Abstract
Background
Local tissue injury from sustained release formulations for local anesthetics can be severe. There is considerable variability in reporting of that injury. We investigated the influence of the intrinsic myotoxicity of the encapsulated local anesthetic (lidocaine, low; bupivacaine, high) on tissue reaction in rats.
Methods
Cytotoxicity from a range of lidocaine and bupivacaine concentrations was measured in C2C12 myotubes over 6 days. Rats were given sciatic nerve blocks with 4 microparticulate formulations of lidocaine and bupivacaine: 10% (w/w) lidocaine poly-lactic-co-glycolic acid (PLGA), 10% (w/w) bupivacaine PLGA, 50% (w/w) lidocaine PLGA, and 50% (w/w) bupivacaine PLGA. Effectiveness of nerve blockade was assessed by a modified hotplate test and weight-bearing measurements. Myotoxicity was scored in histologic sections of injection sites. Bupivacaine and lidocaine release kinetics from the particles were measured.
Results
Median sensory blockade duration for 50% (w/w) lidocaine was 255 (90–540) min versus 840 (277–1215) min for 50% (w/w) bupivacaine (P=0.056). All microparticulate formulations resulted in myotoxicity. The choice of local anesthetic did not influence the severity of myotoxicity. Median myotoxicity scores for 50% (w/w) lidocaine compared to 50% (w/w) bupivacaine at 4 days was 3.4 (2.1–4.2) vs. 3.3 (2.9–3.5)(P=0.44) and at 14 days 1.9 (1.8–2.4) versus 1.7 (1.3–1.9)(P=0.23) respictively.
Conclusions
Lidocaine and bupivacaine PLGA microspheres resulted in similar degrees of myotoxicity, irrespective of drug loading. Intrinsic myotoxicity did not predict tissue injury from sustained release of these anesthetics. Caution is warranted in the use of such devices near muscle and nerve.
Introduction
A very broad range of controlled release formulations have been developed to provide prolonged duration local anesthesia, including polymeric microspheres,1–7 surgically implantable pellets,8 microcrystals,9 liposomes,10–15 (including a formulation undergoing human clinical trials16,17) lipospheres,18 cross-linkable hyaluronic acid matrices,19 lipid-protein-sugar particles,20,21 cyclodextrin complexes,22,23 liposomes loaded with cyclodextrin complexes24 and implantable membrane matrices.25,26 Such systems have extended the duration of nerve block to varying degrees ranging from hours to weeks, but have not been widely adopted clinically. A major limitation has been adverse tissue reaction, which has included inflammation, myotoxicity and neurotoxicity. All three are well-recognized sequelae of amino-amide and amino-ester local anesthetics.27–32 The degree of toxicity is agent specific.28,30 In a comparison of multiple drugs in rats, single injections of lidocaine produced milder muscle damage than bupivacaine.30 Local anesthetic myotoxicity from single injections has not generated much clinical concern, although the consequences from multiple large doses33,34 or from continuous infusions can be severe and are related to the duration of exposure.35,36 The presence of particles themselves enhances local anesthetic myotoxicity in vivo6 and can cause inflammatory responses at the nerve that may considerably outlast the duration of blockade.6,21,37 Conventional local anesthetics are also neurotoxic, but generally at higher concentrations than those that cause myotoxicity and inflammation.32,38–40
Reporting of local tissue injury from local anesthetic controlled release formulations has been variable, in both animal and human studies. Most do not describe myotoxicity2–5,7–9,11–15,18,22–24 (including the liposomal formulation undergoing human trials16,17), while some others document mild muscle injury comparable to single injections of unencapsulated drug.10,25,26 In our own work, we have found muscle injury to be a ubiquitous finding in a wide range of extended-release bupivacaine formulations independent of the delivery vehicle6,19,21,41 or co-encapsulated agent,32,37,42,43 and it is sometimes severe. That tissue injury can be a crucial issue with sustained release formulations is seen in the example of a sustained-release bupivacaine-dexamethasone formulation;3 nerve and muscle injury in preclinical animal studies and clinical human trials led to withdrawal of its Investigational New Drug application (IND#53,441).44
There are many possible reasons for the variability in reporting of local anesthetic myotoxicity. There is marked heterogeneity in the literature of delivery systems, drug selection, experimental designs, and histopathologic descriptions. Furthermore, the particular drug encapsulated varies; as noted above, that could have an effect on local tissue reaction.
Here we sought to determine whether tissue reaction to a particular formulation is dependent on the specific drug that is encapsulated, by encapsulating a local anesthetic with either high (bupivacaine) or low (lidocaine) potency and myotoxic potential. Such differential toxicity might explain why some lidocaine-releasing formulations have been reported to cause relatively minimal toxicity (comparable to free drug),25 which has not been our experience with bupivacaine-containing formulations. We also examined whether drug loading affects tissue reaction. We selected microspheres composed of high molecular weight poly (lactic-co-glycolic) acid (PLGA), a biodegradable polymer widely used in drug delivery and other applications. Blank PLGA particles produce minimal toxicity in cultured C2C12 myotubes and when injected at the sciatic nerve.6
Methods
Materials
Bupivacaine hydrochloride, lidocaine free base, Tween 80 and sodium carboxymethyl cellulose were purchased from Sigma-Aldrich (St. Louis, MO), poly(lactic-co-glycolic) acid (lactide/glycolide = 65:35, MW 110) (PLGA110) from Medisorb (Alkermes; Cambridge, MA), and poly(vinyl alcohol) (88% hydrolyzed, MW 20,000) from Polysciences, Inc. (Warrington, PA). Bupivacaine hydrochloride was made into the free base by alkaline precipitation and filtration.
Microparticle preparation and characterization
Microspheres with 10% (w/w) and 50% (w/w) drug loading were prepared using the single emulsion method.4,20 Drug free base (50 mg bupivacaine or 175 mg lidocaine for 10% loaded particles; 160 mg bupivacaine or 2 g lidocaine for 50% loaded particles) and PLGA (100 mg for all formulations except 50% lidocaine, which required 600 mg) were dissolved in methylene chloride (3 mL for 50% loaded lidocaine particles; 1.26 mL for all others), and the mixture homogenized (Silverson L4RT-A; Longmeadow, MA) in 50 mL 0.5% polyvinyl alcohol in 100 mM Trizma buffer pH 10.5 for one minute. Lidocaine microparticles required more free base in the starting materials than bupivacaine, owing to the lower octanol:buffer partition coefficient of lidocaine. The resulting suspension was decanted into 100 mL 0.05% polyvinyl alcohol in 100 mM Trizma pH 10.5 and stirred to evaporate the methylene chloride. Microspheres 20 µm to 106 µm in diameter were isolated by wet sieving then resuspended in 50 ml of water. The suspension was washed three times by centrifugation at 4000 rpm for 5 minutes.
Particle size was determined with a Coulter multisizer (Coulter Electronics Ltd., Luton, United Kingdon). Drug loading was determined by dissolving 10 mg of microspheres in 1 mL of methylene chloride, and comparing the resulting UV absorbance (272 nm for bupivacaine, 265 nm for lidocaine) to a standard curve. Blank PLGA microspheres showed negligible absorbance at 272 nm and 265 nm.
The surface morphology of 50% loaded particles was examined using a JEOL Model 6320 FV field emission scanning electron microscope (provided by the Massachusetts Institute of Technology Department of Materials Science and Engineering Electron Microscopy Center). Particles were mounted onto stubs and coated with a layer of gold/palladium. Samples were scanned at a voltage of 5kV at a probe current setting of 3 and working at a distance of 7 millimeters.
