Abstract
The purpose of this study was to examine relations among adrenocortical regulation, eating in the absence of hunger, and body mass index (BMI) in children ages 5 to 9 years (N = 43). Saliva was collected before and after the Trier Social Stress Test for children (TSST-C), and was later assayed for cortisol. Area under the curve with respect to increase (AUCi) was used as a measure of changes in cortisol release from baseline to 60 minutes post-TSST-C. Age- and sex-specific BMI scores were calculated from measured height and weight, and eating in the absence of hunger was assessed using weighed food intake during a behavioral procedure. We also included a measure of parents’ report of child impulsivity, as well as family demographic information. Participants were stratified by age into younger (5 to 7 years) and older (8 to 9 years) groups. In younger children, parents’ reports of child impulsivity were significantly and positively associated with BMI; cortisol AUCi was not associated with BMI or eating in the absence of hunger. In older children, however, greater stress-related cortisol AUCi was related to higher BMI scores and greater energy intake in the absence of hunger. The results suggest that cortisol AUCi in response to psychosocial stress may be linked to problems with energy balance in children, with some variation by age.
Keywords: Stress, psychobiological, disinhibited eating, obesity, binge, self-regulation
INTRODUCTION
In the past two decades, a major focus in developmental science has been to advance our understanding of why, when exposed to stressful experiences or circumstances, some children are placed at risk for emotional and behavioral problems but others are resilient (Boyce & Ellis, 2005; Cicchetti, 2002; Rutter, Dunn, Plomin, Simonoff, & et al., 1997). The effects of stress on children’s health and developmental outcomes are well-documented (Evans & English, 2002; Gunnar & Quevedo, 2007), and these effects extend across the lifespan (Anda, et al., 2006; Dube, Felitti, Dong, Giles, & Anda, 2003; Evans & Kim, 2007). Obesity is one such health outcome whose biobehavioral underpinnings have been linked with stress (Bjorntorp, Rosmond, 2000; Chrousos, 2000; Gundersen, Mahatmya, Garasky, & Lohman, 2011).
Research attention has been drawn to the adrenocortical component of the physiological stress response as a potential mechanism in the development of obesity through its effects on the accumulation of central adiposity and dysregulated eating behavior, or eating in the absence of hunger (Bjorntorp & Rosmond, 2000; Drapeau, Therrien, Richard, & Tremblay, 2003; Kyrou, Chrousos, & Tsigos, 2006; Rogers, 1999). Disruptions in the hypothalamic pituitary adrenocortical (HPA) axis and sympathetic nervous system (SNS) functioning, as indicated by cortisol and cardiovascular responses to stress, are linked to the accumulation of central adiposity, increased energy intake, and appetite regulation (Adam & Epel, 2007; Bjorntorp, 1997; Gluck, 2006; Gluck, Geliebter, Hung, & Yahav, 2004; Nieuwenhuizen & Rutters, 2008; Tataranni, et al., 1996; Wallerius, Rosmond, Ljung, Holm, & Bjorntorp, 2003). Adrenocortical regulation (i.e., cortisol) may be an underlying mechanism for the development of energy balance problems in children (Spencer & Tilbrook, 2011).
Findings from a study in preadolescent youth (average age ~10 years) show that cardiovascular reactivity (i.e., heart rate) to a laboratory-based stressor was linked to a higher percent body fat; increases in perceived stress after exposure to the stressor was also related to higher levels of body fat (Roemmich, Smith, Epstein, & Lambiase, 2007). Dockray, Susman and Dorn (2009) confirmed a link between cortisol reactivity and obesity in 8- to 13-year-old boys and girls. A large majority of the studies that have provided evidence for a link between cortisol and obesity or eating behavior have been conducted with adult populations of men and women (Epel, et al., 2000; Evans, Boxhill, & Pinkaya, 2008; Gluck, Geliebter, & Lorence, 2004; Rowland & Antelman, 1976; Steptoe, Kunz-Ebrecht, Brydon, & Wardle, 2004). These studies suggest that cortisol reactivity may be linked to dysregulated eating, and eating in response to stress (Epel, Lapidus, McEwen, & Brownell, 2001; Gluck, Geliebter, Hung, et al., 2004).
Eating in the absence of hunger, a measure of dysregulated eating behavior, has consistently been associated with weight gain, overweight, and obesity, in both adults and children (Bellisle, et al., 2004; Fisher, Birch, 2002). As such, eating in the absence of hunger has been proposed as a behavioral phenotype of obesity (Faith, 2006). Findings from a study by Rutters and colleagues (Rutters, Nieuwenhuizen, Lemmens, Born, & Westerterp-Plantenga, 2009) revealed that men and women ate more in the absence of hunger after exposure to an acute stressor; these effects were particularly strong for those participants with a disinhibited eating style. Similar findings have been reported more recently, in a sample of non-overweight women (Born, et al., 2010). Stress, both perceived and manipulated, also has been found to affect eating/dietary choices and behaviors in youth (Cartwright, et al., 2003; Jenkins, Rew, & Sternglanz, 2005; Roemmich, Wright, & Epstein, 2002) and adults (Born, et al., 2010; Gibson, 2006; O'Connor, Jones, Conner, McMillan, & Ferguson, 2008; Zellner, et al., 2006). Roemmich and colleagues (2002) found that preadolescent boys and girls (average age ~9yrs) who reported high levels of dietary restraint, reported higher levels of perceived stress, and increased energy intake on days in which they were exposed to stress, compared to non-stress days. Findings from a study with boys and girls as young as age 8 show that perceived stress was positively related to children’s reports of unhealthy eating behaviors, and the use of food as a coping mechanism for stress, nervousness and worry (Jenkins, et al., 2005). There is a dearth of information on the biobehavioral processes underlying these dysregulated eating behaviors in young children. Understanding individual differences in the sensitivity of the physiological stress response may provide more information on the factors that contribute to the development of dysregulated eating and obesity in children.
