Abstract
Background
A single study shows that contraceptive vaginal ring (CVR) use for up to 35 days in women with a normal BMI maintains serum hormone levels sufficient to suppress ovulation. This study is intended to confirm those results and to evaluate prolonged CVR use up to 42 days in both normal BMI and obese women.
Study Design
Twenty women with a normal BMI and 20 obese women enrolled in a prospective open label clinical study of ethinyl estradiol (EE) and etonogestrel (ENG) pharmacokinetics during six weeks of use of a single CVR. Participants underwent twice weekly evaluations to determine serum hormone concentrations, ovarian follicle development, endometrial thickness and bleeding patterns.
Results
Thirty-seven women completed follow-up including eighteen women with a normal BMI and nineteen obese women. EE and ENG concentrations remained in therapeutic range for all women. Follicular development and endometrial proliferation were minimal. By the sixth week, 30% of participants reported spotting or bleeding.
Conclusions
A single CVR used for six weeks demonstrates therapeutic serum levels of EE and ENG among women with normal and obese BMI. Women who forget to remove the CVR at day 21 may well have continued contraceptive protection during the next three weeks.
Keywords: contraceptive vaginal ring, pharmacokinetics, pharmacodynamics, obesity
1. Introduction
The combined hormonal contraceptive vaginal ring (CVR), NuvaRing® (Merck & Co., Inc., Whitehouse, NJ), has been available since 2001. The CVR is prescribed for one cycle of use where the ring is inserted vaginally for three weeks continuously, followed by a week-long ring-free interval; however, package labeling suggests that no backup form of contraception is needed if the ring is left in for an additional seven days beyond day 21. This contraceptive device releases an average of 15 mcg ethinyl estradiol (EE) and 120 mcg of etonogestrel (ENG) each day from a flexible, transparent ring that is 54 mm in diameter and 4 mm in thickness. The primary mechanism of CVR action is inhibition of ovulation [1].
Incorrect and/or inconsistent contraceptive use accounts for just over 40% of all unintended pregnancies each year in the United States. [2] In clinical trials, CVR users demonstrated 80–90% adherence to the contraceptive regimen [3,4]. Women who deviate from the standard regimen report delays in re-insertion after a temporary removal, prolonging the 7-day ring-free interval, as well as forgetting to remove the ring as scheduled, thus prolonging use beyond three weeks [5]. Deviations from standard contraceptive use may increase a woman’s risk for pregnancy. Among 16 normal weight (BMI <27 kg/m2) CVR users, EE and ENG serum concentrations remained sufficient to suppress ovarian function during five weeks of continuous use; thus, women who forget to remove the ring up to two weeks after the usual three-week use period may not be at greater risk for unintended pregnancy during that interval [6].
As more than 50% of women in the United States are overweight or obese, we sought to study extended CVR use in a more representative sample of women [7]. We have previously shown that ENG pharmacokinetics are similar among obese and normal BMI women during 21 days of CVR use [8]. Our goal in this analysis was to evaluate serum concentrations of EE and ENG and markers of ovarian suppression among women with normal and obese BMI during a six-week extended cycle of CVR use. We also evaluated the use of a single CVR for 12 weeks in a subset of four participants.
2. Materials and methods
The Columbia University Institutional Review Board approved the investigation, and all participants provided written informed consent prior to enrolling in this open label clinical trial. Participant-related activities took place between July and December 2008. Women aged 18–35 years who had completed a similar study of oral contraceptives, and who had a measured BMI of 19 to 24.9 kg/m2 (normal) or 30.0 to 39.9 kg/m2 (obese) were eligible for participation in the CVR study. [9] Volunteers who reported regular, spontaneous menses underwent a baseline transvaginal ultrasound evaluation; only those with normal appearing ovaries were invited to enroll. We excluded women with any medical contraindications to the use of combined hormonal contraception, and women using medications known to affect the CYPp450 system [10].
Participants received two CVRs upon enrollment. All participants used the first ring for a standard 28-day cycle (21 days of continuous use, followed by a 7-day ring-free interval). Insertion of the second ring began the study cycle. During the study cycle, participants used the second ring for six continuous weeks and underwent twice weekly visits for collection of blood to measure serum hormone levels, a vaginal examination to confirm CVR use, and ultrasound to assess ovarian follicle development and endometrial proliferation.