In Vitro Release of Bupivacaine and Lidocaine from Microparticles
Fifty milligrams of PLGA microspheres were suspended in 1 mL phosphate-buffered saline, pH 7.4, at 37°C and inserted into the lumen of a Spectra/Por 1.1 Biotech Dispodialyzer (Spectrum Laboratories, Ranchodominguez, CA) with an 8,000 molecular weight cutoff. The dialysis bag was placed into 20 mL phosphate-buffered saline, pH 7.4, and incubated at 37°C with continuous stirring. At predetermined time intervals, the dialysis bag was transferred to a test tube with fresh phosphate-buffered saline. The bupivacaine and lidocaine concentrations in the dialysate were quantified by spectrophotometric means.
Cell Culture
All cell culture supplies were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted. C2C12 mouse myoblasts (American Type Culture Collection CRL-1772, Manassas, VA) were cultured to proliferate in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum and 1% penicillin streptomycin. Cells were then plated in 96 well tissue culture plates with 50,000 cells/mL/well in DMEM, supplemented with 2% horse serum and 1% penicillin-streptomycin, and allowed to differentiate into myotubes for 10–14 days. During differentiation the media were exchanged every 2–3 days. Cells were maintained at 37°C in 5% CO2 with the remainder being ambient air. The hydrochloride salt of bupivacaine or lidocaine was added to DMEM at a concentration of 0.125% (w/v) and serially diluted to prepare the remaining concentrations.
Cell Viability Assay
To quantitatively assess cell viability after adding drug-containing media, a colormetric assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium [MTS] kit, cat. no. G358A; Promega Biosciences, CA, USA) was performed at predetermined time points. In live cells, the yellow tetrazolium salt is metabolized to purple formazen crystals and the color then quantified by spectrophotometric means. Absorbance of each well was measured at 485 nm using a Synergy Mx multi-mode microplate reader (BioTek Instruments, Inc., Winooski, VT).
Animal Care
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 310–420 g were housed in groups, in a 6 am–6 pm light-dark cycle. Animals were cared for in accordance with protocols approved by the Animal Care and Use Committee at the Massachusetts Institute of Technology (Cambridge, Massachusetts), and the Guide for the Care and Use of Laboratory Animals of the United States National Research Council.
For all neurobehavioral and histologic studies, the observer was blinded to the type of particle injected.
Sciatic Blockade Technique
Nerve block injections were performed with a 20-gauge needle under brief isofluorane-oxygen anesthesia as described.20 In brief, each rat was injected with 75 mg of microspheres suspended in 0.6 mL of 1% sodium carboxymethyl cellulose and 0.1% Tween 80 after gentle agitation. The needle was introduced posteromedial to the greater trochanter pointing in an anteromedial direction. Once bone was contacted, the particle-containing solution was injected.20,45
Assessment of Nerve Blockade
The effectiveness of block was measured at predetermined time points, using methods previously described.8,20,45 Hindpaws were exposed in sequence (left, then right) to a 56°C hotplate (model 39D Hot Plate Analgesia meter; IITC Inc., Woodland Hills, CA), and the time until paw withdrawal (thermal latency) was measured. The data are reported in terms of thermal latency (intensity) and duration of block. Thermal latency is a measure of the degree of analgesia. If the animal did not remove its paw within 12 seconds, it was removed to avoid injury or the development of hyperalgesia. Latency was measured in the uninjected leg and used as a control for systemic effects. The duration of thermal nociceptive block was calculated as the time required for thermal latency to return to a value of 7 seconds from a higher value. Seven seconds is the midpoint between maximal block and normal thermal latency (approximately 2 seconds) in adult rats, and a maximal latency of 12 seconds.20
Motor strength was assessed with a weight-bearing test. The animal was held over a digital balance allowing it to bear weight with one hindpaw at a time. The maximum weight that it could bear without its ankle touching the balance was recorded. The duration of motor blockade was defined as the time for weight bearing to return halfway to normal from maximal.
Tissue Harvesting and Histology
After euthanasia with carbon dioxide the sciatic nerve and surrounding muscle were harvested and processed to produce hematoxylin and eosin-stained slides, using standard techniques. A dissection score was given based upon the observed tissue reaction as follows: 0 = tissue planes obvious and easily separated; 1 = tissue planes obvious but separated with some difficulty; 2 = tissue planes effaced and separated with some difficulty; 3 = tissue planes completely obliterated, could not separate surrounding tissues from nerve without cutting through them.21 The samples were scored for inflammation (0–4) and myotoxicity (0–6).42 The inflammation score was a subjective assessment of severity. The myotoxicity score reflected two characteristic features of local anesthetic myotoxicity: nuclear internalization and regeneration. Nuclear internalization is characterized by myocytes normal in size and chromicity, but with nuclei located away from their usual location at the periphery of the cell. Regeneration is characterized shrunken myocytes with basophilic cytoplasm. Scoring was as follows: 0 = normal; 1 = perifascicular internalization; 2 = deep internalization (>5 cell layers), 3 = perifascicular regeneration, 4 = deep regeneration, 5 = hemifascicular regeneration, 6 = holofascicular regeneration.
Statistical Analysis
Most data are reported as medians with 25th and 75th percentiles and are compared using the Mann-Whitney U test. This method was selected because the data were ordinal (dissection scores, inflammation scores, myotoxicity scores), or because they were not normally distributed (neurobehavioral data). Results of MTS assays were described with parametric measures (mean, standard deviation, 1-way repeated measures ANOVA with Bonferonni’s multiple correction test). LC50 values were calculated using nonlinear least squares regression analysis of percent cell viability values. These tests were unpaired except when comparing sensory versus motor blockade in the same rat. To avoid type 1 error for gross dissection, inflammation and myotoxicity scoring, multiple comparisons were done in a planned manner (i.e., comparisons were selected individually), and the P value required for statistical significance (α) was determined by dividing 0.05 by the number of comparisons. Therefore, for all 3 tissue scoring parameters (4 comparisons), α = 0.05/4 = 0.013, so P < 0.0013 was required for statistical significance. For duration of blockade, there was only one comparison, so the α remained 0.05. We reported exact P-values.
Results
Cytotoxicity of bupivacaine and lidocaine
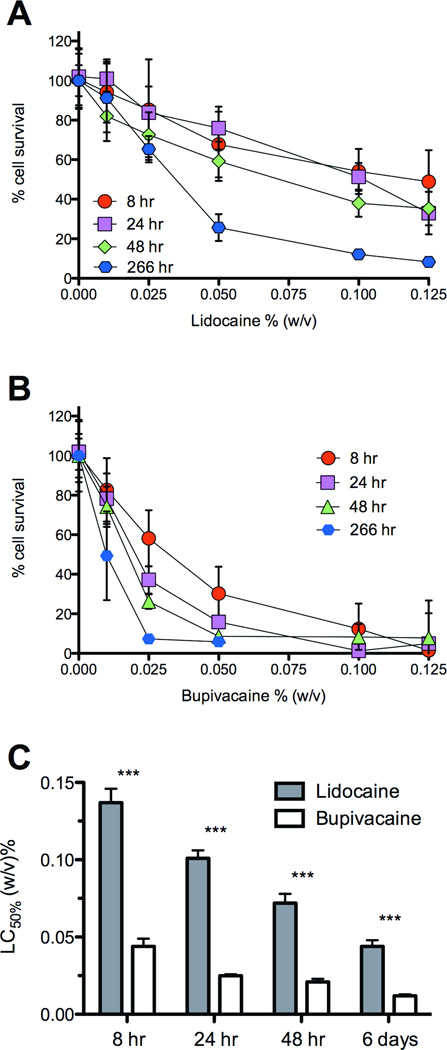
The myotoxicities of lidocaine and bupivacaine were compared in vitro using differentiated C2C12 myotubes.6,32,46 Myotoxicity was less for cells grown in media with lidocaine than with bupivacaine (Figs. 1a and b), at all durations of exposure for concentrations of 0.01% and larger (n=8, largest corrected P<0.050). The LC50 for bupivacaine (the concentration required to kill half of the cells) was lower than for lidocaine at all time points (P<0.00010 for all time points)(Figure 1c).