Findings from recent studies show that eating appears to be a coping response to stress in youth (Balantekin & Roemmich, 2012; Roemmich, Lambiase, Lobarinas, & Balantekin, 2011). In these studies, adolescents ages 8–12 years consumed more calories on a stress day compared to a non-stress day, and this finding was particularly pronounced in adolescents with greater adiposity, and those who reported lower levels of dietary restraint. Similar findings from animal and human models studies suggest that eating may be a coping response to stress, particularly when the food is a comfort food (Dallman, et al., 2003; Greeno & Wing, 1994; Tomiyama, Dallman, & Epel, 2011). Ulrich-Lai and colleagues (2010) showed that intake of palatable foods can buffer the biobehavioral effects of stress via reward pathways in the brain. The maladaptive, long-term outcome of this biobehavioral dysregulation is the weight gain and fat accumulation that may result from consumption, or overconsumption, of energy-dense foods.
Using a longitudinal sample of youth in the U.S., Francis and Susman (2009) examined links between behavioral regulation (measured using laboratory-based self-control and delay of gratification tasks) and BMI changes from age 3 to 12 years. Results revealed that youth who showed self-regulation failure at ages 3 and 4 had the most rapid increases in BMI from age 3 to age 12, compared to those who showed evidence of self-regulatory success at ages 3 and 4. We propose that cortisol output in response to a stressor is not only a marker for HPA-axis functioning, but may also be linked to regulation across domains, particularly as it relates to biobehavioral regulation of eating and body mass. Given that childhood obesity is a major public health concern, with more than 30% of children ages 6 to 11 years in the U.S. classified as overweight or obese (Ogden, Carroll, Kit, & Flegal, 2012), a better understanding of the biobehavioral factors involved in the etiology of childhood obesity is needed. The purpose of this study was to examine adrenocortical regulation (as indexed by cortisol output in response to psychosocial stress) as an underlying mechanism for the development of problems with energy balance in 5- to 9-year-old children, through its effects on eating in the absence of hunger and body mass index (BMI). We tested the hypothesis that greater cortisol output in response to psychosocial stress would be linked to more eating in the absence of hunger and higher BMI scores.
METHODS AND PROCEDURES
Participants
Participants included 43 children (61% boys; 68% White, 16% Black, 16% other) ages 5- to 9-years (mean age = 7.1 ± 1.5 yrs) and their biological parents, recruited for a study on growth and development in young children in a mid-Atlantic state; the sample was not recruited or selected based on weight status, stress levels, or eating behavior. Eligibility criteria for participation included living with at least one biological parent, and the absence of any biological, physical or developmental problem that would preclude participation in the food procedures, activities, or interviews (e.g., food allergies, heart abnormality or developmental delays). Families were recruited using flyers and newspaper advertisements. Parents were well-educated and predominantly middle income; most parents reported having attained a bachelor’s degree or higher (69.2%) and more than 75% of families reported combined family incomes over $50,000/yr.
Procedure
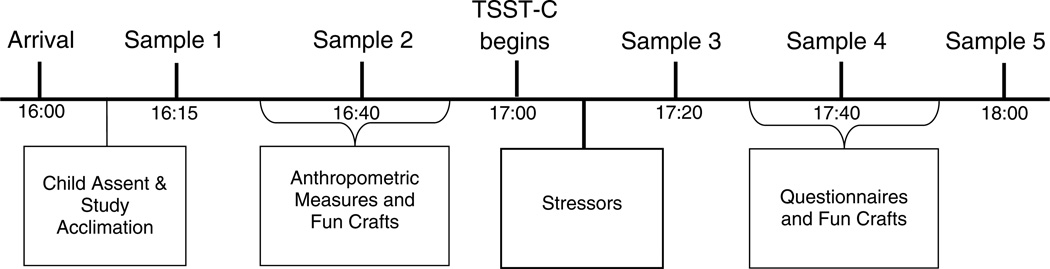
The study protocol appears in Table 1. Families visited a university General Clinical Research Center (GCRC) at approximately 1600 hrs. Parents provided written consent for their family’s participation, and completed scales on child health (e.g., respiratory infection, stomach ache, medications, etc.), and whether or not the child ate or drank anything within the past 2 hours. After providing assent, children were taken to a private location to rotate through six 20- to 25-min interview sessions. To reduce boredom and break up the monotony of the interview sessions, children participated in 15- to 20-min craft and activity breaks after each interview session, unless the interview session included a fun activity (e.g., television-viewing procedure, not discussed here). The study was approved by the university’s Office for Research Protections
Table 1.
