 |
| Current Smoker, 1+ Packs Per Day | 9.87 | 7.86 |
| Total | 29.66 | 33.10 |
Note: Based on hazard ratios from a model controlling for survey year, age at baseline, race, BMI, marital status, educational attainment, family income, and region of residence.
We estimate that 29.7 % and 33.1 % of deaths among white and black men, respectively, were attributable to smoking. Among whites, heavy smokers made the largest contribution (9.9 %); contributions of the other smoking categories are only about half or less than half this size. In contrast, among blacks, current light smokers made the largest contribution (13.0 %), followed by current heavy smokers (7.9 %). Contributions of most other smoking categories among blacks were less than half this size. We further estimate the percentage of the B-W gap in expected years lived between ages 50 and 85 that is attributable to smoking to be 31.3 % based on the NHIS. This figure is very similar to the 2002 estimate of the contribution of smoking to the B-W gap in expected years lived between ages 50 and 85 based on the indirect method using vital statistics and census data (29.4 %, calculations by the authors).
Discussion and Conclusions
Smoking continues to be a leading cause of morbidity and mortality in the United States. Recent evidence suggests that the relative risk of death among current and former smokers has continued to increase over time (Mehta and Preston 2012). We employed multiple methods and data sources to provide a comprehensive assessment of its contribution to trends in black and white mortality and to B-W differences in mortality at ages 50 and above between 1980 and 2005. Smoking reduced life expectancy at age 50 by up to 2.86 years (1990) for white men and 4.62 years (1989) for black men. By 2005, these figures declined to 2.21 and 3.09 years, respectively. The contribution of smoking to the B-W male life expectancy gap at age 50 peaked in 1984 at 1.69 years (accounting for approximately 48 % of the gap), but was reduced to 0.88 years by 2005 (to approximately 23 % of the gap).
Smoking made only a minor contribution to the B-W gap in female life expectancy at age 50. Smoking-attributable mortality reduced white female life expectancy at age 50 by 0.74 years in 1980, compared with 0.80 years among black women. By 2005, these contributions had increased to 1.72 and 1.66 years, respectively.
We estimated the percentage of deaths attributable to smoking above age 50 to be 24 % to 28 % among black males and 19 % to 21 % among white males between 1997 and 2005 based on the indirect method. In 2002, the B-W difference in the percentage of deaths attributable to smoking for men was 5.8 % (25.1 % to 19.3 %). These estimates are consistent with the results of Preston et al. (2010), who estimated the smoking-attributable fraction among all U.S. males above age 50 to be 24 % in 2003, and Fenelon and Preston (2012), who estimated the smoking-attributable fraction of deaths among men aged 50–84 to be 21 % in 2004.
Our estimates of the population attributable fraction of deaths due to smoking based on the NHIS were 33.1 % for black men and 29.7 % for white men. These estimates apply to the period 1997–2006 and exceed the indirect estimates corresponding to the midpoint of this period, 2002. However, the magnitude of the B-W difference was similar (3.4 % vs. 5.8 %). In contrast, we find that the proportion of the B-W gap in expected number of years lived between ages 50 and 85 attributable to smoking based on the NHIS (31.3 %) was very similar to the same figure based on indirect estimates (29.4 % in 2002). Indirect estimates of the proportion of the B-W gap in expected number of years lived between ages 50 and 85 ranged from 25.7 % to 34.5 % between 1997 and 2005. Thus, we obtained highly consistent findings regarding the contribution of smoking to B-W mortality differences between ages 50 and 85.
Based on the NHIS, we found that the black excess mortality risk among men was reduced by 20 % when baseline smoking status was included in the model. However, the risk reduction was much greater when sociodemographic characteristics were added (62 %). Thus, while B-W differences in smoking among men do contribute to B-W disparities in male mortality, B-W differences in SES play a much larger role (see also Hayward et al. 2000). Finally, we speculated that the association between smoking and mortality would vary between blacks and whites but found no support for this hypothesis. This result is consistent with Pampel and Rogers (2004), who found that an interaction between race and smoking status, controlling for SES, was not a significant predictor of morbidity, mortality, or self-rated health. Danaei et al. (2010:4) also concluded that “the current evidence indicates that while the absolute effects (e.g., excess mortality rate) of risk factors vary by race, their proportional effects (i.e., relative risks) did not vary appreciably by race and ethnicity.”