Fig. 1.
Percentage of C2C12 cell survival after exposure to (A) free lidocaine or (B) bupivacaine for various durations. Also shown are the LC50 values (C) derived from the percentage survival curves in (A) and (B). The LC50 values are significantly lower for bupivacaine (***for all time points, largest P < 0.00010). Data are means ± standard deviation.
Particle characteristics
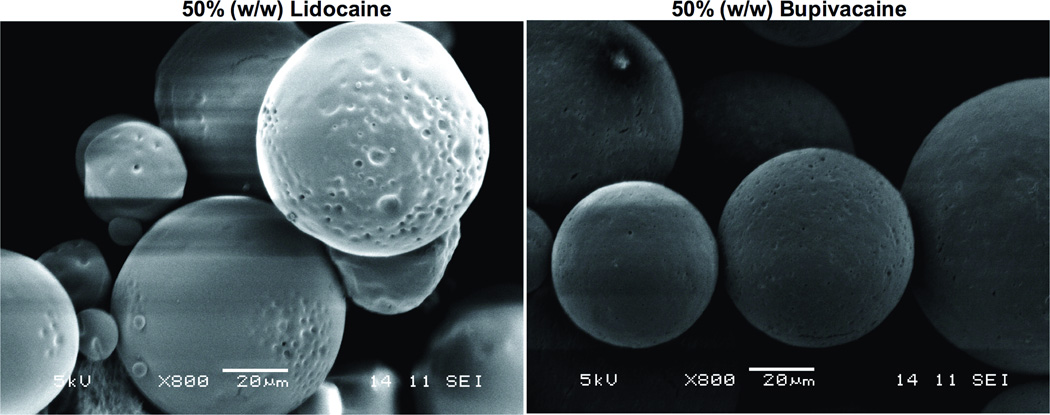
Particles were produced containing 10 or 50% (w/w) lidocaine or bupivacaine (Table 1). Ten percent (w/w) and 50% (w/w) loaded microspheres were approximately 50 and 60 µm in diameter respectively. Particles examined by scanning electron microscopy were spherical (Figure 2).
Table 1.
Characteristics of poly-lactic-co-glycolic acid (PLGA)-based microspheres
| Microsphere drug content (w/w)a | n | Diameter (µm) b | Drug content (%) b, c |
|---|---|---|---|
| Lidocaine 10% | 4 | 47.5 ± 13.9 | 10.0 ± 2.4 |
| Bupivacaine 10% | 4 | 49.2 ± 14.4 | 13.4 ± 2.1 |
| Lidocaine 50% | 4 | 65.2 ± 20.7 | 54.0 ± 3.0 |
| Bupivacaine 50% | 5 | 57.8 ± 16.3 | 51.7 ± 3.7 |
Theoretical
Values are means ± standard deviations
Measured
Fig. 2.
Scanning electron micrograph of microspheres (50% (w/w) drug loading). Scale bar is 20 µm in both panels.
Drug release from microspheres
Release of lidocaine or bupivacaine from 50 mg of 10% (w/w) or 50% (w/w) microspheres (Figure 3) was slowed compared to unencapsulated drug (1 mL of 0.5% (w/v) drug, or 5 mg). Bupivacaine release was slower than that of lidocaine. For example at 215 hours the 50% (w/w) bupivacaine particles had released 53.4 (50.6–57.2) % of their content compared to 74.2 (70.1–78.5) % for 50% (w/w) lidocaine particles (p=0.032). Similar trends were seen with the 10% (w/w) formulations.
Fig. 3.
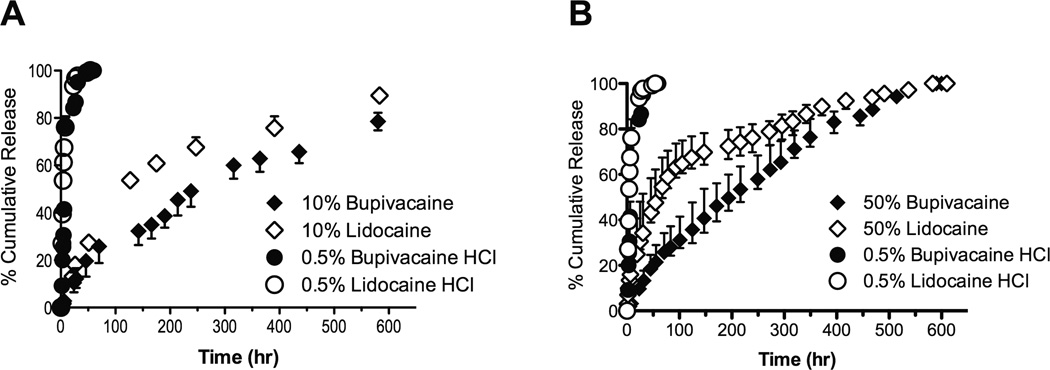
Cumulative release of encapsulated bupivacaine or lidocaine eluted from (A) 10% (w/w) or (B) 50% (w/w) drug loaded microspheres. Also shown is the release of unencapsulated 0.5% (w/v) lidocaine or bupivacaine. Data are medians with 25th and 75th percentiles.
Effect of drug selection on duration of sciatic nerve block
Animals received injections with 75 mg of particle at the sciatic nerve (Figure 4). Maximal sensory block (i.e., a thermal latency of 12 sec) was achieved in 6 of 8 animals injected with 50% (w/w) lidocaine particles and 8 of 8 animals injected with 50% (w/w) bupivacaine particles (p=0.13, Chi-square). By 210 minutes, sensory block had resolved completely in the 50% (w/w) lidocaine group, whereas the 50% (w/w) bupivacaine group remained fully blocked. However, the median duration of thermal nociceptive block from 50% (w/w) lidocaine particles was 255 (90–540) min versus 840 (277–1215) min from 50% (w/w) bupivacaine particles; the difference was not statistically significant (p=0.056; n = 8).
Fig. 4.
Duration of sensory versus motor blockade after injection with local anesthetic-containing microspheres. Percentages are drug loading (w/w). Data are medians with 25th and 75th percentiles.
To evaluate whether 50% (w/w) drug loading was so high that differences in quality of nerve blockade were obscured, eight animals were injected with 10% (w/w) loaded particles. The resulting duration of sensory block for rats injected with 10% (w/w) lidocaine was 15 (0–38) min (n=4). Only two met our definition of sensory nerve block (one lasting 30 minutes, the other 60 minutes). Of the four animals injected with 10% (w/w) bupivacaine particles, three developed sensory block lasting from 90–180 minutes with a median duration 105 minutes (23–180). The difference in median durations of block between lidocaine and bupivacaine groups was not statistically significant (p=0.37).