Schedule of study procedures
| TIME | CHILD | PARENT |
|---|---|---|
|
3:30pm – 4:00pm |
Arrival times will vary | |
| Meet interviewing buddy | Child health and wellness assessment; consent forms and health history form |
|
| Anthropometric Measurements – Questionnaires (~40min) |
Parent Questionnaires Anthropometric Measurements |
|
| Stress Procedure Questionnaires/Crafts (75 min) | ||
| Dinner (25 min) | ||
| Activity Break (20 min) | ||
| Food Preference Procedure (15 min) | ||
| Break (15 min) | ||
| TV Viewing Procedure (30 min) | ||
|
7:30pm – 8:00pm |
Questionnaires/Crafts (20 min) |
|
| Games, activities, incentives and pickup | ||
Measures
Adrenocortical Output
was assessed by cortisol output in response to the Trier Social Stress Test for Children (TSST-C) (Kirschbaum, Pirke, & Hellhammer, 1993). The TSST-C modification for children consists of an anticipation period (5 min.) and a test period (10 min.) in which children were asked to deliver a 4-min speech and perform a mental arithmetic (serial subtraction) task for 4 minutes. We substituted the block design task from the Wechsler’s Intelligence Scale for Children-Revised (WISC-R) for the serial subtraction task so as to be more appropriate for use with children under 8 years old (Tsukayama, Duckworth, & Geier, 2010). Analyses revealed no significant age differences in cortisol levels at each time point during the procedure. The TSST-C tasks were completed in the presence of two confederate judges.
Five saliva samples were collected via passive drool (Granger, Kivlighan, el-Sheikh, Gordis, Stroud, 2007) at baseline (Times 1 and 2), and after the TSST-C (Times 3, 4 and 5). Using the passive drool saliva collection protocol, children are instructed to gently force saliva pooling in their mouths into a collection vial for 2 minutes, or until the saliva has reached the fill line. Cortisol samples were collected over the same time frame for all children; the entire protocol is shown in Figure 1. To avoid sample contamination (Granger, et al., 2012), participants were asked to refrain from eating, drinking (other than water), and tooth brushing for at least 2 hours before arriving at the laboratory. The first baseline measure was collected at approximately 1615 hrs, and the second baseline measure was collected approximately 25 minutes later. The TSST-C began at approximately 1700 hrs; 3 saliva samples were collected approximately 20 minutes apart following exposure to the final stressor. Saliva samples were assayed in duplicate using highly-sensitive enzyme immunoassays for salivary cortisol without modification to the manufacturer’s recommended protocol (Salimetrics LLC, State College, PA). The test used 25 ul of saliva and had a lower limit of sensitivity of .003 ug/dl, and a range of sensitivity from .003 to 3.0 ug/dl, and average intra- and inter-assay coefficients of variation of less than 5% and 10%.
Figure 1.
Saliva sample collection periods. Two baseline samples were collected prior to administration of the Trier Social Stress Test for Children (TSST-C). Three additional samples were collected 20 minutes apart after the TSST-C ended.
Eating in the Absence of Hunger
was measured using the Free Access Procedure (Fisher & Birch, 1999a). Approximately 20 minutes following a standard, ad-libitum lunch, children were individually interviewed and asked to indicate their hunger level on a 3-point scale (hungry, in-between or full. Following the hunger assessment, children were given free access to generous portions of 10 snack foods, along with a variety of toys/activities, and were told that that they can use the toys/activities or eat any of the foods while the interviewer took care of some homework in the adjacent room for 10 minutes. Using manufacturer’s nutrient information, caloric intake during the 10-minute period was calculated by summing the caloric intake of all the snack foods consumed.
Weight Status
Height and weight measurements were obtained by trained research assistants in order to determine body mass indices (BMI percentiles and z-scores) using age- and gender-specific reference data for children (Kuczmarski, 2000). Children with BMI percentiles ≥95 were classified as overweight. BMI z-score (BMIz) was used as the main BMI criterion variable in regression models.
Child Temperament and Behavior
Caregivers’ reports on various dimensions of their child’s self-regulation and behavior were assessed using the Children’s Behavior Questionnaire (CBQ, (Rothbart, Ahadi, Hershey, & Fisher, 2001) For the purposes of this study, the inhibitory control and impulsivity subscales were used. Inhibitory control is defined as “the capacity to plan and to suppress inappropriate approach responses under instructions or in novel or uncertain situations.” An example of an item from the inhibitory control scale is: “My child is usually able to resist temptation when told he/she is not supposed to do something.” Impulsivity is defined as “the speed of response initiation,” or the inability to think before you act. An example of an item from the impulsivity subscale is, “My child usually rushes into an activity without thinking about it.” Internal consistency estimates for caregivers in this sample were 0.72 and 0.77 for the inhibitory control and impulsivity subscales, respectively. Both inhibitory control and impulsivity have been linked to BMI and dysregulated eating in young children (Anzman & Birch, 2009; Nederkoorn, 2006).
