Abstract
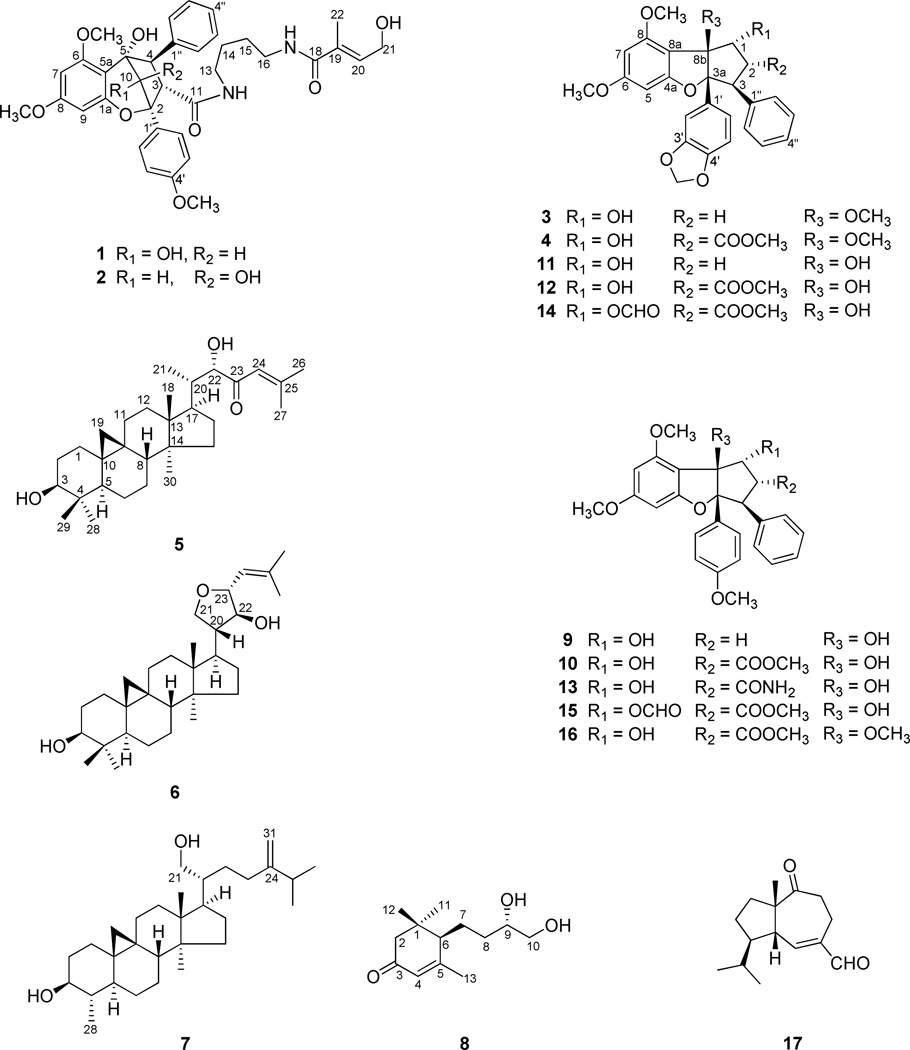
Eight new compounds, including two cyclopenta[b]benzopyran derivatives (1, 2), two cyclopenta[b]benzofuran derivatives (3, 4), three cycloartane triterpenoids (5–7), and an apocarotenoid (8), together with 16 known compounds, were isolated from the chloroform-soluble partitions of separate methanol extracts of a combination of the fruits leaves and twigs, and of the roots of Aglaia perviridis collected in Vietnam. Isolation work was monitored using human colon cancer cells (HT-29) and facilitated with an LC/MS dereplication procedure. The structures of the new compounds (1–8) were determined on the basis of spectroscopic data interpretation. The Mosher ester method was employed to determine the absolute configurations of 5–7, and the absolute configurations of the 9,10-diol unit of compound 8 was established by a dimolybdenum tetraacetate [Mo2(AcO)4] induced circular dichroism (ICD) procedure. Seven known rocaglate derivatives (9–15) exhibited significant cytotoxicity against the HT-29 cell line, with rocaglaol (9) being the most potent (ED50 0.0007 µM). The new compounds 2–4 were also active against this cell line, with ED50 values ranging from 0.46 to 4.7 µM. The cytotoxic compounds were evaluated against a normal colon cell line, CCD-112CoN. In addition, the new compound perviridicin B (2), three known rocaglate derivatives (9, 11, 12), as well as a known sesquiterpene, 2-oxaisodauc-5-en-12-al (17), showed significant NF-κB (p65) inhibitory activity in an ELISA assay.