To determine if formulations were sensory or motor selective, duration of sensory and motor blockade was compared. For 50% (w/w) bupivacaine particles the duration of sensory blockade was 840 (277–1215) min versus motor blockade of 570 (300–1140) min (p=0.63). 50% (w/w) lidocaine particles produced sensory blockade lasting 255 (90–540) min compared to motor blockade of 540 (420–870) min (p=0.025). Ten percent (w/w) bupivacaine particles resulted in 105 (23–180) min of sensory blockade versus 105 (60–165) min of motor blockade (p=0.32), and for 10% (w/w) lidocaine particles sensory duration was 15 (0–38) min compared to 30 (0–98) min (p=0.51). The durations of sensory and motor blockade of these formulations were not significantly different, except that in 50% (w/w) of lidocaine particles, motor blockade outlasted sensory blockade by 450 (330–728) min (p=0.025) (Figure 4).
Tissue Reaction
Groups of rats were injected at the sciatic nerve with 50% (w/w) lidocaine particles (n=8) or 50% (w/w) bupivacaine particles (n=8). The sciatic nerves were removed 4 days (n=4) or 2 weeks (n=4) after injection and processed for histology. Animals were also injected with 10% (w/w) lidocaine particles (n=4) or 10% (w/w) bupivacaine particles (n=4) to determine whether the high loading of drug in the 50% (w/w) loaded groups obscured differences in anesthetic effect or tissue toxicity. This was only done at 4 days, the time of maximal injury in the 50% (w/w) drug groups.
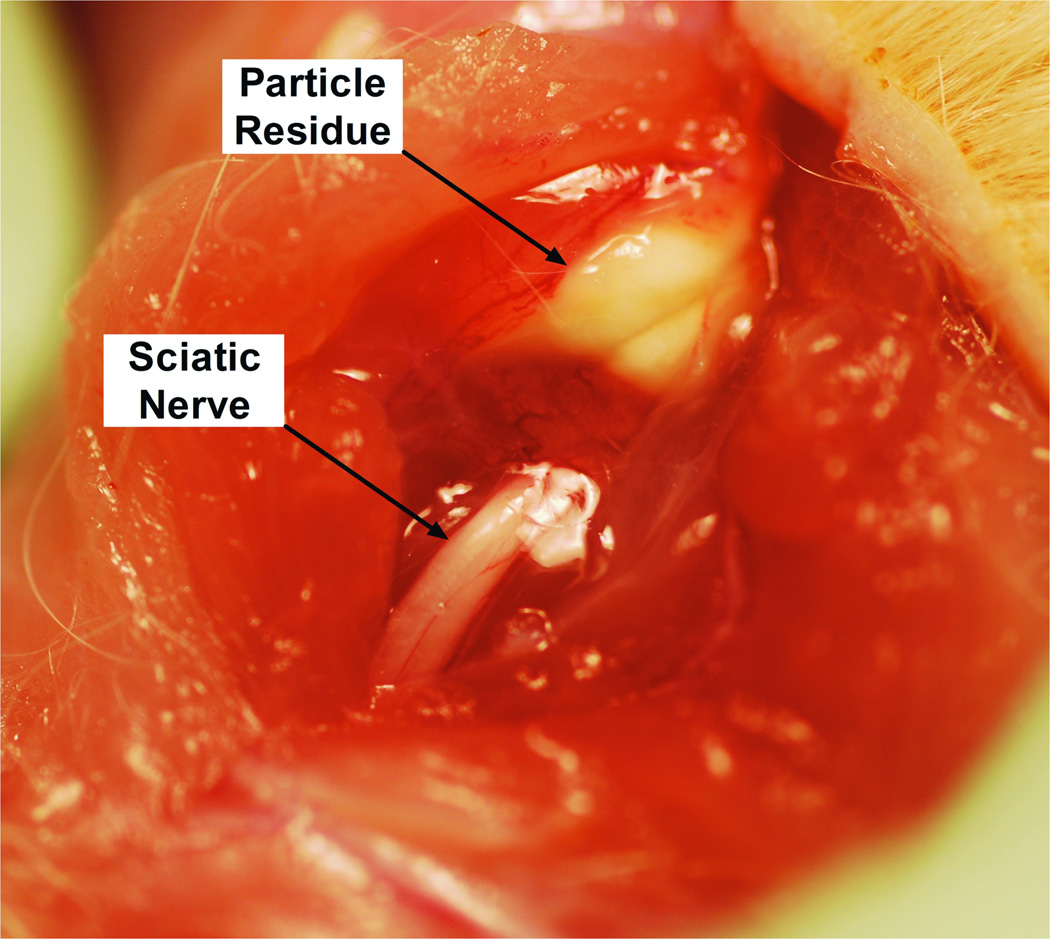
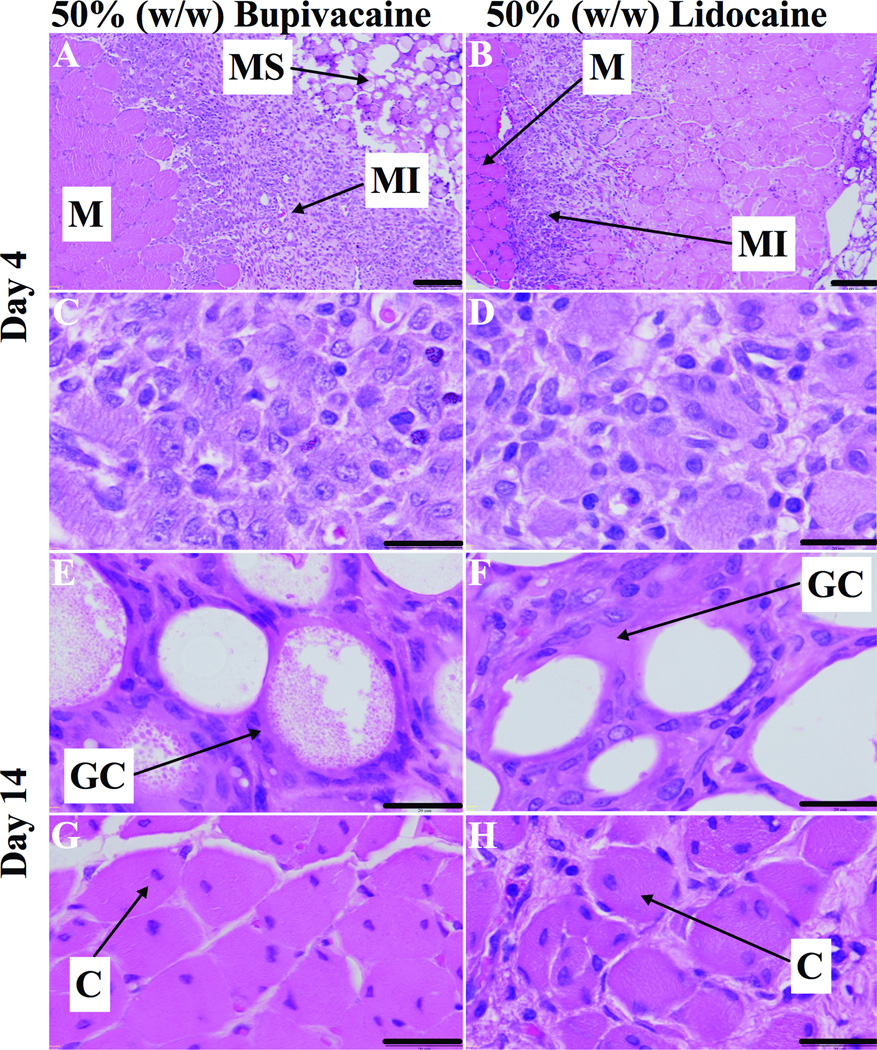
All injected rats had firm, white, globular deposits of particle in discrete pockets directly adjacent to the sciatic nerve (Figure 5), without spread to distant sites. The particle deposits appeared similar at 4 days and 2 weeks. The tissues immediately adjacent to the particle mass were adherent to the residue, but the latter was clearly separate from both muscle and nerve, i.e., there was no evidence of intraneural or intramuscular injection (on gross dissection or on subsequent microscopic examination), and tissue planes were easily identified and separated. Gross appearance was similar for all particle formulations and durations of exposure. On histologic examination, there was evidence of inflammation and myotoxicity in all animals (Table 2, Figure 6).