The deleterious effects of smoking change only modestly when we include controls for marital status, educational attainment, family income, and region. The excess risks associated with smoking are striking. Current smokers, 93 % of whom have smoked for more than 30 years, are 2.3–2.7 times more likely to die than never-smokers. Very recent quitters have elevated risks slightly higher than current light smokers, which may be due to quitting in response to smoking-related conditions, and the risk diminishes considerably as time since quitting increases. Those who stopped smoking at least 30 years prior to the baseline survey have mortality risks similar to never-smokers. Most of these individuals had quit prior to age 40. These results are consistent with those of Peto et al. (2000), who concluded that smokers quitting before middle age avoid more than 90 % of smoking-attributable lung cancer risk, and Oza et al. (2011), who found that smokers’ risk of dying from cardiovascular disease converges to that of never smokers after 10 years of having quit smoking.
Black-white differences in cohort patterns of smoking prevalence and cessation are consistent with the widening and subsequent narrowing of the B-W gap in smoking-attributable mortality. Nevertheless, given the relatively small B-W differences in ever-smoking prevalence (Fig. 2), questions remain about why black males experience higher smoking-attributable mortality than white males. Our results support the hypothesis that lower smoking cessation rates and longer smoking durations among blacks are a contributing factor. In our sample, 50- to 84-year-old non-Hispanic black and white male ever-smokers reported having smoked 35.0 and 31.0 years (age-standardized), respectively—a difference of 4 years, on average. The B-W difference in years smoked before age 50 is only 1.9 years. This is consistent with Fig. 4, which shows that cohort differences in current smoking prevalence widen with age and are particularly large after age 50. These B-W differences are more likely to be due to B-W differences in quitting success, access to effective smoking cessation interventions, and experiences of daily stressors than a lack of information about the benefits of quitting. B-W differences in exposure to toxins per cigarette resulting from slower clearance of nicotine and cotinine (reviewed earlier), depth of inhalation, amount of each cigarette smoked, and type of cigarettes smoked may also contribute (Chen 1993; Sellers 1998; Williams and Collins 1995). Other factors, such as the impact of cumulative disadvantage over the life course and differential access to timely and high-quality health care and other health-enhancing resources, are also likely to play a role. Furthermore, blacks are more likely than whites to be diagnosed at a later stage for smoking-related cancers, and blacks also have a greater burden of comorbidities.
The main strengths of our paper lie in the pairing of direct and indirect estimation approaches to assess smoking-attributable mortality and our inclusion of detailed information on individual smoking histories. The indirect method has the potential to produce more accurate estimates of smoking-attributable mortality because it does not rely on self-reported smoking behavior at a single point in time. It allows for straightforward estimation of smoking-attributable deaths by age and their impact on life expectancy at age 50. This assessment is particularly instructive because mortality attributable to smoking varies by age, and deaths at younger ages contribute more to life expectancy than those at older ages (Rogers et al. 2005). The comparison with the results based on the NHIS are also instructive. The NHIS allows us to control for potential confounders and verify that our results are robust to the inclusion or exclusion of Hispanics (see Table 4) or alternate model specifications. Finally, our NHIS measure of smoking incorporates more information on smoking behavior than most prior studies, which often control only for whether the individual is a never-smoker, former smoker, or current smoker. We capture both smoking duration and intensity among current smokers and time since quitting among former smokers.
Table 4.