Aglaia Lour., containing more than 120 species, is the largest genus of the plant family Meliaceae.1 Aglaia species have attracted considerable interest in the area of natural products research, since they are a rich source of the “flavagline” class of bioactive agents. Flavagline derivatives have been proposed to be biogenetically derived from the coupling of a flavonoid unit and a cinnamic acid amide moiety, and can be divided into three subtypes, namely, the cyclopenta[b]benzofurans, the cyclopenta[b]benzopyrans, and the benzo[b]oxepines.2–5 More than 100 flavaglines have been isolated from over 30 Aglaia species to date.2–5
In a search for new anticancer agents from tropical plants, several Aglaia species, including Aglaia crassinervia,6 A. edulis,7 A. elliptica,8 A. foveolata,9–11A. ponapensis,12 and A. rubiginosa,13 mainly collected from Indonesia, have been investigated previously as promising candidate plants in our laboratories for phytochemical investigation. Cyclopenta[b]benzopyran derivatives, with most of them being rocaglaol and related analogues, and triterpenoids, mainly of the glabretal-, baccharane-, and dammarane-types, were isolated as the major constituents from the above-mentioned taxa.6–13 Among the flavaglines from Aglaia species, two cyclopenta[b]benzofurans have been reported to exhibit inhibitory activity in vivo in tumor-bearing experimental animals,2–5 namely, rocaglamide14 and silvestrol.9,15–18 Silvestrol was isolated and fully structurally characterized from A. foveolata Pannell9, obtained from Kalimantan, Indonesia, and also obtained from A. stellatopilosa Pannell,15,19 collected in Sarawak, Malaysia. It may be noted that the taxonomic resolution of the complex species A. leptantha in Borneo resulted in the recognition of three separate species, namely, A. leptantha Miq., A. glabriflora Hiern, and the new A. stellatopilosa Pannell, with the latter endemic to Borneo.19 Silvestrol has been synthesized by the Porco20 and Rizzacasa21,22 groups, and found to be a translation inhibitor.23 Silvestrol has been accepted for preclinical development through the NeXT program of the U.S. National Cancer Institute as a result of its potential use in treating B-cell malignancies.17,18,24
Aglaia perviridis Hiern (Meliaceae), a tree up to 15 m tall, is distributed in the forest regions of southern mainland China, Bangladesh, Bhutan, India, the Indian Ocean islands, Laos, Malaysia, Thailand, and Vietnam.25 Previous phytochemical studies on this plant have led to the isolation of bisamides, lignans, sterols, sesquiterpenes, and triterpenes.26–29 Among these compounds, two bisamides were reported as being biologically active for inhibition of their NO production.28 Thus far, no antiproliferative agents have been isolated from this plant. In the present investigation, a CHCl3 extract of a combination of leaves, twigs, and