Serum samples to measure EE and ENG were collected approximately every three days ± one day throughout the study cycle. Specimens were allowed to clot for at least 10 min at room temperature, and then separated at 3400 rpm by centrifuge. The serum was stored in aliquots at −80 °C until analysis. We report EE and ENG concentrations from serum specimens using liquid chromatography tandem mass spectrometry (UPLC-MS/MS) by the Biomarkers Core Laboratory of the Irving Institute of Clinical and Translational Research at Columbia University Medical Center. [8]
We present the average serum levels of EE and ENG levels for weeks 1–6. We did not assess the serum levels immediately prior to ring insertion, and assume the day 0 levels were 0 for both hormones. Since participants did not undergo blood draws on exactly the same cycle days, we interpolated levels for days 0–42 for each woman, and used these values to calculate daily serum concentrations for the groups. Calculations regarding hormone levels were carried out on a log scale; all resulting values were exponentiated for ease of interpretation. We calculated the slope describing the rate of change of mean serum EE and ENG levels from day 14 through day 42 using these data.
A subset of four participants used a third ring for 12 consecutive weeks after a washout period of several months. These women underwent venipuncture at the end of week 3, and then weekly at the end of weeks 6 through 12 to measure EE and ENG concentrations.
Transvaginal ultrasound with a 7.5 mHz probe (TITAN, Sonosite, Inc., Bothell, WA) was performed twice weekly to assess ovarian follicle-like structures. We measured follicles in two perpendicular diameters (FD) and recorded the dimensions of follicles with a mean diameter of at least 8 mm; we also measured anterior-posterior endometrial thickness during each sonogram evaluation. We assessed serum progesterone levels for all participants who had any ovarian follicle with a diameter of 13 mm or greater. The Core Laboratory of the Irving Institute of Clinical and Translational Research at CUMC performed progesterone assays using the RIA kit Coat-A-Count Progesterone (Siemens Medical Solution Diagnostics, Malvern, PA).
Participants used paper diaries to record daily bleeding and spotting during the study, which were verified at each follow-up visit. The loss of blood requiring the use of a sanitary pad or tampon was noted as “bleeding” and the loss of blood requiring a panty liner or no protection as “spotting.”
Throughout the study, sexually active participants received latex condoms to use during any sexual acts after day 21 of the study cycle.
We evaluated baseline characteristics with frequency distributions, chi-squared, and Fisher’s Exact tests of association to evaluate for differences between BMI groups. We calculated the geometric mean serum EE and ENG levels, the proportion of participants that achieved a maximum FD greater than 8mm, the mean endometrial thickness, and the total number of bleeding and spotting days throughout the study cycle. We used two-sided t-tests to evaluate for differences of these variables between BMI groups. Slopes for the time-concentration curves of EE and ENG were calculated from individual regression lines, and we employed the Wilcoxon rank sum test to compare these slopes between BMI groups. Finally, we performed a 2-way ANOVA to evaluate an association between endometrial thickness and number of bleeding/spotting days.
A final sample size of 17 participants in each group was planned a priori in order to have 80% power to identify a one standard deviation difference in the mean serum levels of the contraceptive hormones, based on expected values for normal weight women [6].
3. Results
Forty women enrolled in the study and 37 women completed follow-up while using a single CVR for six continuous weeks. Of the 444 visits scheduled during the study cycle, women completed 435 visits or 98% of follow-up. Four of these women (two normal and two obese BMI) used a third ring for 12 continuous weeks.
Study Participants included 18 normal weight women with a mean BMI of 21.5 ± SD 1.3 and 19 obese women with a mean BMI of 34.3 ± SD 3.0. Most participants identified as Hispanic (38%) or non-Hispanic African American (32%), had never been pregnant (51%) and were non-smokers (86%). The mean age of women in this study was 26.6 ± SD 4.5. Participants differed with regard to BMI by design; there were no other differences in baseline characteristics noted among normal weight and obese women in this study [9].