Comparison of population attributable fractions (%) from indirect estimation and Cox regression models for black and white males
| Blacks | Whites | Difference (B-W) | |
|---|---|---|---|
| Indirect Estimation (2002) | 25.08 | 19.29 | 5.79 |
| Direct Estimation (1997–2006) | |||
| Eight-category smoking status (non-Hispanics)a | 33.10 | 29.66 | 3.43 |
| Three-category smoking status (non-Hispanics)b | 31.30 | 29.30 | 1.99 |
| Eight-category smoking status (including Hispanics)a | 32.33 | 29.13 | 3.20 |
| Three-category smoking status (including Hispanics)b | 30.76 | 28.79 | 1.97 |
Categories: Never-smoker, former smoker quit 30+ years ago, former smoker quit 20–29 years ago, former smoker quit 10–19 years ago, former smoker quit 5–9 years ago, former smoker quit 0–4 years ago, current smoker <1 pack per day, and current smoker 1+ packs per day
Categories: Never-smoker, former smoker, and current smoker.
Our study also has limitations. First, the indirect method relies on the strength of the association between smoking and lung cancer mortality. Racial identity seems to affect the classification of certain causes of death (e.g., cirrhosis and homicide) (Noymer et al. 2011). If the quality of death certification for lung cancer varies between blacks and whites, some bias could be introduced. However, because of the strong link between lung cancer and smoking and the high quality of lung cancer death certification in the United States, misclassification is unlikely to be a major problem. Despite their lower health care access (which may make them less likely to be diagnosed), black males have a higher incidence of lung cancer. They also experience lower survival from lung cancer relative to white males. Black males’ higher mortality rates are consistent with these observations, which suggests that any certification bias is unlikely to be large. Potential bias may be introduced if the coefficients used to estimate smoking-attributable mortality differ between blacks and whites. We do not expect that such differences would alter our findings. Additionally, the expected lung cancer death rates among nonsmokers were drawn from the CPS-II, a study based on volunteers who were more likely to be white, middle-class, and college-educated than the general U.S. population. However, the rates of lung cancer among nonsmokers in the CPS-II were similar to those observed in other samples, and the CPS-II is the best available source of these estimates because it “remains the largest epidemiological study of its kind ever attempted in the history of medical science,” having enrolled 1.2 million participants (Klausner 1997:iv; Preston et al. 2010).
The primary limitation of the direct estimates based on the NHIS is the reliance on self-reported smoking information from a cross-sectional survey. Recall bias and misclassification of smoking status could result in less accurate estimates of smoking-attributable mortality. The extent to which these factors vary between blacks and whites is ambiguous. Because of data limitations, we cannot control for intensity of smoking among former smokers, use of cigars and smokeless tobacco, or occupational exposures. In addition, because smoking status is measured at baseline, we do not know whether respondents’ behavior changed during follow-up or how accurately our measures capture lifetime smoking intensity. For example, individuals entering the survey at ages 50 and older may now smoke fewer cigarettes than in the past or may have altered their behavior in response to illness or changes in cigarette taxes, state laws, and tar and nicotine yields in cigarettes (Bergen and Caporaso 1999). We may also overestimate the proportion of all-cause mortality attributable to smoking if blacks are more concentrated in high-risk occupations. B-W differences in occupational exposures and lung cancer risk are not well studied. Haiman et al. (2006) found that controlling for occupational exposure did not account for blacks’ greater lung cancer risk, nor did the authors observe a significant association between occupation and lung cancer risk. B-W differences in occupational exposure to lung carcinogens were small in their sample (11.1 % among blacks vs. 10.2 % among whites) (Haiman et al. 2006).
Overall, our findings are consistent with the previous observations that blacks suffer disproportionately from smoking-related diseases despite lower levels of ever-smoking prevalence and smoking intensities among current smokers. Some have suggested that these black-white differences in smoking-related diseases are due to longer smoking durations and lower smoking cessation rates among blacks (Burns et al. 1997). Our study supports this hypothesis. It also emphasizes the need to consider detailed categorizations of smoking status. Former smokers are a highly heterogeneous group and should be differentiated when estimating the effects of smoking on mortality. Simply comparing levels of ever-smoking prevalence without considering differences in smoking duration, intensity, and cessation can mask differences among subgroups and may not adequately capture the impact of smoking on mortality. We find that PAF estimates based on a three-category smoking variable are lower than estimates based on our more detailed categorization of smoking status (Table 4).