fruits of A. perviridis collected in Vietnam was found to exhibit cytotoxic activity (IC50 3.0 µg/mL) against human colon cancer (HT-29) cells. Subsequent bioassay-guided fractionation conducted using the same cell line led to the isolation of two new cyclopenta[b]benzopyrans, perviridisins A and B (1 and 2), three new cycloartane triterpenoids, perviridisinols A–C (5–7), a new apocarotenoid, (6R,9S)-9,10-dihydroxy-4-megastigmen-3-one (8), together with 12 known compounds, including five rocaglate derivatives, rocaglaol (9),30 methyl rocaglate (10),30 4′-demethoxy-3′,4′-methylenedioxyrocaglaol (11),7 methyl 4′-demethoxy-3′,4′-methylenedioxyrocaglate (12),7 didesmethylrocaglamide (13),31 a bisamide, gigantamide A,32 a sesquiterpene, 2-oxaisodauc-5-en-12-al (17),33,34 scopoletin,35 5,7,4′-tri-O-methylkaempferol,36 as well as three triterpenes, cabraleahydroxylactone,6 24-methylenecycloartan-3β,21-diol,37 and argenteanol.38
The CHCl3 extract of the roots of A. perviridis was also found to be very active when evaluated for cytotoxicity against HT-29 cells (ED50 0.2 µg/mL). To avoid the re-isolation of only the same cytotoxic agents as from the plant parts investigated above, the CHCl3-soluble extract of A. perviridis root was subjected to an LC-MS dereplication procedure, which revealed the presence of four rocaglaol derivatives (9–12). In addition, the occurrence of unknown cytotoxic compounds was suggested with possible molecular formulas of C29H28O9 and C27H26O7. Further fractionation on this extract led to the purification of six additional rocaglaol derivatives, including two new cyclopenta[b]benzofurans, 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglaol (3) and methyl 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglate (4), along with four rare known rocaglates, methyl 1-formyloxy-4′-demethoxy-3′,4′-methylenedioxyrocaglate (14),7 methyl 1-formyloxyrocaglate (15),39 8b-O-methylrocaglaol,40 and methyl 8b-O-methylrocaglate (16).40 The isolates were tested for their cytotoxicity against the HT-29 cancer cell line as well as the normal colon cell line, CCD-112CoN. Finally, the NF-κB (p65) inhibitory effects of these compounds were also evaluated using an ELISA assay.
RESULTS AND DISCUSSION
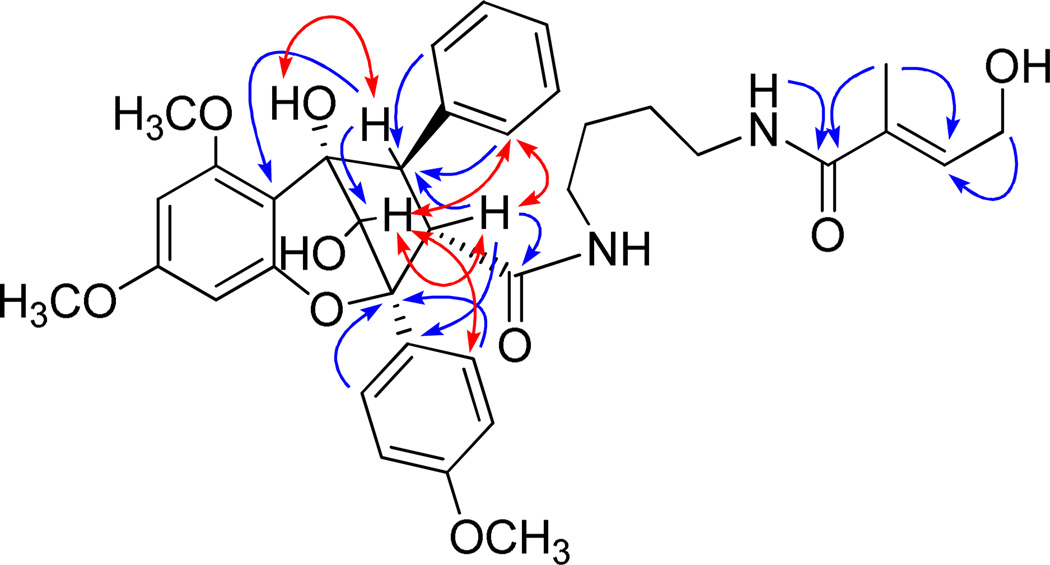
The molecular formula of compound 1 was determined as C36H42N2O10 based on the [M + Na]+ ion peak at m/z 669.2809 (calcd 669.2788) in the HRESIMS. In the 1H NMR spectrum, the 11 protons in the low-field region could be attributed to three aromatic rings, including a monosubstituted benzene ring at δH 7.29–7.40 (5H, m, H-2″-6″), a 1,4-disubstituted benzene ring at δH 7.06 (2H, d, J = 8.9 Hz, H-3′, 5′) and 7.70 (2H, d, J = 8.9 Hz, H-2′, 6′), as well as a 1,2,3,5 tetrasubstituted benzene ring at δH 6.16 (1H, d, J = 2.2 Hz, H-7) and 6.19 (1H, d, J = 2.3 Hz, H-9). Also observed were an oxygenated