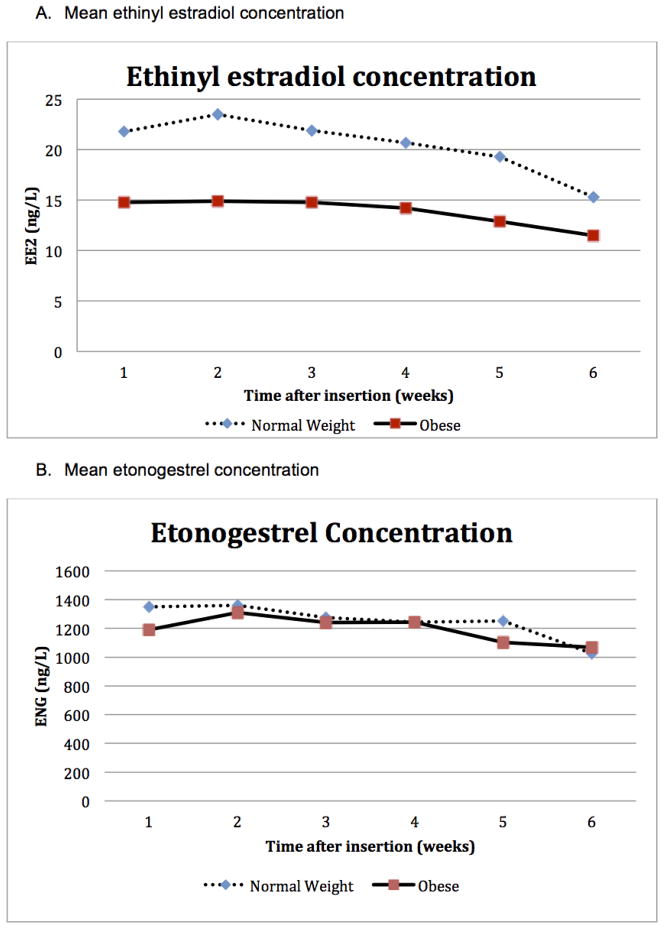
Table 1 shows the mean EE and ENG levels during six weeks of continuous CVR use. The average concentration OF EE was 15.3 ng/L for normal BMI women and 11.5 ng/L for obese women (p = 0.10) at the end of week 6. The average concentration of ENG was 1026 ng/L for normal BMI women and 1068 ng/L for obese women (p = 0.70), also at the end of week (Table 1).
Table 1.
Follicular development and mean EE and ENG levels by week
| Week | Follicular Diameter ≥ 8mm n(%) | Ethinyl estradiol (ng/L) Mean (± standard deviation1) |
Etonogestrel (ng/L) Mean (± standard deviation) |
||||
|---|---|---|---|---|---|---|---|
| Normal (n=18) | Obese (n=19) | p | Normal (n=18) | Obese (n=19) | p | ||
|
| |||||||
| 1 | 11 (30) | 21.8 (13.9, 34.0) | 14.8 (10.2, 21.4) | 0.012 | 1349 (1026, 1773) | 1190 (752, 1884) | 0.74 |
|
| |||||||
| 2 | 5 (14) | 23.5 (16.2, 34.1) | 14.9 (10.0, 22.1) | 0.002 | 1360 (1046, 1768) | 1311 (899, 1912) | 0.82 |
|
| |||||||
| 3 | 4 (11) | 21.9 (14.6, 32.8) | 14.8 (10.5, 20.9) | 0.007 | 1275 (971, 1674) | 1240 (841, 1830) | 0.97 |
|
| |||||||
| 4 | 4 (11) | 20.7 (13.6, 31.5) | 14.2 (10.2, 19.6) | 0.019 | 1243 (905, 1707) | 1244 (871, 1778) | 0.97 |
|
| |||||||
| 5 | 3 (8) | 19.1 (12.5, 29.2) | 13.3 (9.6, 18.4) | 0.016 | 1252 (956, 1638) | 1107 (824, 1486) | 0.52 |
|
| |||||||
| 6 | 1 (3) | 16.2 (10.1, 25.8) | 12.5 (8.1, 19.5) | 0.088 | 1063 (730, 1548) | 1096 (730, 1646) | 0.82 |
All EE and ENG values represent the levels on the last day of each week. These standard deviations are not symmetrical because all calculations were done using log transformed values. However, the standard deviations above are presented in the original units for ease of interpretation.
Fig. 1 pictures the concentration curves for EE and ENG during the study cycle. Mean serum concentrations of EE were lower among obese CVR users than normal BMI users throughout the study cycle; whereas, mean ENG levels showed little difference between the two groups over time. Despite lower mean EE levels among obese participants, there were minimal differences in the decline of mean serum EE concentration over time (−0.24 ng/L/day (7.4% decline/week) for normal BMI and −0.11 ng/L/day (4.8% decline per week) for obese women. Mean serum concentrations of ENG were similar among normal weight and obese women throughout the study cycle with a similar decline over time (−6.8 ng/L/day (3.9% decline/week) for normal BMI and −10.0 ng/L/day (5.1% decline/week) for obese women.
Fig. 1.
There was minimal follicular development noted during six weeks of continuous CVR use among both normal BMI and obese women. The proportion of women with maximum follicular diameters greater than or equal to 8 mm did not increase over time or differ between groups. The largest follicles were seen within the first week of the second cycle (Table 1). Only nine women were noted to have any follicles measuring >13 mm during the study cycle; these large follicles were noted in the first weeks of use. No woman with follicular development >13 mm ovulated based on all measured progesterone levels ≤1.0 ng/L. Even among women with the lowest ENG levels at six weeks (320 ng/L normal BMI and 482 ng/L obese, respectively), no follicular development was noted at the end of the study cycle.