Statistical Analyses
Data were available on 43 children, and 38 caregivers. Using the formula described by Pruessner and colleagues (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003), area under the curve was calculated to provide a repeated-measures indicator of total cortisol secretion over the course of the procedure. Area under the curve with respect to the ground (AUCg) is a measure of the total area under the curve for all 5 cortisol measurements. As described by Fekedulegn et al. (2007) and Pruessner et al. (2003), AUCg provides a measure of total hormonal output by calculating the differences between each cortisol measurement (sensitivity), as well as intensity, which is calculated as the distance of all cortisol measures from zero, or the point at which change occurs. Area under the curve with respect to the increase (AUCi) does not take the distance from zero of all measurements into account. This measure highlights changes over time from baseline, thus providing a measure of person-specific changes in cortisol output over the course of the procedure, which is related to sensitivity of the system (Granger, et al., 2012). For the purposes of this paper, we focused analyses on AUCi.
Five children did not have a fifth saliva sample collected, thus, the AUCi score includes only four measures for these children. Given the abnormal distribution of the data, natural logarithmic transformations were performed on cortisol values, before AUC calculations (Gordis, Granger, Susman, & Trickett, 2006), and high cortisol values for one extreme outlier were changed to 3 SD above the mean. Analyses were conducted with and without this outlier, and the main pattern of results did not differ. Given that the stressor we used was validated on children as young as 8 years, we chose to stratify the sample by age in order to examine within-group associations between AUCi, eating in the absence of hunger, and BMIz. Children were group into younger (5 to 7 years; N=32) and older (8 to 9 years; N=11) age groups. Because we were interested in grouping children based on age, the small sample size limited our ability to conduct further analyses by gender or race.
Descriptive and correlation analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC). Multiple-group regression analysis was conducted using AMOS version 18 (SPSS Inc., Chicago, IL), and was used to examine relations between AUCi, eating in the absence of hunger, and BMI. Using this approach, identical regression models are run separately and simultaneously by group. Regression models were adjusted for the potential influence of parental education.
RESULTS
Sample Characteristics
Table 2 presents descriptive data on children in the sample. Overall, children in the sample were within the normal weight range based on BMI percentiles; approximately 19% of children in the sample were categorized as overweight (BMI percentile ≥ 85th). On average, children consumed just over 250 kcals during the Free Access procedure, although some children consumed very little (20 kcals) while other consumed upwards of 700 kcals during the 10-minute period.
Table 2.
Descriptive Sample Information
| Variable | Mean (SD) | Range |
|---|---|---|
| Child Age (yrs) | 6.6 (1.4) | 5.0 – 9.0 |
| Child BMIz | 0.3 (1.3) | −3.1 – 4.4 |
| Child BMI percentile | 58.8 | 0.1 – 99.9 |
|
Child eating in the absence of hunger (kcals) |
257.1 (164.8) | 20.0 – 704.1 |
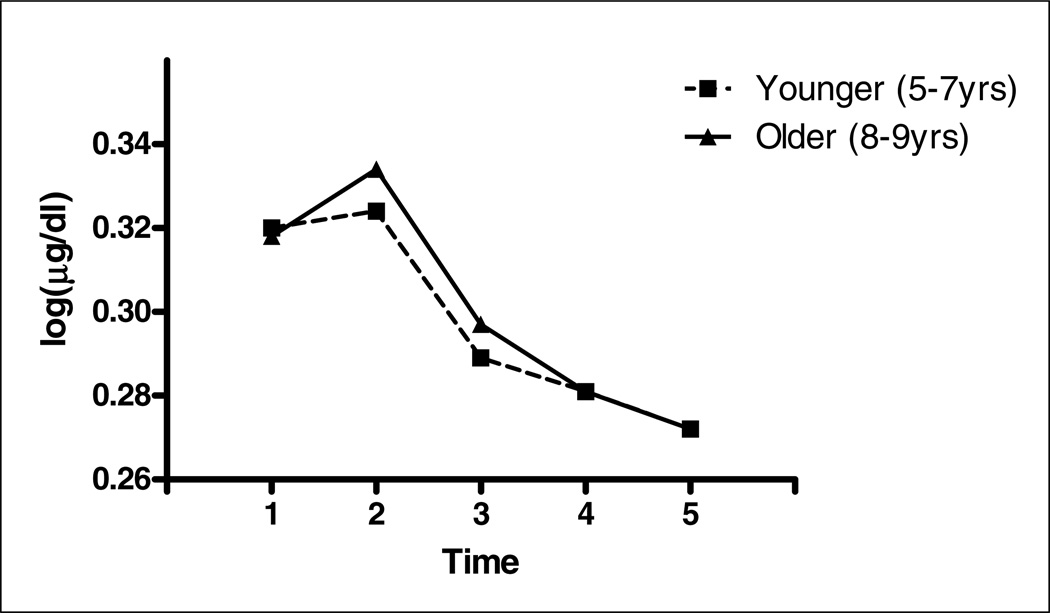
Figure 2 displays the overall pattern of cortisol reactivity and recovery by groupings based on child age (younger/older). Preliminary analyses revealed that there were no significant differences in baseline cortisol by age (F=0.39; p=.54). In general, regardless of age, most children appeared to initially react to the novel, clinical setting upon arrival. Differential patterns of adrenocortical reactivity showed that some children did not exhibit high levels of cortisol upon arrival. Approximately 26% (11/43) of children showed at least a 10% increase in cortisol in response to the stressor (baseline to ~40 min post-stressor).