methine singlet at δH 4.67 (1H, s, H-10), two methine protons at δH 3.50 (1H, d, J = 5.2 Hz, H-3) and 4.30 (1H, d, J = 5.4 Hz, H-4), which coupled to each other in the 1H-1H COSY spectrum as well as three methoxy groups at δH 3.88 (3H, s, OCH3-6), 3.87 (3H, s, OCH3-4′), and 3.76 (3H, s, OCH3-8) (Table 1). These proton signals suggested that 1 is based on a typical cyclopenta[b]benzopyran skeleton as found previously in several other Aglaia species.3,10,12,41,42 Besides the signals ascribed to the general cyclopenta[b]benzopyran skeletal feature, a putrescinyl 4-hydroxytiglate moiety was recognized. The latter was based on the carbon signals of four CH2 groups at δC 38.3 (C-13), 27.9 (C-14), 26.2 (C-15) and 39.8 (C-16), a carbonyl group at δC 169.6 (C-18), a trisubstituted double bond at δC 133.3 (C-19) and 133.7 (C-20), an oxygenated methylene at δC 59.8 (C-21), as well as a methyl group at δC 13.4 (C-22). These signals were consistent with the proton resonances of two alkyl vicinal methylenes at δH 1.35 (2H, m, H-14) and 1.44 (2H, m, H-15), two N-vicinal methylenes at δH 2.87 and 3.30 (each 1H, m, H-13), and 3.23 (2H, m, H-16), an olefinic proton at δH 6.27 (1H, brt, J = 5.9 Hz, H-20), an oxygenated methylene at δH 4.27 (2H, brd, J = 5.8 Hz, H-21), as well as a methyl group located on the double bond at δH 1.82 (3H, s, H-22) (Table 1). The location of this putrescinyl 4-hydroxytiglate group on C-3 through an amide linkage was deduced by the HMBC correlation between H-3 with the carbonyl group C-11. Only two cyclopenta[b]benzopyran derivatives isolated from A. dasyclada have been reported to possess the same amidic putrescinyl 4-hydroxytiglate group.43 The connection of the aromatic ring with C-4 was confirmed by HMBC correlations of H-2″ and H-6″ with C-4. Further key HMBC correlations of H-4 with C-3, C-5, C-5a, and C-10, H-3 with C-4 and C-1′, H-10 with C-5 and C-5a, H-2′ and 6′ with C-2, as well as H-9 with C-9a supported the proposed cyclopenta[b]benzopyran skeleton of 1 (Figure 1). According to the literature, H-3β and H-4α substituents could be suggested based on the coupling constant of 5.4 Hz between H-3 and H-4.10,44 In addition, if there were a C-4 α-oriented phenyl ring, the 6-OCH3 protons would be shielded from around δH 3.88 to approximately 3.11.10,43 In the 1H NMR spectrum of compound 1, the 6-OCH3 protons appeared at δH 3.88, which implied the absence of any shielding effect from the benzene ring, and confirmed the β orientation of the C-4 phenyl group. The observed NOESY cross peaks of H-10/H-3, H-2′(6′) and H-2″(6″), as well as H-3/H-2′(6′) and H-2″(6″), further supported the 3-βH and 4-αH orientation and established the endo relationship between H-10 and H-3 (Figure 1). Thus, the structure of compound 1 was elucidated as shown, and this substance has been accorded the trivial name, perviridisin A.
Table 1.