Fig. 5.
Representative photograph of particle residue (here, with 50% (w/w) bupivacaine microspheres 4 days after injection). Residue appearance was similar in all particle formulations and time points.
Table 2.
Values for the three measures of biocompatibility for each microparticulate formulation
| Score | |||
|---|---|---|---|
| Scale | Day 4 | Day 14 | |
| Gross dissection | Lidocaine 10% | 1.0 (0.8–1.0) | -- |
| (0–3) | Bupivacaine 10% | 1.0 (1.0–1.3) | -- |
| P value* | 0.13 | ||
| Lidocaine 50% | 1.0 (1.0–1.3) | 1.0 (1.0–1.5) | |
| Bupivacaine 50% | 2.0 (1.8–2.0) | 2.0 (2.0–2.3) | |
| P value* | 0.12 | 0.11 | |
| Inflammation | Lidocaine 10% | 1.0 (1.0–1.2) | -- |
| (0–4) | Bupivacaine 10% | 1.4 (1.2–1.6) | -- |
| P value* | 0.27 | ||
| Lidocaine 50% | 3.0 (2.1–3.5) | 1.4 (1.3–1.6) | |
| Bupivacaine 50% | 2.7 (2.5–2.9) | 0.7 (0.5–1.1) | |
| P value* | 0.44 | 0.10 | |
| Myotoxicity | Lidocaine 10% | 1.2 (0.9–1.7) | -- |
| (0–6) | Bupivacaine 10% | 1.7 (1.5–1.9) | -- |
| P value* | 0.19 | ||
| Lidocaine 50% | 3.4 (2.1–4.2) | 1.9 (1.8–2.4) | |
| Bupivacaine 50% | 3.3 (2.9–3.5) | 1.7 (1.3–1.9) | |
| P value* | 0.44 | 0.23 | |
Values are medians with 25th and 75th percentiles in parentheses; n = 4 for all groups
Particles with 10% loading were tested at 4 days only
P value of Mann-Whitney U test comparing lidocaine to bupivacaine for equally loaded particles
Fig. 6.
Representative light microscopy of hematoxylin/eosin-stained sections of muscles (M), and surrounding tissues at the sight of injection of 50% (w/w) bupivacaine or lidocaine loaded poly-lactic-co-glycolic acid (PLGA) microspheres (MS). (A and B) 4 days after injection, muscle injury (MI) extends deep into the muscle fascicle. (C and D) Tissue reaction was characterized by myocyte injury with abundant macrophages and occasional lymphocytes. (E and F) 2 weeks after injection, microspheres were surrounded by lymphocytes, macrophages and foreign-body giant cells (GC). (G and H) Tissue reaction was characterized by myocytes with nuclear centralization (C) surrounded by spasely distributed lymphoctes and macrophages. Scale bars 100 µm (A and B), 20 µm (C–H).
Tissue reaction at 4 days
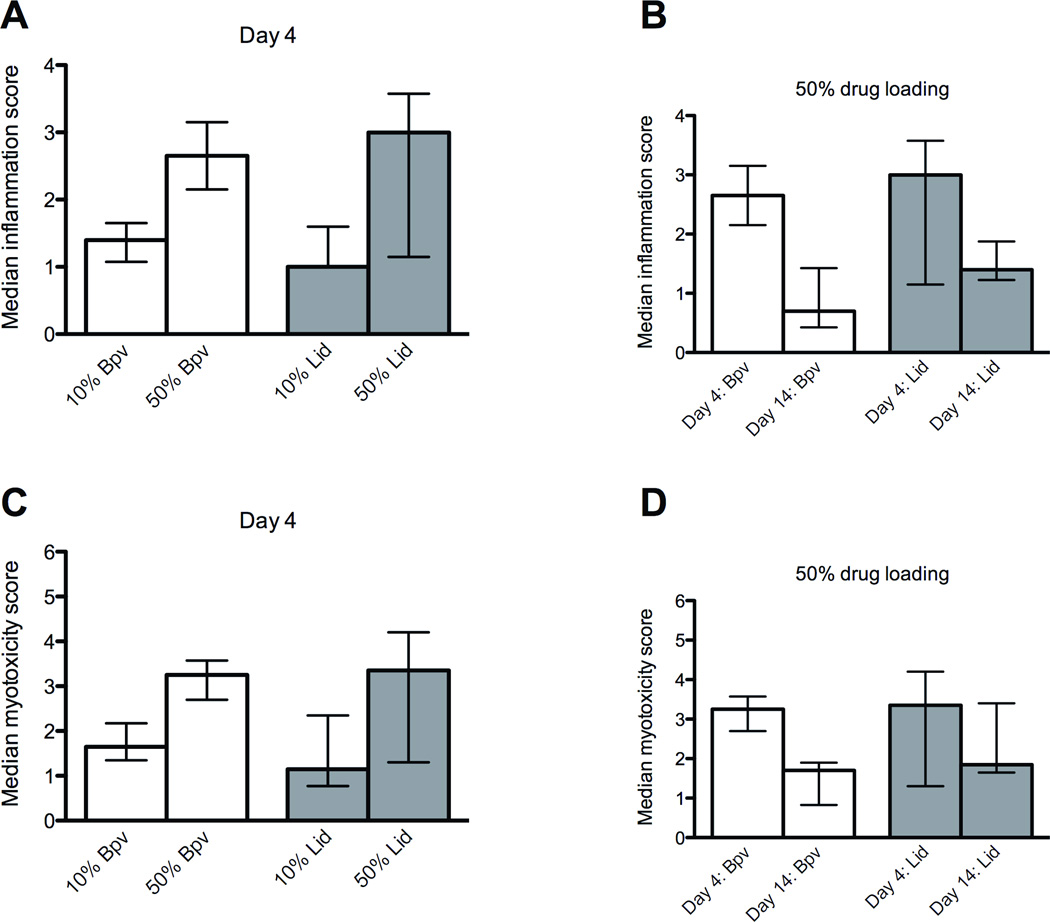
Tissue reaction was evaluated in all groups at 4 days after injection, when inflammation and tissue injury is the most intense.21 On necropsy, gross appearance was similar between groups, as reflected in the lack of difference in dissection scores irrespective of drug or drug loading. On histological examination, the cellular response for all formulations was characterized by inflammatory cells consisting of neutrophils, lymphocytes and macrophages. PLGA microspheres were visible as round structures 40–60 µm across. There was evidence of myotoxicity in all animals (Figures 6a–d), characterized by shrunken myofibers with basophilic cytoplasm, in the cell layers proximal to the microspheres. Muscle injury occurred in a perifascicular pattern, worse in areas adjacent to pockets of particle residue, indicating extrinsic tissue injury originating from the particles. Deeper layers demonstrated edematous cells with centralized nuclei (i.e., a lesser degree of myotoxicity). There was no statistically significant difference in the inflammatory or myotoxicity scores between particles loaded with lidocaine or bupivacaine at either concentration (Figure 7; Table 2).