Supplementary Material
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R21HD060175) and the National Science Foundation Graduate Research Fellowship (Grant No. DGE-0822). We would like to thank Neil Mehta, Sam Preston, Hans-Peter Kohler, Mark Hayward, and three anonymous reviewers for comments on earlier drafts of this article.
Footnotes
These sites are the kidney, larynx, liver, lung, myeloid leukemia, nasal cavity and paranasal sinuses, nasopharynx, oro- and hypopharynx, esophagus, oral cavity, pancreas, stomach, ureter, urinary bladder, and uterine cervix (IARC 2004).
Quit rates are calculated from a question asking respondents how long it had been since they last smoked regularly. The authors estimated a survival model using age at cessation as time of event and a base population of all ever-smokers in a given birth cohort/race/gender subgroup. Only respondents who had successfully quit at least two years prior to a survey were counted as having events in order to minimize the inclusion of failed quit attempts.
“Never-smokers” are defined as those who reported smoking less than 100 cigarettes in their lifetimes.
The median value in the open-ended category is estimated using the Pareto curve. The slope of this curve, v, is estimated as [log(nt + nt−1)− log(nt)]/[log(xt)− log(xt−1)], where nt is the number of people in the open-ended (last) income category, nt − 1 is the number of people in the next-to-last income category, xt is the lower bound of the open-ended income category, and xt − 1 is the lower bound of the next-to-last income category. The median value for the open-ended category is then estimated as 10(0.301/v) × xt.
(1.49 − 1.39)/(1.49 − 1.00) = .20.
Contributor Information
Jessica Y. Ho, Email: yjho@sas.upenn.edu, Population Studies Center, University of Pennsylvania, 239 McNeil Building, 3718 Locust Walk, Philadelphia, PA 19104-6298
Irma T. Elo, Population Studies Center, University of Pennsylvania
References
- Allison P. Survival analysis using SAS: A practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- Altman DG, Schooler C, Basil MD. Alcohol and cigarette advertising on billboards. Health Education Research. 1991;6:487–490. [Google Scholar]
- Arias E. National Vital Statistics Reports. 9. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2011. United States life tables, 2007. [PubMed] [Google Scholar]
- Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. Vital and Health Statistics. 148. Vol. 2. Hyattsville, MD: National Center for Health Statistics; 2008. The validity of race and Hispanic origin reporting on death certificates in the United States. [PubMed] [Google Scholar]
- Barbeau EM, Krieger N, Soobader M. Working class matters: Socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. American Journal of Public Health. 2004;94:269–278. doi: 10.2105/ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Caporaso N. Cigarette smoking. Journal of the National Cancer Institute. 1999;91:1365–1375. doi: 10.1093/jnci/91.16.1365. [DOI] [PubMed] [Google Scholar]
- Blaxter M. Health and lifestyles. London, UK: Tavistock; 1990. [Google Scholar]
- Burns DM, Lee L, Shen LZ, Gilpin E, Tolley HD, Vaughn J, Shanks TG. Cigarette smoking behavior in the United States. In: Burns DM, Garfinkel L, Samet J, editors. Smoking and tobacco control monograph no 8. Bethesda, MD: Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health; 1997. pp. 13–112. [Google Scholar]
- Caraballo RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, Maurer KR. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. Journal of the American Medical Association. 1998;280:135–139. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention/National Center for Health Statistics. Life tables. 2010 Retrieved from http://www.cdc.gov/nchs/products/life_tables.htm.
- Centers for Disease Control and Prevention/National Center for Health Statistics. About the National Health Interview Survey. 2012 Retrieved from http://www.cdc.gov/nchs/nhis/about_nhis.htm.