Figure 2.
Cortisol output by age.
Cortisol, BMIz, and Eating in the Absence of Hunger by Age
Standardized regression estimates appear in Table 3. Results revealed that AUCi was significantly and positively associated with BMIz and energy intake in the absence of hunger, but only in older children. That is, older children who exhibited greater cortisol release over the course of the procedure had higher BMI z-scores scores, and consumed a greater number of calories in the absence of hunger. Parental education was also a significant predictor of BMIz in older children, with higher BMIz scores being associated with lower reported levels of parental education (p<.01). Impulsivity was the only variable associated BMIz in younger children; children with higher BMIz had parents who rated them higher in impulsivity; no other associations were seen for younger children. For younger children, the model R2 estimates were .41 and .01 for BMIz and eating in the absence of hunger, respectively, and .52 and .50 for older children.
Table 3.
Regression coefficients for relations among area under the curve with respect to increase (AUCi), child body mass index z-score (BMIz), eating in the absence of hunger, and impulsivity; by age group.
| YOUNGER (5–7yrs) (N=32) |
OLDER (8–9yrs) (N=11) |
|||
|---|---|---|---|---|
| B (SE) | β | B (SE) | β (SE) | |
| Relations with AUCi | ||||
| BMIz (kg/m2) | 0.07 (.20) | .05 | 1.38 (.47)** | .89** |
|
Eating in the Absence of Hunger (kcals) |
8.54 (27.98) | .06 |
253.68 (109.43)* |
.69* |
| Relations with Parents’ Report of Child IMPULSIVITY | ||||
| BMIz (kg/m2) | 0.96 (.22)*** | .63*** | 0.47 (.53) | .31 |
|
Eating in the Absence of Hunger (kcals) |
12.78 (32.21) | .08 | 40.20 (124.26) |
.11 |
| R2 for BMIz | .41 | .52 | ||
|
R2 for Eating in the Absence of Hunger |
.01 | .50 | ||
Note: All models are adjusted for parental combined education.
SE = Standard error of measurement
p≤.05,
p≤.01,
p≤.001
We also dissected the cortisol curve to better understand pre- and post-stress responses (data not shown). In older children, greater cortisol output from baseline to immediate post-stress was related to higher levels of eating in the absence of hunger (r=.66, p≤.05). Furthermore, greater cortisol output after the stressor had passed (low recovery) was associated with higher levels of eating in the absence of hunger (r=.71, p≤.05).
DISCUSSION
This study was designed to examine associations between individual differences in cortisol output in response to stress, eating in the absence of hunger and BMI z-scores in 5- to 9-year-old children. The results from the study provide evidence for adrenocortical output as a potential marker for problems beyond general behavioral and psychological domains of child development. Area under the curve with respect to increase (AUCi) was related to children’s eating in the absence of hunger and BMI, but only in children ages 8 to 11 years. Specifically, 8- to 11-year old children who exhibited greater AUCi over the course of the stress procedure had higher BMI z-scores and exhibited greater eating in the absence of hunger. In 5- to 7-year-old children greater levels of mother-reported impulsivity were related higher BMI z-scores. Although we did not experimentally examine the effects of exposure to an acute stressor on energy intake in this study, all children were exposed to the stressor, and later participated in the eating in the absence of hunger protocol.
We found no similar published studies on stress reactivity and eating in the absence of hunger in young children, however, findings from studies with adult samples provide partial corroboration for our results. In an experimental study on stress eating in adults, Epel and colleagues (2001) found that women who exhibited greater cortisol output on a day in which they encountered a psychosocial stressor, consumed more calories during a snacking procedure compared to women who exhibited lower cortisol output on the stress day. Similarly, Gluck and colleagues (2004) reported that women with binge-eating disorder (BED) exhibited greater cortisol output in response to a physiological stressor, compared to non-BED women. Similar findings have also been reported in women with other disordered eating profiles, and show that cardiovascular reactivity also distinguishes between disordered and non-disordered eaters (Koo-Loeb, Costello, Light, & Girdler, 2000; Koo-Loeb, Pedersen, & Girdler, 1998). Results from another study revealed that peak cortisol levels after an injection of corticotropin-releasing hormone (CRH) were related to increased energy intake in healthy, non-obese adults (George, Khan, Briggs, & Abelson, 2010); this study provides evidence for the causal effects of cortisol on food intake. Taken together, these findings highlight cortisol output as an important factor in the energy balance equation.