Fig. 7.
Comparison of the effect of drug loading (A and C) and time after injection (B and D) on inflammation and myotoxicity. Data are medians with 25th and 75th percentiles.
Particle drug loading did not influence inflammation or myotoxicity scores (Figures 7a and c). The median inflammation and myotoxicity scores for 10% (w/w) lidocaine particles were 1.0 (1.0–1.2) and 1.2 (0.9–1.7) respectively, compared to 3.0 (2.1–3.5) and 3.4 (2.1–4.2) for 50% (w/w) lidocaine particles (smallest p=0.30). For 10% (w/w) bupivacaine particles the inflammation and myotoxicity scores were 1.4 (1.2–1.6) and 1.7 (1.5–1.9) respectively, compared to 2.7 (2.5–2.9) and 3.3 (2.9–3.5) for 50% (w/w) bupivacaine particles (smallest p=0.029). These differences were not significant by our predetermined α of p<0.013.
Tissue reaction 2 weeks after injection
For the 50% loaded particles, in which tissue reaction had been more severe, we followed tissue reaction out to 2 weeks after injection. The acute inflammatory reaction had been replaced by a chronic reaction with macrophages and lymphocytes. The microspheres were surrounded by multinucleated foreign-body giant cells. In all samples, adjacent muscle showed myofiber regeneration in more advanced stages than was observed in the 4-day cohort. In the layers close to the microspheres, regenerating myofibers remained shrunken, but demonstrated normal chromicity. Deeper myofibers had normal morphology with nuclei located at the periphery of the cell (Figures 6e–h).
The cellular inflammatory infiltrate resulting from both drug formulations was less severe and did not involve as much of the muscle fascicle as noted on day 4 (Figure 6g and h). For 50% (w/w) bupivacaine particles, the median inflammation scores decreased from 2.7 (2.5–2.9) on day 4 to 0.7 (0.5–1.1) at two weeks, and the myotoxicity scores decreased from 3.3 (2.9–3.5) to 1.7 (1.3–1.9) (smallest p<0.029). For 50% (w/w) lidocaine particles, the median inflammation scores decreased from 3.0 (2.1–3.5) on day 4 to 1.4 (1.3–1.6) at two weeks, and the myotoxicity scores decreased from 3.3 (2.9–3.5) to 1.7 (1.3–1.9) (smallest p=0.34). These differences were not significant by our predetermined α of p<0.013 (Figures 7b and d).
There were no statistically significant differences in the low inflammation or myotoxicity scores between the lidocaine and bupivacaine formulations at two weeks (Table 2). The inflammation scores for 50% (w/w) lidocaine particles were 1.4 (1.3–1.6) versus 0.7 (0.5–1.1) for 50% (w/w) bupivacaine particles (p=0.10). The myotoxicity scores were 1.9 (1.8–2.4) compared to 1.7 (1.3–1.9) for bupivacaine particles (p=0.23).
Conclusions
The key question addressed in this work, from a practical pharmaceutical point of view, was whether the selection of local anesthetic with respect to myotoxicity affects tissue injury from a controlled release system. Our results, using a very common, well established drug delivery vehicle, suggest that it does not. One might have expected the lidocaine microspheres to result in less severe tissue injury than bupivacaine, based on previous in vivo research documenting differences in the myotoxic potential of these two drugs,30 and based on some reports using sustained-release vehicles.25 Furthermore, our myotoxicity experiments with a mouse myoblast cell line (C2C12) demonstrated bupivacaine to be more toxic than lidocaine. These findings were similar to previous research, where bupivacaine was documented to be approximately 7-fold more cytotoxic than lidocaine.46 In contrast, in PLGA microspheres, the choice of drug had little influence on the severity of inflammation and myotoxicity. This lack of difference may have been due to the relatively high concentrations that may have been obtained in the first few hours, and/or from long duration of exposure, both of which can have marked effects on toxicity.6 (Note that the concentrations used for in vitro toxicity were well below what is used clinically [0.25–0.75% for bupivacaine and 0.5–2% for lidocaine] since clinical concentrations caused immediate cell death [data not shown]). Such high concentrations might have been sufficiently toxic with both drugs that no difference could be seen. However, this lack of difference in toxicity was noted even in the 10% (w/w) loaded particles, in which there was relatively minimal nerve blockade (and therefore presumably not very high drug levels). By way of comparison, 0.5% (w/v) bupivacaine reliably yields a nerve block lasting approximately 150 min,19,47 with minimal tissue toxicity.42,48 These observations are not consistent with the lack of difference in toxicity being simply due to excessive levels of local anesthetic.
We have previously noted that the mere presence of a sustained release vehicle can exacerbate local tissue toxicity19,42 in vivo, even though the vehicle might have no effect on local anesthetic cytotoxicity in cell culture.6 The mechanism for this effect is unknown. It is possible that the inflammation caused by the particles, or the particles themselves, worsens the severity of myotoxicity from agents that would otherwise be mild.6,21,37 Particles alone can also cause inflammation at the nerve that can considerably outlast the duration of blockade.21,41,43 The specific nature of the delivery vehicle itself may therefore have an impact on local tissue injury from local anesthetics. As with myotoxicity, the severity of inflammation appeared to correlate with drug loading (Figure 7a) and decrease over time.
Conventional local anesthetics are also neurotoxic.32,38,39,49 There are reports of bupivacaine-containing PDLA formulations where no neurotoxicity was documented.1,32 However, as noted in the introduction, neurotoxicity was a major factor leading to withdrawal of the Investigational New Drug application (IND#53,441)44 of a sustained-release bupivacaine-dexamethasone formulation.3 The hematoxylin-eosin staining used here was not sufficiently sensitive to detect any but severe nerve injury. Nonetheless, because amino-amide (and amino ester) local anesthetics cause injury to both tissues in a concentration-dependent manner, the presence of myotoxicity suggests the potential for neurotoxicity.
As noted in the Results section, dissection revealed the presence of microparticulate residue immediately adjacent to the sciatic nerve. This observation would suggest that block durations as presented here are likely reflections of the local anesthetic capabilities of the microparticulate formulations rather than operator error (failed blocks). The lack of statistically significant difference in the durations of block from bupivacaine and lidocaine microspheres may seem surprising given that lidocaine solution provides shorter nerve blocks than bupivacaine.6,49,50 This lack of difference may, in part, have arisen from the relatively short duration of effect of the drugs used here compared to the timeframes over which the microspheres can release drugs. (Note the extremely long durations of block that can be achieved with these microspheres using synergistic drug combinations).37 The great variability in nerve block durations from polymeric microsphere formulations may also have made differences hard to detect. That variability was consistent with our prior experience with PLGA microspheres for nerve blockade.6,20,37,43
It is interesting to consider the risk of PDLA-related local toxicity in the context of ultrasound-guided regional anesthesia, which is now common practice. In many circumstances, ultrasound-guidance may allow PDLA formulations to be deposited in locales where they are adjacent to nerves but remote from major muscle groups. There will also be circumstances where that is not possible. It would remain to be seen how far muscle groups need to be from such devices to be unaffected. Of course, ultrasound guidance is not perfect: there is always the possibility of inadvertent injection into or near muscle. Many PDLA formulations could be used for infiltration anesthesia or field blocks, in which case ultrasound guidance would likely not be used, even in areas near muscles (perianal procedures, hernia repairs, etc.). It also bears emphasizing that one principal virtue of ultrasound-guidance, that it can deposit anesthetics right on the nerve, also has the potential to increase the probability of one of the most serious potential complications of PDLA: nerve injury.