- Chen VW. Smoking and the health gap in minorities. Annals of Epidemiology. 1993;3:159–164. doi: 10.1016/1047-2797(93)90130-v. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Ries LAG. Race and ethnicity. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. Cancer survival among adults: US SEER Program, 1988–2001, patient and tumor characteristics. Bethesda, MD: National Cancer Institute, SEER Program, NIH; 2007. pp. 263–276. [Google Scholar]
- Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. Journal of Health Economics. 2010;29:1–28. doi: 10.1016/j.jhealeco.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJL, Ezzati M. The promise of prevention: The effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Medicine. 2010;7:e1000248. doi: 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLancey JOL, Thun MJ, Jemal A, Ward EM. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- Denney JT, Rogers RG, Hummer RA, Pampel FC. Education inequality in mortality: The age and gender specific mediating effects of cigarette smoking. Social Science Research. 2010;39:662–673. doi: 10.1016/j.ssresearch.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. British Journal of Cancer. 2005;92:426–429. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Ries LAG. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo IT. New African American life tables from 1935–1940 to 1985–1990. Demography. 2001;38:97–114. doi: 10.1353/dem.2001.0002. [DOI] [PubMed] [Google Scholar]
- Fenelon A, Preston SH. Estimating smoking-attributable mortality in the United States. Demography. 2012;49:797–818. doi: 10.1007/s13524-012-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Novotny TE, Pierce JP, Hatziandreu EJ, Patel KM, Davis RM. Trends in cigarette smoking in the United States: The changing influence of gender and race. Journal of the American Medical Association. 1989;261:49–55. [PubMed] [Google Scholar]
- Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: Results from Cancer Prevention Study II. Cancer Research. 2003;63:6556–6562. [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. Journal of the American Medical Association. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Novotny TE. Trends in black/white differences in current smoking among 18- to 24-year-olds in the United States, 1983–1993. American Journal of Preventive Medicine. 1998;14:19–24. doi: 10.1016/s0749-3797(97)00009-3. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, Eriksen MP. Surveillance for selected tobacco-use behaviors—United States, 1900–1994. Morbidity and Mortality Weekly Report. 1994;43(3):1–43. [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, Le Marchand L. Ethnic and racial differences in the smoking-related risk of lung cancer. New England Journal of Medicine. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. Journal of the National Cancer Institute. 1993;85:457–464. doi: 10.1093/jnci/85.6.457. [DOI] [PubMed] [Google Scholar]
- Harper S, Lynch J, Burris S, Smith GD. Trends in the black-white life expectancy gap in the United States, 1983–2003. Journal of the American Medical Association. 2007;297:1224–1232. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65:910–930. [Google Scholar]
- Howard G, Anderson RT, Russell G, Howard VJ, Burke GL. Race, socioeconomic status, and cause-specific mortality. Annals of Epidemiology. 2000;10:214–223. doi: 10.1016/s1047-2797(00)00038-7. [DOI] [PubMed] [Google Scholar]
- Hummer RA, Rogers RG, Nam CB, LeClere FB. Race/ethnicity, nativity, and U.S. adult mortality. Social Science Quarterly. 1999;80:136–153. [Google Scholar]
- Ingram DD, Lochner KA, Cox CS. Vital and Health Statistics. 147. Vol. 2. Hyattsville, MD: National Center for Health Statistics; 2008. Mortality experience of the 1986–2000 National Health Interview Survey linked mortality files participants. [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: International Agency for Research on Cancer; 2004. Tobacco smoke and involuntary smoking. [PMC free article] [PubMed] [Google Scholar]
- Kiefe CI, Williams OD, Lewis CE, Allison JJ, Sekar P, Wagenknecht LE. Ten-year changes in smoking among young adults: Are racial differences explained by socioeconomic factors in the CARDIA study? American Journal of Public Health. 2001;91:213–218. doi: 10.2105/ajph.91.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. The “race” concept in smoking: A review of the research on African Americans. Social Science & Medicine. 1997;45:1075–1087. doi: 10.1016/s0277-9536(97)00035-x. [DOI] [PubMed] [Google Scholar]