Early childhood self-regulatory problems (i.e., self-control and delay of gratification) have been shown to be important determinants of risk for overweight (Graziano, Calkins, & Keane, 2010). Of note is that self-regulatory problems were shown to be longitudinal predictors of rapid weight gain from early childhood through early adolescence (Seeyave, et al., 2009; Tsukayama, Toomey, Faith, & Duckworth, 2010). Given that adrenocortical reactivity is considered a biomarker for self-regulation, the findings from the current study suggest that self-regulation failure in early childhood may predispose children to excessive weight gain, indicating a shared mechanism between the behavioral and energy balance domains of development. Our results show that children’s cortisol response to stress was related to children’s eating in the absence of hunger, a context in which energy-dense food is plentiful and easily accessible, but this was only true for older children (ages 8 to 9 years). If we were to extrapolate from the eating in the absence of hunger paradigm to the larger, obesigenic environment in which palatable, energy-dense foods are highly available, the results from the study suggest that children with maladaptive responses to stress are at a heightened risk for developing problems with obesity, partly due to dysregulated eating behavior.
Findings from several rodent studies provide evidence for the link between stress, HPA-axis activity, increased food intake, and adiposity (Dallman, Pecoraro, & la Fleur, 2005; Ely, et al., 1997; Hagan, et al., 2002; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004; Rowland & Antelman, 1976; Wallach, Dawber, Mcmahon, & Rogers, 1977). These studies show that both acute (e.g., tail pinch) and chronic stressors (e.g., noise) can result in increased or hyperphagic eating. Furthermore, factors such as previous restriction or hunger can exacerbate these effects (Hagan, Chandler, Wauford, Rybak, & Oswald, 2003; Hagan, et al., 2002). Translated to human development and behavior, it is possible that such factors as living in high-stress environments (e.g., poverty, crime), and exposure to restriction (e.g., food insecurity, or controlling child-feeding practices) may increase eating in the absence of hunger, and increase children’s risk for obesity. These environmental factors have previously been found to be related to children’s dysregulated eating behavior (Birch, Fisher, & Davison, 2003; Fisher & Birch, 1999a, 1999b) and risk for obesity (Faith, 2006; Fisher, Birch, 2002; Francis, Ventura, Marini, & Birch, 2007; Larson & Story, 2011).
We are limited by our small, racially and economically homogenous sample, which precludes generalizability of the findings to more diverse populations. In addition, whereas approximately 26% of children in this study showed more than a 10% increase in cortisol in response to the stressor, the large majority of children showed decreases from baseline to immediate post-stressor. The decrease in reactivity suggests that children may have been reacting to the novel, research environment upon arrival. We also did not have a self-report measure of children’s perceived stress following the stressor, which may have provided an opportunity to better understand characteristics of children who appeared to be most sensitive to the stressor. Although some developmental studies have yielded consistent mean-level differences in patterns of cortisol response following exposure to a stressful or novel event, a wide range of inter-individual differences in stress-related reactivity is expected. Granger and colleagues note that in response to the mild-to-moderate type stressors typically employed in developmental science, approximately 20–30% of individuals will exhibit a salivary cortisol increase over pre-task levels that are at least greater than 10% of the pre-task level (Evans, Fuller-Rowell, & Doan, 2012; Granger, et al., 2012). Unfortunately, there are many individual differences in prior life experience that we were unable to assess in the present study (e.g. insensitive parenting; (Roisman, et al., 2009). Those background differences would certainly contribute to how children individually approached, responded to and coped with the stressors.
In sum, the findings from this study indicate that individual differences in HPA-axis reactivity in response to psychosocial stress may be related, in different ways, to eating in the absence of hunger and BMI in young children. The approach to understanding relations between cortisol output and regulation of food intake and body weight is a promising area of inquiry with regard to obesity. Our findings confirm a link between cortisol output and energy balance in young children, and corroborate similar findings in adults and animal models. The results also highlight the promise of a minimally invasive technique to measure individual differences in salivary hormones, which afforded the opportunity to sample and study this same relationship in children. This collection technique enables us to measure this phenomenon in children’s everyday social worlds. Increased attention has been focused on developing a better understanding of individual differences in biological sensitivity to context, which suggests that an individual’s response to stress is highly dependent on the context in which they exist (Boyce & Ellis, 2005; Ellis & Boyce, 2008,Ellis & Boyce, 2008; Ellis, Essex, & Boyce, 2005; Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010). While the current study was not designed to test this theory, the findings suggest the need to account for an individual’s biological and behavioral reactivity in a specific context in order to better understand health and developmental outcomes, as well as their antecedents and correlates.
Highlights.
We examined links between stress reactivity, eating behavior and body mass index in 5- to 9-year-old children
Greater increases in cortisol in response to stress were related to dysregulated eating and higher body mass
Stress reactivity may be a marker for energy balance dysregulation
ACKNOWLEDGEMENTS
We would like to thank the families who participated in this study for their time. The services provided by the General Clinical Research Center of The Pennsylvania State University are greatly appreciated. The study was supported by funding from the Children, Youth and Families Consortium at The Pennsylvania State University and NIH Grant M01 RR 10732. We appreciate the feedback we received on this manuscript from Drs. Laura Klein and Sheila West at the Pennsylvania State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
In the interest of full disclosure, Dr. Granger is the founder and Chief Scientific and Strategy advisor at Salimetrics LLC (State College, PA) and this relationship is managed by the policies of the committee on conflict of interest at the Johns Hopkins University School of Medicine. The authors have no additional conflicts of interest to declare.