1H and 13C NMR Spectroscopic Data of Compounds 1 and 2a
| position | 1 | 2 | ||
|---|---|---|---|---|
| δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | |
| 2 | 84.7 | 87.1 | ||
| 3 | 3.50, d (5.2) | 60.0 | 4.28, d (6.7) | 60.3 |
| 4 | 4.30, d (5.4) | 54.7 | 4.40, d (6.5) | 58.1 |
| 5 | 80.0 | 79.9 | ||
| 5-OH | 5.38, s | 5.23, s | ||
| 5a | 108.4 | 112.0 | ||
| 6 | 158.5 | 156.2 | ||
| 7 | 6.16, d (2.2) | 93.4 | 6.14, d (2.1) | 92.6 |
| 8 | 160.8 | 160.4 | ||
| 9 | 6.19, d (2.3) | 93.9 | 6.16, d (2.2) | 94.0 |
| 9a | 153.8 | 153.0 | ||
| 10 | 4.67, s | 76.2 | 4.20, s | 82.4 |
| 10-OH | nd | 2.97, brs | ||
| 11 | 168.7 | 169.5 | ||
| NH-12 | 5.04, brt (6.2) | 5.21, brt (6.4) | ||
| 13 | 2.87, m | 38.3 | 2.86, m | 38.5 |
| 3.30, m | 3.17, m | |||
| 14 | 1.35, m | 27.9 | 1.33, m | 27.3 |
| 15 | 1.44, m | 26.2 | 1.60, m | 25.6 |
| 16 | 3.23, m | 39.8 | 3.26, m | 39.4 |
| NH-17 | 6.15, brt (6.0) | 6.10, brt (5.2) | ||
| 18 | 169.6 | 169.1 | ||
| 19 | 133.3 | 133.0 | ||
| 20 | 6.27, brt (5.9) | 133.7 | 6.27, brt (6.0) | 133.1 |
| 21 | 4.27, brd (5.8) | 59.8 | 4.27b | 59.4 |
| 22 | 1.82, s | 13.4 | 1.82, s | 13.0 |
| 1′ | 131.3 | 129.6 | ||
| 2′,6′ | 7.70, d (8.9) | 127.2 | 7.84, d (8.9) | 128.3 |
| 3′,5′ | 7.06, d (8.9) | 115.0 | 7.05, d (8.9) | 114.0 |
| 4′ | 160.0 | 159.6 | ||
| 1″ | 141.6 | 140.7 | ||
| 2″,6″ | 7.36b | 129.4 | 7.52, d (7.3) | 129.9 |
| 3″,5″ | 7.40b | 129.0 | 7.36, t (7.4) | 128.3 |
| 4″ | 7.29, m | 127.4 | 7.29b | 126.8 |
| OCH3-6 | 3.88, s | 56.4 | 3.89, s | 56.2 |
| OCH3-8 | 3.76, s | 55.8 | 3.77, s | 55.4 |
| OCH3-4′ | 3.87, s | 55.9 | 3.87, s | 55.5 |
1H NMR spectrum measured at 400 MHz, 13C NMR spectrum measured at 100 MHz; obtained in CDCl3 with TMS as internal standard. Assignments supported with 2D NMR spectra.
Overlapping signals.
Figure 1.
Selected key HMBC ( ) and NOESY (
) and NOESY ( ) correlations observed for perviridisin A (1)
) correlations observed for perviridisin A (1)
The same molecular formula as that of 1, C36H42N2O10, was assigned to compound 2 based on the [M + Na]+ ion peak at m/z 669.2763 (calcd 669.2788) in the HRESIMS. The 1H and 13C NMR spectroscopic data of these two compounds were found to be quite comparable. Thus, it was evident that no rearrangements had occurred in the cyclopenta[b]benzopyran system, and the phenyl ring and the amidic putrescinyl 4-hydroxytiglate moiety at C-4 and C-3, respectively, were both identical to those of 1 (Table 1). In the 1H NMR spectrum of 2, a singlet appearing at δH 2.97 was found to show HMBC correlations with C-2, C-10, and C-5, and was assigned subsequently as a hydroxy group at C-10. In the NOESY spectrum, no NOE effect between H-3 and H-10 was observed, while the 10-H proton correlated with H-3, H-2′(6′) and H-2″(6″). This analysis suggested OH-10 and H-3 to be spatially close, consistent with a downfield shift of 0.78 ppm observed for H-3 caused by the deshielding effect from OH-10. Thus, the structure of compound 2 (perviridisin B) was deduced as the C-10 epimer of 1, as shown.