Five women (3 normal BMI and 2 obese) reported 9 ring removals lasting from 15 min up to 3 h in a 24-h hour period. These women were not noted to have enlarged follicles ≥ 8 mm during the weeks when these unscheduled removals occurred.
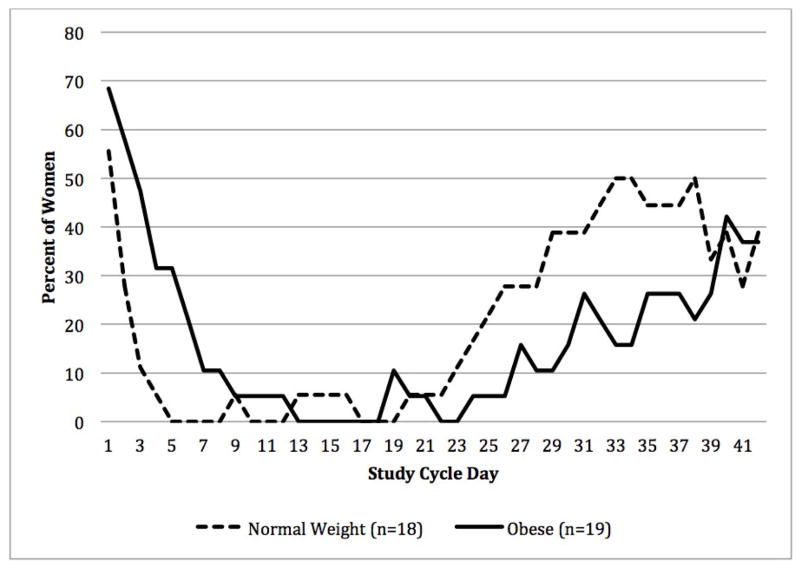
Fig. 2 illustrates the percent of women who reported bleeding or spotting in their diaries for each day of the study cycle. Obese women reported bleeding or spotting at the start of the six week extended CVR cycle more than women with a normal BMI. In contrast, more normal BMI women reported bleeding or spotting at the end of six weeks. Overall, there was no difference in the total number of bleeding or spotting days reported by the two groups (normal BMI: 8.7 ± SD 7.3 days, obese: 7.7 ± SD 6.0 days p= 0.64), and approximately 30% of all women reported bleeding or spotting at the end of six weeks.
Fig. 2.
Percent of women with bleeding or spotting
We found similar mean endometrial thickness IN normal BMI and obese women during three weeks of use (3.9 ± SD 1.6 mm, 4.4 ± SD 1.8 mm, respectively, p = 0.36) and at the end of continuous six week use (3.9 ± SD 1.3 mm, 4.7 ± SD 1.8 mm, respectively, p = 0.14). There was no difference in endometrial thickness among women who reported bleeding and spotting versus those who did not in both GROUPS (ANOVA, p=0.3).
At the end of six weeks of continuous CVR use, no serum ENG concentration was below 200 ng/L. The four women continuing CVR use for 12 weeks had serum ENG concentrations from 342 ng/L to 755 ng/L during the last week of use.
4. Discussion
These results are consistent with a previous report regarding 16 normal weight women using the CVR continuously for 35 days [6]. Our normal BMI CVR users demonstrated similar mean serum concentrations of EE and ENG and gradually decreasing mean serum levels over time. Ovarian suppression with minimal follicle development and endometrial proliferation along with the absence of ovulation was consistent between studies; these findings support the notion of steady hormone levels during extended use of the CVR. [6,11]
While obese women have lower mean EE levels throughout 42 days, they have similar mean ENG levels to women with normal BMI. Obese women also have the same lack of new follicle development as normal BMI women. A very gradual decline in serum EE and ENG levels was observed among both normal BMI and obese participants from day 14 to day 42 of ring use. Exploratory data from just four women with 12 week use supports the concept of a gradual and steady decrease in hormone levels.
Steady state mean concentrations for the ENG contraceptive implant in the first year following insertion have been reported to be 200 ng/L (range 150–260 ng/L) with only a slight decline to 156 ng/L (range 150–260 ng/L) at the end of the third year of use [12]. Studies demonstrate predictable ovarian suppression in 95% of women during this approved interval of implant use [13,14]. Our findings of robust ovarian suppression among continuous six week CVR users at ENG levels three times those among implant users may not be surprising. In addition, the four women who used the contraceptive ring continuously for 12 weeks had mean serum levels similar to the steady state concentration of ENG found at three years of contraceptive implant use. This suggests the possibility that contraceptive protection may extend beyond six weeks use of a single CVR.