We have observed myotoxicity with a wide range of delivery systems with very different compositions of matter, including PLGA,6,21,37,51 lipid-sugar-protein particles,21,41,43,51 polysaccharide-based gels,19,52 and thermosensitive nanogels.53 This has raised the concern that myotoxicity may be an unavoidable consequence of increased concentrations or prolonged exposures to conventional local anesthetics, regardless of drug selection.6 The observation that even particles with low drug loading (10% in this study) and little anesthetic benefit generate myotoxicity is consistent with that view. Given the potentially severe tissue toxicity (muscle and nerve injury) that has been documented with sustained release of local anesthetics,44 these results suggest that caution is warranted in the application of sustained release formulations of all amino-amide and amino-ester local anesthetics, particularly around muscle and nerve.
Acknowledgments
Funding: This research was funded by National Institutes of Health grant number R01 GM073626.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
DISCLOSURES:
Name: J. Brian McAlvin, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: J. Brian McAlvin has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Gally Reznor, BS, MBS
Contribution: This author helped conduct the study
Attestation: Gally Reznor has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Sahadev A. Shankarappa, MPH, MBBS, PhD
Contribution: This author helped conduct the study
Attestation: Sahadev A. Shankarappa has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Cristina F. Stefanescu, BS
Contribution: This author helped conduct the study
Attestation: Cristina F. Stefanescu has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Daniel S. Kohane, MD, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Daniel S. Kohane has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
This manuscript was handled by: Marcel E. Durieux, MD, PhD
Contributor Information
J. Brian McAlvin, Department of Medicine, Medicine Critical Care Program, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts; Laboratory for Biomaterials and Drug Delivery, Department of Anesthesiology, Division of Critical Care Medicine, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts; Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Cambridge, Massachusetts.
Gally Reznor, Laboratory for Biomaterials and Drug Delivery, Department of Anesthesiology, Division of Critical Care Medicine, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts.
Sahadev A. Shankarappa, Laboratory for Biomaterials and Drug Delivery, Department of Anesthesiology, Division of Critical Care Medicine, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts; Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Cambridge, Massachusetts.
Cristina F. Stefanescu, Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Cambridge, Massachusetts.
Daniel S. Kohane, Laboratory for Biomaterials and Drug Delivery, Department of Anesthesia, Division of Critical Care Medicine, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts; Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Cambridge, Massachusetts.
References
- 1.Tanaka PP, Torres MF, Tenorio SB. Analysis of the acute cytotoxic potential of bupivacaine and 50% enantiomeric excess bupivacaine (s75-r25) incorporated into microspheres in rat sciatic nerves. Rev Bras Anestesiol. 2012;62:223–234. doi: 10.1016/S0034-7094(12)70120-4. [DOI] [PubMed] [Google Scholar]
- 2.Le Corre P, Estebe JP, Clement R, Du Plessis L, Chevanne F, Ecoffey C, Le Verge R. Spray-dryed bupivacaine-loaded microspheres: in vitro evaluation and biopharmaceutics of bupivacaine following brachial plexus administration in sheep. Int J Pharm. 2002;238:191–203. doi: 10.1016/s0378-5173(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 3.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, Wilder R, Langer R, Berde C. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–1166. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Curley J, Castillo J, Hotz J, Uezono M, Hernandez S, Lim JO, Tigner J, Chasin M, Langer R, Berde C. Prolonged regional nerve blockade. Injectable biodegradable bupivacaine/polyester microspheres. Anesthesiology. 1996;84:1401–1410. doi: 10.1097/00000542-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Wakiyama N, Juni K, Nakano M. Preparation and evaluation in vitro and in vivo of polylactic acid microspheres containing dibucaine. Chem Pharm Bull (Tokyo) 1982;30:3719–3727. doi: 10.1248/cpb.30.3719. [DOI] [PubMed] [Google Scholar]
- 6.Padera R, Bellas E, Tse JY, Hao D, Kohane DS. Local myotoxicity from sustained release of bupivacaine from microparticles. Anesthesiology. 2008;108:921–928. doi: 10.1097/ALN.0b013e31816c8a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager C, Benziger D, Gao F, Berde CB. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–979. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Masters DB, Berde CB, Dutta SK, Griggs CT, Hu D, Kupsky W, Langer R. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 1993;79:340–346. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Boedeker BH, Lojeski EW, Kline MD, Haynes DH. Ultra-long-duration local anesthesia produced by injection of lecithin-coated tetracaine microcrystals. J Clin Pharmacol. 1994;34:699–702. doi: 10.1002/j.1552-4604.1994.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 10.Richard BM, Ott LR, Haan D, Brubaker AN, Cole PI, Nelson KG, Ross PE, Rebelatto MC, Newton PE. The safety and tolerability evaluation of DepoFoam bupivacaine (bupivacaine extended-release liposome injection) administered by incision wound infiltration in rabbits and dogs. Expert Opin Investig Drugs. 2011;20:1327–1341. doi: 10.1517/13543784.2011.611499. [DOI] [PubMed] [Google Scholar]
- 11.Richard BM, Newton P, Ott LR, Haan D, Brubaker AN, Cole PI, Ross PE, Rebelatto MC, Nelson KG. The Safety of EXPAREL (R) (Bupivacaine Liposome Injectable Suspension) Administered by Peripheral Nerve Block in Rabbits and Dogs. J Drug Deliv. 2012;2012 doi: 10.1155/2012/962101. 962101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard BM, Rickert DE, Newton PE, Ott LR, Haan D, Brubaker AN, Cole PI, Ross PE, Rebelatto MC, Nelson KG. Safety Evaluation of EXPAREL (DepoFoam Bupivacaine) Administered by Repeated Subcutaneous Injection in Rabbits and Dogs: Species Comparison. J Drug Deliv. 2011;2011 doi: 10.1155/2011/467429. 467429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant GJ, Vermeulen K, Langerman L, Zakowski M, Turndorf H. Prolonged analgesia with liposomal bupivacaine in a mouse model. Reg Anesth. 1994;19:264–269. [PubMed] [Google Scholar]
- 14.Mowat JJ, Mok MJ, MacLeod BA, Madden TD. Liposomal bupivacaine. Extended duration nerve blockade using large unilamellar vesicles that exhibit a proton gradient. Anesthesiology. 1996;85:635–643. doi: 10.1097/00000542-199609000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Richard BM, Rickert DE, Doolittle D, Mize A, Liu J, Lawson CF. Pharmacokinetic Compatibility Study of Lidocaine with EXPAREL in Yucatan Miniature Pigs. ISRN Pharm. 2011;2011 doi: 10.5402/2011/582351. 582351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552–1559. doi: 10.1097/DCR.0b013e318232d4c1. [DOI] [PubMed] [Google Scholar]
- 17.Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam(R) bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776–788. doi: 10.1007/s12325-011-0052-y. [DOI] [PubMed] [Google Scholar]