The HRESIMS of compound 3 gave a sodiated molecular ion peak at m/z 485.1582 [M + Na]+, consistent with a molecular formula of C27H26O7. The NMR data of 3 proved to be similar with values published for 4′-demethoxy-3′,4′-methylenedioxyrocaglaol (11),7 a known compound isolated in this investigation. In the 1H NMR spectrum, signals for a monosubstituted benzene ring at δH 6.82 (2H, m, H-2″ and H-6″) and 7.09 (3H, m, H-3″, H-4″, and H-5″), a 1,3,4-trisubstituted benzene ring at δH 6.77 (1H, brs, H-2′), 6.61 (1H, d, J = 8.2 Hz, H-5′), 6.74 (1H, brd, J = 8.2 Hz, H-6′), and a 1,2,3,5-tetrasubstituted benzene ring at δH 6.31 (1H, d, J = 1.9 Hz, H-5) and 6.19 (1H, d, J = 1.9 Hz, H-7) were observed. An (–OCH-CH2-CH-) spin system was evident based on the coupling patterns of the methylene protons at δH 1.97 (1H, dd, J = 13.8, 6.7, H-2α) and 2.67 (1H, ddd, J = 14.2, 14.2, 6.5 Hz, H-2β), as well as two methine protons at δH 4.90 (1H, d, J = 7.1, H-1) and 3.79 (1H, dd, J = 14.3, 6.7, H-3), which was confirmed by the analysis of the 1H-1H COSY spectrum. In addition, two aromatic methoxy groups at δH 3.86 (3H, OCH3-6) and 3.93 (3H, OCH3-8), and methylenedioxy protons at δH 5.87 and 5.88 (each 1H, d, J = 1.4, OCH2O) (Table 2) were observed. Besides the above characteristic protons assigned to a 4′-demethoxy-3′,4′-methylenedioxyrocaglaol moiety, an extra methoxy group signal appeared at δH 2.46 (3H, OCH3-8b), which suggested the hydroxy group at C-8b of 4′-demethoxy-3′,4′-methylenedioxyrocaglaol (11) to be methylated in 3. In comparison with the 13C NMR spectrum of 11, a downfield shift of 5 ppm for C-8b, as well as upfield shifts of ca.2 and 3 ppm for C-3a and C-8a, respectively, were observed for compound 3 due to this substitution. In the HMBC spectrum, a key correlation between the methoxy group at δH 2.46 with C-8b, was observed. The β-orientation of this methoxy group was supported by the NOE cross peak of OCH3-8b/ H-2′and H-6′. According to a previous study, only two naturally occurring rocaglaol analogues from A. duppereana, which were also isolated from A. perviridis in the present investigation as compounds 8b-O-methylrocaglaol and methyl 8b-O-methylrocaglate (16), have been reported to have the OH group at C-8b substituted by a methoxy group.40 Furthermore, analysis of the additional NOE effects gave supporting evidence that the relative configuration of compound 3 is identical with those of previously reported rocaglaol derivatives.7,40 Thus, the structure of 3 was determined as 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglaol.
Table 2.