- King G, Polednak A, Bendel RB, Vilsaint MC, Nahata SB. Disparities in smoking cessation between African Americans and Whites: 1990–2000. American Journal of Public Health. 2004;94:1965–1971. doi: 10.2105/ajph.94.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD. Foreword. In: Burns DM, Garfinkel L, Samet J, editors. Smoking and tobacco control monograph no 8. Bethesda, MD: Cancer Control and Population Sciences, National Cancer Institute, U.S. National Institutes of Health; 1997. pp. iii–v. [Google Scholar]
- Landrine H, Klonoff EA, Fernandez S, Hickman N, Kashima K, Parekh B, Jensen JA. Cigarette advertising in black, Latino, and white magazines, 1998–2002: An exploratory investigation. Ethnicity & Disease. 2005;15:63–67. [PubMed] [Google Scholar]
- Lawlor DA, Frankel S, Shaw M, Ebrahim S, Smith GD. Smoking and ill health: Does lay epidemiology explain the failure of smoking cessation programs among deprived populations? American Journal of Public Health. 2003;93:266–270. doi: 10.2105/ajph.93.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;35:80–94. [PubMed] [Google Scholar]
- Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: Modeling total exposure and intensity. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- Lutfey K, Freese J. Toward some fundamentals of fundamental causality: Socioeconomic status and health in the routine clinic visit for diabetes. American Journal of Sociology. 2005;110:1326–1372. [Google Scholar]
- Massey DS, Denton N. American apartheid: Segregation and the making of the underclass. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Mehta NK, Chang VW. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46:851–872. doi: 10.1353/dem.0.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Preston S. Continued increases in the relative risk of death from smoking. American Journal of Public Health. 2012 doi: 10.2105/AJPH.2011.300489. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Population Center and State Health Access Data Assistance Center. Integrated Health Interview Series: Version 3.0. Minneapolis: University of Minnesota; 2010. Retrieved from http://www.ihis.us. [Google Scholar]
- Murray RP, Connett JE, Buist AS, Gerald LB, Eichenhorn MS. Experience of black participants in the Lung Health Study smoking cessation intervention program. Nicotine & Tobacco Research. 2001;3:375–382. doi: 10.1080/14622200110081435. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (NCHS) Vital statistics of the United States, 1993. Hyattsville, MD: NCHS; 1997. Preprint of Vol. II mortality, Part A, Sec. 6, life tables. [Google Scholar]
- National Center for Health Statistics (NCHS) Mortality tables. Hyattsville, MD: NCHS; 2010a. Retrieved from http://www.cdc.gov/nchs/nvss/mortality_tables.htm. [Google Scholar]
- National Center for Health Statistics (NCHS) Health, United States, 2009: With special feature on medical technology. Hyattsville, MD: NCHS; 2010b. [PubMed] [Google Scholar]
- Novotny TE, Warner KE, Kendrick JS, Remington PL. Smoking by blacks and whites: Socioeconomic and demographic differences. American Journal of Public Health. 1988;78:1187–1189. doi: 10.2105/ajph.78.9.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noymer A, Penner AM, Saperstein A. Cause of death affects racial classification on death certificates. PLoS ONE. 2011;6(1):e15812. doi: 10.1371/journal.pone.0015812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi M, Winant H. Racial formation in the United States: from the 1960s to the 1990s. New York: Routledge; 1994. [Google Scholar]
- Oza S, Thun MJ, Henley SJ, Lopez AD, Ezzati M. How many deaths are attributable to smoking in the United States? Comparison of methods for estimating smoking-attributable mortality when smoking prevalence changes. Preventive Medicine. 2011;52:428–433. doi: 10.1016/j.ypmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annual Review of Sociology. 2010;36:349–370. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampel FC, Rogers RG. Socioeconomic status, smoking, and health: A test of competing theories of cumulative advantage. Journal of Health and Social Behavior. 2004;45:306–321. doi: 10.1177/002214650404500305. [DOI] [PubMed] [Google Scholar]
- Parker RN, Fenwick R. The Pareto curve and its utility for open-ended income distributions in survey research. Social Forces. 1983;61:873–885. [Google Scholar]
- Percy CL, Miller BA, Ries LAG. Effect of changes in cancer classification and the accuracy of cancer death certificates on trends in cancer mortality. Annals of the New York Academy of Sciences. 1990;609:87–97. doi: 10.1111/j.1749-6632.1990.tb32059.x. [DOI] [PubMed] [Google Scholar]