The experience of unscheduled bleeding and spotting episodes is often a reason for discontinuation of hormonal contraception, particularly in association with extended use regimens [15,16] Assessment of continuous NuvaRing® use (back–to-back 3 week regimens of serial rings) has shown good cycle control; however, longer durations of continuous ring use precipitate more unscheduled bleeding and spotting days for some women [15]. In our study, minimal bleeding or spotting occurred up to day 21; bleeding or spotting that was reported during this interval most commonly occurred during the first week of the study cycle, and reflects the end of the previous episode of scheduled withdrawal bleeding. In the second three weeks of continuous CVR use, more women, especially normal weight women, experienced bleeding or spotting.
All of these results, taken together, suggest that inadvertent ring continuation past day 21 through day 42 may be associated with continued contraceptive protection in both normal BMI and obese women. The experience of breakthrough bleeding, however, may be a marker that a woman has inadvertently continued CVR use past 21 days and prompt appropriate initiation of a new contraceptive ring.
Acknowledgments
FINANCIAL SUPPORT FOR THE STUDY
NIH Grant R01 HD04578
NIH CTSA Grant UL1RR024156
Anonymous Foundation
Merck donation of Nuvarings and Laboratory assays
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nuvaring® [package insert] Roseland, NJ: Organon, a subsidiary of Merck & Co., Inc; 2001. p. 2010. [Google Scholar]
- 2.Frost JJ, Darroch JE, Remez L. In Brief. Vol. 1. New York: Guttmacher Institute; 2008. Improving contraceptive use in the United States. [PubMed] [Google Scholar]
- 3.Ahrendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 mcg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451–7. doi: 10.1016/j.contraception.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Oddsson K, Leifels-Fischer B, de Melo N, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176–82. doi: 10.1016/j.contraception.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Roumen F, op ten Berg M, Hoomans E. The combined contraceptive vaginal ring (NuvaRing): first experience in daily clinical practice in The Netherlands. Eur J Contracept Reprod Health Care. 2006;11:14–22. doi: 10.1080/13625180500389547. [DOI] [PubMed] [Google Scholar]
- 6.Timmer C, Mulders T. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233–42. doi: 10.2165/00003088-200039030-00005. [DOI] [PubMed] [Google Scholar]
- 7.McDowell MAFC, Ogden CL, Flegal KM. National health statistics reports. Vol. 10. Hyattsville, MD: National Center for Health Statistics; 2008. Anthropometric reference data for children and adults: United States, 2003–2006. [PubMed] [Google Scholar]
- 8.Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal weight and obese women. Amer J Obstet Gynecol. 2012;207:39–45. doi: 10.1016/j.ajog.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhoff CL, Torgal AH, Mayeda ER, et al. Ovarian suppression in normal weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116:275–283. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Department of Reproductive Health and Research. Medical eligibility criteria for contraceptive use. 4. World Health Organization; Geneva: 2010. [Google Scholar]
- 11.Mulders T, Dieben T. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865–70. doi: 10.1016/s0015-0282(01)01689-2. [DOI] [PubMed] [Google Scholar]
- 12.Coelingh Bennink HJ. The pharmacokinetics and pharmacodynamics of Implanon®, a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5 (Suppl 2):12–20. [PubMed] [Google Scholar]
- 13.Olsson SE, Odlind V, Johanssen E. Clinical results with subcutaneous implants containing 3-ketodesogestrel. Contraception. 1990;42:1–11. doi: 10.1016/0010-7824(90)90087-c. [DOI] [PubMed] [Google Scholar]
- 14.Diaz S, Pavez M, Moo-Young AJ, et al. Clinical trial with 3-keto-desogestrel subdermal implants. Contraception. 1991;44:393–408. doi: 10.1016/0010-7824(91)90030-j. [DOI] [PubMed] [Google Scholar]
- 15.Miller LMD, Verhoeven CHJP, Hout JitMS. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol. 2005;106:473–82. doi: 10.1097/01.AOG.0000175144.08035.74. [DOI] [PubMed] [Google Scholar]
- 16.Barrieros FA, Guazzelli CAF, Barbosa R, de Assis F, Fernando de Araujo F. Extended regimens of the CVR- evaluation of clinical aspects. Contraception. 2010;81:223–25. doi: 10.1016/j.contraception.2009.10.007. [DOI] [PubMed] [Google Scholar]