1H and 13C NMR Spectroscopic Data of Compounds 3 and 4a
| position | 3 | 4 | ||
|---|---|---|---|---|
| δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | |
| 1 | 4.90, d (7.1) | 80.1 | 5.09, d (7.1) | 80.3 |
| 2 | 1.97, α, dd, (13.8, 6.7) | 35.5 | 3.79, dd (14.8, 7.1) | 49.7 |
| 2.67, β, ddd (14.2, 14.2, 6.5) | ||||
| 3 | 3.79, dd (14.3, 6.7) | 53.9 | 4.11, d (14.4) | 55.2 |
| 3a | 101.1 | 99.4 | ||
| 4a | 161.2 | 161.4 | ||
| 5 | 6.31, d (1.9) | 89.7 | 6.31, d (1.9) | 89.9 |
| 6 | 164.0 | 164.4 | ||
| 7 | 6.19, d (1.9) | 92.4 | 6.17, d (1.8) | 92.8 |
| 8 | 157.4 | 157.6 | ||
| 8a | 104.0 | 104.0 | ||
| 8b | 100.6 | 99.8 | ||
| 1′ | 128.9 | 128.8 | ||
| 2′ | 6.77, brs | 107.8 | 6.75b | 108.0 |
| 3′ | 146.7 | 147.4 | ||
| 4′ | 146.3 | 146.7 | ||
| 5′ | 6.61, d (8.2) | 107.0 | 6.62, brs | 107.4 |
| 6′ | 6.74, brd (8.2) | 120.5 | 6.75b | 120.6 |
| 1″ | 137.8 | 136.5 | ||
| 2″,6″ | 6.82, m | 128.2 | 6.76b | 128.0 |
| 3″,5″ | 7.09b | 127.5 | 7.06b | 127.8 |
| 4″ | 7.09b | 126.6 | 7.08, m | 127.0 |
| OCH2O | 5.88, d (1.4) | 100.7 | 5.87, brs | 101.0 |
| 5.87, d (1.4) | 5.88, brs | |||
| OCH3-6 | 3.86, s | 55.7 | 3.86, s | 55.9 |
| OCH3-8 | 3.93, s | 55.9 | 3.90, s | 56.0 |
| OCH3-8b | 2.46, s | 51.7 | 2.47, s | 52.1 |
| COOCH3 | 170.2 | |||
| COOCH3 | 3.60, s | 52.0 | ||
1H NMR spectrum measured at 400 MHz, 13C NMR spectrum measured at 100 MHz; obtained in CDCl3 with TMS as internal standard. Assignments supported with 2D NMR spectra.
Overlapping signals.
Compound 4 gave a molecular formula of C29H28O9, as determined by a sodiated molecular ion peak at m/z 543.1638 [M + Na]+ in the HRESIMS. The NMR spectra of 3 and 4 were closely comparable, with the major differences focused on signals of the cyclopentane ring. In the 1H NMR spectrum of 4, instead of two geminal protons ascribed to H-2 of compound 3, a methine signal appeared at δH 3.79 (dd, J = 14.8 and 7.1 Hz), which showed COSY correlations with two methines at δH 5.09 (1H, d, J = 7.1 Hz) and 4.11 (1H, d, J = 14.4 Hz), respectively. In addition, a methyl ester group was recognized at δH 3.60 (3H, s, COOCH3). Correspondingly, in the 13C NMR spectrum, a methine group at δC 49.7 (C-2) and a methyl group at δC 52.0 (COOCH3), as well as a carbonyl group at δC 170.2 (COOCH3), were evident (Table 2). These observations suggested that a methoxycarbonyl group is located at C-2 in compound 4, which was confirmed by key HMBC correlation between H-2 and the carbonyl carbon at δC 170.2. Thus, the structure of 4 was elucidated as methyl 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglate.
Compound 5 was obtained as a white powder. Its molecular formula was assigned as C30H48O3 based on the [M + Na]+ ion peak at m/z 479.3502 (calcd 479.3501) in the HRESIMS. In the high-field region of the 1H NMR spectrum, besides proton signals for four tertiary methyl groups at δH 0.81 (3H, s, H-29), 0.88 (3H, s, H-30), 0.97 (3H, s, H-28), and 0.98 (3H, s, H-18), a secondary methyl group at δH 0.94 (3H, d, J = 6.0 Hz, H-21) as well as two methyl groups located at a vinylic carbon at δH 1.97 (3H, s, H-26) and 2.19 (3H, s, H-27), two protons attributed to a typical cyclopropyl methylene group were recognized at δH 0.33 (1H, d, J = 4.0 Hz, H-19α) and 0.55 (1H, d, J = 4.0 Hz, H-19β). In the low-field region of the 1H NMR spectrum, proton signals for two oxygenated methines were evident at δH 3.28 (1H, dd, J = 11.0, 4.4 Hz, H-3) and 4.10 (1H, brs, H-22), and an olefinic proton at δH 6.13 (1H, s, H-24). The 13C NMR spectrum of 5 showed 30 carbon signals, which were classified from the DEPT and HSQC spectra into seven methyls, nine methylenes, four alkyl methines, five alkyl quaternary carbons, two oxygenated methines (δC 78.8, C-3 and 81.0, C-22), a trisubstituted double bond (δC 120.7, C-24 and 158.0, C-25), and a carbonyl group (δC 201.5, C-23) (Table 3). These characteristic NMR data suggested that 5 possesses a cycloartane skeleton, which has been reported as one of the major classes of triterpenes isolated from Aglaia species.38,44–48 In the HMBC spectrum, observed correlations from H-2, H3-28, and H3-29 to C-3, as well as H-22 to C-21 and C-17, and H-21 to C-22 supported the location of hydroxy groups at C-3 and C-22, respectively. The protons of two geminal methyls, H3-26 and H3-27, showed correlations with the Δ24 double bond carbons, respectively. In addition, the olefinic H-24, correlated with the C-23 carbonyl group, as well as C-26 and C-27. These observations confirmed the presence of a terminal dimethylvinyl moiety conjugated with a carbonyl functionality in the side chain.