- Percy C, Stanek E, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. American Journal of Public Health. 1981;71:242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Stable EJ, Herrera B, Jacob P, Benowitz NL. Nicotine metabolism and intake in black and white smokers. Journal of the American Medical Association. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Peto R, Darby S, Deo H, Silcock P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C. Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- Preston SH, Elo IT. Black mortality at very old ages in official U.S. life tables: A skeptical appraisal. Population and Development Review. 2006;32:557–565. [Google Scholar]
- Preston SH, Glei D, Wilmoth J. A new method for estimating smoking-attributable mortality in high-income countries. International Journal of Epidemiology. 2010;39:430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Glei D, Wilmoth J. Contribution of smoking to international differences in life expectancy. In: Crimmins E, Preston SH, Cohen B, editors. International differences in mortality at older ages: Dimensions and sources. Washington, DC: National Academy Press; 2011. pp. 105–131. [Google Scholar]
- Preston SH, Heuveline P, Guillot M. Demography: Measuring and modeling population processes. Oxford, UK: Blackwell Publishers; 2001. [Google Scholar]
- Preston SH, Wang H. Sex mortality differences in the United States: The role of cohort smoking patterns. Demography. 2006;43:631–646. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. American Journal of Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality attributable to cigarette smoking in the United States. Population and Development Review. 2005;31:259–292. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM. Pharmacogenetics and ethnoracial differences in smoking. Journal of the American Medical Association. 1998;280:179–180. doi: 10.1001/jama.280.2.179. [DOI] [PubMed] [Google Scholar]
- Siahpush M, Singh GK, Jones PR, Timsina LR. Racial/ethnic and socioeconomic variations in duration of smoking: Results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. Journal of Public Health. 2010;32:210–218. doi: 10.1093/pubmed/fdp104. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- Smelser NJ, Wilson WJ, Michell F, editors. America becoming: Racial trends and their consequences. Washington, DC: National Academies Press; 2001. [Google Scholar]
- Surveillance Epidemiology and End Results (SEER) Fast Stats: An interactive tool for access to SEER cancer statistics. Washington, DC: SEER, National Cancer Institute; 2010. Retrieved from http://seer.cancer.gov/faststats. [Google Scholar]
- U.S. Department of Health and Human Services (U.S. DHHS) DHHS Pub No (CDC) 89-8411. Atlanta, GA: DHHS, Public Health Service, Centers for Disease Control and Prevention, and National Center for Chronic Disease; 1989. Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General. [Google Scholar]
- U.S. Department of Health and Human Services (U.S. DHHS) DHHS Publication No (CDC) 90-8416. Washington, DC: U.S. DHHS, Public Health Service, Centers for Disease Control and Prevention, Center for Chronic Disease Prevention and Health Promotion, and Office on Smoking and Health; 1990. The health benefits of smoking cessation. [Google Scholar]
- U.S. Department of Health and Human Services (U.S. DHHS) Tobacco use among US racial/ethnic minority groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A report of the Surgeon General. Atlanta, GA: U.S. DHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, and Office on Smoking and Health; 1998. [Google Scholar]
- U.S. Department of Health and Human Services (U.S. DHHS) Healthy people 2010: Understanding and improving health. 2. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- U.S. Department of Health and Human Services (U.S. DHHS) Women and smoking: A report of the Surgeon General. Rockville, MD: U.S. DHHS, Public Health Service, and Office of the Surgeon General; 2001. [Google Scholar]
- Williams DR, Collins C. U.S. socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology. 1995;21:349–386. [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MD, Ettner SL, Boscardin WJ, Shapiro MF. The contribution of cancer incidence, stage at diagnosis and survival to racial differences in years of life expectancy. Journal of General Internal Medicine. 2009;24:475–481. doi: 10.1007/s11606-009-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Robert SA. The contributions of race, individual socioeconomic status, and neighborhood socioeconomic context on the self-rated health trajectories and mortality of older adults. Research on Aging. 2008;30:251–273. [Google Scholar]
- Zuberi T. Thicker than blood: How racial statistics lie. Minneapolis: University of Minnesota Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.