REFERENCES
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology and Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood - A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzman SL, Birch LL. Low Inhibitory Control and Restrictive Feeding Practices Predict Weight Outcomes. Journal of Pediatrics. 2009;155:651–656. doi: 10.1016/j.jpeds.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balantekin KN, Roemmich JN. Children's coping after psychological stress. Choices among food, physical activity, and television. Appetite. 2012;59:298–304. doi: 10.1016/j.appet.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Clement K, Le Barzic M, Le Gall A, Guy-Grand B, Basdevant A. The Eating Inventory and body adiposity from leanness to massive obesity: a study of 2509 adults. Obesity Research. 2004;12:2023–2030. doi: 10.1038/oby.2004.253. [DOI] [PubMed] [Google Scholar]
- Birch LL, Fisher JO, Davison KK. Learning to overeat: Maternal use of restrictive feeding practices promotes girls' eating in the absence of hunger. American Journal of Clinical Nutrition. 2003;78:215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. International Journal of Obesity. 2000;24:S80–S85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond P. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Born JM, Lemmens SGT, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, Westerterp-Plantenga MS. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. International Journal of Obesity. 2010;34:172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Cartwright M, Wardle J, Steggles N, Simon AE, Croker H, Jarvis MJ. Stress and dietary practices in adolescents. Health Psychology. 2003;22:362–369. doi: 10.1037/0278-6133.22.4.362. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. International Journal of Obesity. 2000;24:S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. The impact of social experience on neurobiological systems: illustration from a constructivist view of child maltreatment. Cognitive Development. 2002;17:1407–1428. [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: A new view of "comfort food". Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behavior and Immunity. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dockray S, Susman EJ, Dorn LD. Depression, Cortisol Reactivity, and Obesity in Childhood and Adolescence. Journal of Adolescent Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau V, Therrien F, Richard D, Tremblay A. Is visceral obesity a physiological adaptation to stress? Panminerva Medica. 2003;45:189–195. [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong MX, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Preventive Medicine. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ely DR, Dapper V, Marasca J, Correa JB, Gamaro GD, Xavier MH, Michalowski MB, Catelli D, Rosat R, Ferreira MBC, Dalmaz C. Effect of restraint stress on feeding behavior of rats. Physiology & Behavior. 1997;61:395–398. doi: 10.1016/s0031-9384(96)00450-7. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Evans GW, Boxhill L, Pinkaya M. Poverty and maternal responsiveness: The role of maternal stress and social resources. International Journal of Behavioral Development. 2008;32:232–237. [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Fuller-Rowell TE, Doan SN. Childhood Cumulative Risk and Obesity: The Mediating Role of Self-Regulatory Ability. Pediatrics. 2012;129:E68–E73. doi: 10.1542/peds.2010-3647. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health - Cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: A genetic marker for childhood obesity in prepubertal boys? Obesity Research. 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Biolanti JM, Hartley TA, Charles LE, Miller DB. Area Under the Curve and Other Summary Indicators of Repeated Waking Cortisol Measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Restricting access to foods and children's eating. Appetite. 1999a;32:405–419. doi: 10.1006/appe.1999.0231. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. American Journal of Clinical Nutrition. 1999b;69:1264–1272. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. American Journal of Clinical Nutrition. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters' increase in BMI and disinhibited overeating from 5 to 13 years. Obesity. 2007;15:1544–1553. doi: 10.1038/oby.2007.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis LA, Susman EJ. Self-Regulation and Rapid Weight Gain in Children from Age 3 to 12 years. Archives of Pediatrics & Adolescent Medicine. 2009;163(4):297–302. doi: 10.1001/archpediatrics.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiology and Behavior. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Gluck ME. Stress response and binge eating disorder. Appetite. 2006;46:26–30. doi: 10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosomatic Medicine. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Lorence M. Cortisol stress response is positively correlated with central obesity in obese women with binge eating disorder (BED) before and after cognitive-behavioral treatment. Biobehavioral Stress Response: Protective and Damaging Effects. 2004;Vol. 1032:202–207. doi: 10.1196/annals.1314.021. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D. Focus on Methodology: Salivary bioscience and research on adolescence: An integrated perspective. Journal of Adolescence. 2012;35:1081–1095. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Graziano PA, Calkins SD, Keane SP. Toddler self-regulation skills predict risk for pediatric obesity. International Journal of Obesity. 2010;34:633–641. doi: 10.1038/ijo.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-Induced Eating. Psychological Bulletin. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Gundersen C, Mahatmya D, Garasky S, Lohman B. Linking psychosocial stressors and childhood obesity. Obesity Reviews. 