- 18.Masters DB, Domb AJ. Liposphere local anesthetic timed-release for perineural site application. Pharm Res. 1998;15:1038–1045. doi: 10.1023/a:1011978010724. [DOI] [PubMed] [Google Scholar]
- 19.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Kohane DS, Lipp M, Kinney RC, Lotan N, Langer R. Sciatic nerve blockade with lipid-protein-sugar particles containing bupivacaine. Pharm Res. 2000;17:1243–1249. doi: 10.1023/a:1026470831256. [DOI] [PubMed] [Google Scholar]
- 21.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J Biomed Mater Res. 2002;59:450–459. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki R, Arai YC, Hamayasu K, Fujita K, Hara K, Yamaguchi T, Sasaguri S. Complex of branched cyclodextrin and lidocaine prolonged the duration of peripheral nerve block. J Anesth. 2009;23:295–297. doi: 10.1007/s00540-008-0720-5. [DOI] [PubMed] [Google Scholar]
- 23.Karashima K, Taniguchi M, Nakamura T, Takasaki M, Matsuo K, Irikura M, Irie T. Prolongation of intrathecal and sciatic nerve blocks using a complex of levobupivacaine with maltosyl-beta-cyclodextrin in rats. Anesth Analg. 2007;104:1121–1128. doi: 10.1213/01.ane.0000260309.15034.52. tables of contents. [DOI] [PubMed] [Google Scholar]
- 24.Bragagni M, Maestrelli F, Mennini N, Ghelardini C, Mura P. Liposomal formulations of prilocaine: effect of complexation with hydroxypropyl-ss-cyclodextrin on drug anesthetic efficacy. J Liposome Res. 2010;20:315–322. doi: 10.3109/08982100903544169. [DOI] [PubMed] [Google Scholar]
- 25.Wang CF, Djalali AG, Gandhi A, Knaack D, De Girolami U, Strichartz G, Gerner P. An absorbable local anesthetic matrix provides several days of functional sciatic nerve blockade. Anesth Analg. 2009;108:1027–1033. doi: 10.1213/ane.0b013e318193596a. [DOI] [PubMed] [Google Scholar]
- 26.Gerner P, Wang CF, Lee BS, Suzuki S, Degirolami U, Gandhi A, Knaack D, Strichartz G. The relationship between functional sciatic nerve block duration and the rate of release of lidocaine from a controlled-release matrix. Anesth Analg. 2010;111:221–229. doi: 10.1213/ANE.0b013e3181dd2690. [DOI] [PubMed] [Google Scholar]
- 27.Benoit PW, Belt WD. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine) J Anat. 1970;107:547–556. [PMC free article] [PubMed] [Google Scholar]
- 28.Benoit PW, Belt WD. Some effects of local anesthetic agents on skeletal muscle. Exp Neurol. 1972;34:264–278. doi: 10.1016/0014-4886(72)90173-2. [DOI] [PubMed] [Google Scholar]
- 29.Yagiela JA, Benoit PW, Buoncristiani RD, Peters MP, Fort NF. Comparison of myotoxic effects of lidocaine with epinephrine in rats and humans. Anesth Analg. 1981;60:471–480. [PubMed] [Google Scholar]
- 30.Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59:727–736. [PubMed] [Google Scholar]
- 31.Brun A. Effect of procaine, carbocain and xylocaine on cutaneous muscle in rabbits and mice. Acta Anaesthesiol Scand. 1959;3:59–73. doi: 10.1111/j.1399-6576.1959.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 32.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci U S A. 2009;106:7125–7130. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouette-Gaulain K, Bringuier S, Canal-Raffin M, Bernard N, Lopez S, Dadure C, Masson F, Mercier J, Sztark F, Rossignol R, Capdevila X. Time course of mitochondrial metabolism alterations to repeated injections of bupivacaine in rat muscle. Can J Anaesth. 2010;57:836–842. doi: 10.1007/s12630-010-9347-8. [DOI] [PubMed] [Google Scholar]
- 34.Hogan Q, Dotson R, Erickson S, Kettler R, Hogan K. Local anesthetic myotoxicity: a case and review. Anesthesiology. 1994;80:942–947. doi: 10.1097/00000542-199404000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Neuburger M, Breitbarth J, Reisig F, Lang D, Buttner J. Complications and adverse events in continuous peripheral regional anesthesia Results of investigations on 3,491 catheters. Anaesthesist. 2006;55:33–40. doi: 10.1007/s00101-005-0920-4. [DOI] [PubMed] [Google Scholar]
- 36.Pere P, Watanabe H, Pitkanen M, Wahlstrom T, Rosenberg PH. Local myotoxicity of bupivacaine in rabbits after continuous supraclavicular brachial plexus block. Reg Anesth. 1993;18:304–307. [PubMed] [Google Scholar]
- 37.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NG, Berde CB, Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104:415–421. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer C, Piepenbrink K, Riest G, Peters J. Cardiotoxic and neurotoxic effects after accidental intravascular bupivacaine administration. Therapy with lidocaine propofol and lipid emulsion. Anaesthesist. 2007;56:449–453. doi: 10.1007/s00101-007-1147-3. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita A, Matsumoto M, Matsumoto S, Itoh M, Kawai K, Sakabe T. A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. Anesth Analg. 2003;97:512–519. doi: 10.1213/01.ANE.0000068885.78816.5B. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Selander D. Neurotoxicity of local anesthetics: animal data. Reg Anesth. 1993;18:461–468. [PubMed] [Google Scholar]
- 41.Colombo G, Langer R, Kohane DS. Effect of excipient composition on the biocompatibility of bupivacaine-containing microparticles at the sciatic nerve. J Biomed Mater Res A. 2004;68:651–659. doi: 10.1002/jbm.a.20074. [DOI] [PubMed] [Google Scholar]
- 42.Padera RF, Tse JY, Bellas E, Kohane DS. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve. 2006;34:747–753. doi: 10.1002/mus.20618. [DOI] [PubMed] [Google Scholar]
- 43.Colombo G, Padera R, Langer R, Kohane DS. Prolonged duration local anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A. 2005;75:458–464. doi: 10.1002/jbm.a.30443. [DOI] [PubMed] [Google Scholar]
- 44.Kohane DSL. R. Biocompatibility and drug delivery systems. Chemical Science. 2010;1:441–446. [Google Scholar]
- 45.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–1025. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Maurice JM, Gan Y, Ma FX, Chang YC, Hibner M, Huang Y. Bupivacaine causes cytotoxicity in mouse C2C12 myoblast cells: involvement of ERK and Akt signaling pathways. Acta Pharmacol Sin. 2010;31:493–500. doi: 10.1038/aps.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–131. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Barnet CS, Louis DN, Kohane DS. Tissue injury from tricyclic antidepressants used as local anesthetics. Anesth Analg. 2005;101:1838–1843. doi: 10.1213/01.ANE.0000184129.50312.C1. [DOI] [PubMed] [Google Scholar]
- 49.Miller RD. Miller's anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010. [Google Scholar]
- 50.Chen PC, Kohane DS, Park YJ, Bartlett RH, Langer R, Yang VC. Injectable microparticle-gel system for prolonged and localized lidocaine release. II. In vivo anesthetic effects. J Biomed Mater Res A. 2004;70:459–466. doi: 10.1002/jbm.a.30101. [DOI] [PubMed] [Google Scholar]
- 51.Kohane DS, Plesnila N, Thomas SS, Le D, Langer R, Moskowitz MA. Lipid-sugar particles for intracranial drug delivery: safety and biocompatibility. Brain Res. 2002;946:206–213. doi: 10.1016/s0006-8993(02)02878-0. [DOI] [PubMed] [Google Scholar]
- 52.Hoare T, Zurakowski D, Langer R, Kohane DS. Rheological blends for drug delivery. I. Characterization in vitro. J Biomed Mater Res A. 2010;92:575–585. doi: 10.1002/jbm.a.32392. [DOI] [PubMed] [Google Scholar]
- 53.Patenaude M, Hoare T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules. 2012;13:369–378. doi: 10.1021/bm2013982. [DOI] [PubMed] [Google Scholar]