2011;12:e54–e63. doi: 10.1111/j.1467-789X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. International Journal of Eating Disorders. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: Key synergistic role of past caloric restriction and stress. Physiology & Behavior. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Jenkins SK, Rew L, Sternglanz RW. Eating behaviors among school-age children associated with perceptions of stress. Issues Compr Pediatr Nurs. 2005;28:175–191. doi: 10.1080/01460860500227580. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test - a Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koo-Loeb JH, Costello N, Light KC, Girdler SS. Women with eating disorder tendencies display altered cardiovascular, neuroendocrine, and psychosocial profiles. Psychosomatic Medicine. 2000;62:539–548. doi: 10.1097/00006842-200007000-00013. [DOI] [PubMed] [Google Scholar]
- Koo-Loeb JH, Pedersen C, Girdler SS. Blunted cardiovascular and catecholamine stress reactivity in women with bulimia nervosa. Psychiatry Research. 1998;80:13–27. doi: 10.1016/s0165-1781(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. American Journal of Clinical Nutrition. 2000;72:1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Stress, Obesity, and Metabolic Syndrome. 2006;Vol. 1083:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- Larson NI, Story MT. Food Insecurity and Weight Status Among US Children and Families A Review of the Literature. American Journal of Preventive Medicine. 2011;40:166–173. doi: 10.1016/j.amepre.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eating Behaviors. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiology and Behavior. 2008;94:169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- O'Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychology. 2008;27:S20–S31. doi: 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological Sensitivity to Context: The Interactive Effects of Stress Reactivity and Family Adversity on Socioemotional Behavior and School Readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. Jama-Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Lambiase MJ, Lobarinas CL, Balantekin KN. Interactive effects of dietary restraint and adiposity on stress-induced eating and the food choice of children. Eating Behaviors. 2011;12:309–312. doi: 10.1016/j.eatbeh.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Smith JR, Epstein LH, Lambiase M. Stress reactivity and adiposity of youth. Obesity. 2007;15:2303–2310. doi: 10.1038/oby.2007.273. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Wright SM, Epstein LH. Dietary restraint and stress-induced snacking in youth. Obesity Research. 2002;10:1120–1126. doi: 10.1038/oby.2002.152. [DOI] [PubMed] [Google Scholar]
- Rogers PJ. Eating habits and appetite control: a psychobiological perspective. Proceedings of the Nutrition Society. 1999;58:59–67. doi: 10.1079/pns19990009. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-LaForce C, Owen MT, Belsky J, Bradley RH, Houts R, Steinberg L Network, N. E. C. C. R. Early Family and Child-Care Antecedents of Awakening Cortisol Levels in Adolescence. Child Development. 2009;80:907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the Children's Behavior Questionnaire. Child Dev. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Antelman SM. Stress-Induced Hyperphagia and Obesity in Rats - Possible Model for Understanding Human Obesity. Science. 1976;191:310–312. doi: 10.1126/science.1246617. [DOI] [PubMed] [Google Scholar]
- Rutter M, Dunn J, Plomin R, Simonoff E, et al. Integrating nature and nurture: Implications of person-environment correlations and interactions for developmental psychopathology. Development & Psychopathology. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Rutters F, Nieuwenhuizen AG, Lemmens SGT, Born JM, Westerterp-Plantenga MS. Acute Stress-related Changes in Eating in the Absence of Hunger. Obesity. 2009;17:72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- Seeyave DM, Coleman S, Appugliese D, Corwyn RF, Bradley RH, Davidson NS, Kaciroti N, Lumeng JC. Ability to Delay Gratification at Age 4 Years and Risk of Overweight at Age 11 Years. Archives of Pediatrics and Adolescent Medicine. 2009;163:303–308. doi: 10.1001/archpediatrics.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Tilbrook A. The glucocorticoid contribution to obesity. Stress-the International Journal on the Biology of Stress. 2011;14:233–246. doi: 10.3109/10253890.2010.534831. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. International Journal of Obesity. 2004;28:1168–1173. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. American Journal of Physiology-Endocrinology and Metabolism. 1996;34:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36:1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukayama E, Duckworth AL, Geier AB. Self-controlled children stay leaner in the transition to adolescence. Appetite. 2010;54:304–308. doi: 10.1016/j.appet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukayama E, Toomey SL, Faith MS, Duckworth AL. Self-control as a Protective Factor Against Overweight Status in the Transition From Childhood to Adolescence. Archives of Pediatrics and Adolescent Medicine. 2010;164:631–635. doi: 10.1001/archpediatrics.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach MB, Dawber M, Mcmahon M, Rogers C. New Anorexigen Assay - Stress-Induced Hyperphagia in Rats. Pharmacology Biochemistry and Behavior. 1977;6:529–531. doi: 10.1016/0091-3057(77)90112-5. [DOI] [PubMed] [Google Scholar]
- Wallerius S, Rosmond R, Ljung T, Holm G, Bjorntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: A preliminary report. Journal of Endocrinological Investigation. 2003;26:616–619. doi: 10.1007/BF03347017. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiology and Behavior. 2006;87:789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]