Table 3.
13C NMR Chemical Shifts of Compounds 5–8a
| position | 5 | 6 | 7 | 8 | ||||
|---|---|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | |
| 1 | 1.55b, α | 31.9 | 1.55b, α | 31.9 | 1.54b, α | 30.7 | 36.3 | |
| 1.22b, β | 1.22b, β | 1.26b, β | ||||||
| 2 | 1.75 b, α | 30.3 | 1.75b, α | 30.3 | 1.99 b, α | 34.8 | 2.00, d (17.3), α | 47.0 |
| 1.57 b, β | 1.57b, β | 1.32 b, β | 2.48, d (17.3), β | |||||
| 3 | 3.28,dd (11.0, 4.4) | 78.8 | 3.28,dd (10.8, 4.4) | 78.8 | 3.22,ddd (10.4, 9.0, 4.5) | 76.5 | 201.3 | |
| 4 | 40.5 | 40.5 | 1.18b | 44.6 | 5.83, brs | 124.4 | ||
| 5 | 1.30b | 47.0 | 1.28b | 46.9 | 1.19b | 43.2 | 168.7 | |
| 6 | 1.59b, α | 21.1 | 1.60b, α | 21.0 | 1.68b, α | 24.6 | 2.03b | 51.3 |
| 0.77b, β | 0.79b, β | 0.58, m, β | ||||||
| 7 | 1.10b, α | 26.0 | 1.11b, α | 26.0 | 1.08b, α | 25.1 | 1.48b, a | 26.1 |
| 1.31b, β | 1.32b, β | 1.38b, β | 2.02b, b | |||||
| 8 | 1.49, dd (12.0, 4.4) | 47.9 | 1.50, dd (12.4, 5.0) | 47.8 | 1.58b | 46.7 | 1.48b, a 1.68, m, b |
33.0 |
| 9 | 19.9 | 19.9 | 23.4 | 3.56, dt (10.4, 5.4) | 72.2 | |||
| 10 | 26.0 | 26.3 | 29.6 | 3.47, d (5.4) | 66.2 | |||
| 11 | 1.96b, α | 26.3 | 1.98b, α | 26.4 | 1.99b,α | 26.9 | 1.04, s | 28.0 |
| 1.11b, β | 1.14b, β | 1.20b, β | ||||||
| 12 | 1.59b | 32.7 | 1.55b, α | 31.6 | 1.68b, α | 32.0 | 1.12, s | 26.5 |
| 1.59b | 1.34b, β | 1.61b, β | ||||||
| 13 | 45.8 | 45.9 | 45.2 | 2.07, d (0.9) | 23.8 | |||
| 14 | 48.5 | 47.9 | 48.9 | |||||
| 15 | 1.30b | 35.9 | 1.40b | 35.9 | 1.31b | 35.2 | ||
| 16 | 1.83b | 27.8 | 1.95b | 26.2 | 1.95b | 27.5 | ||
| 1.23b | 1.70b | 1.32b | ||||||
| 17 | 2.00b | 47.6 | 1.93, m | 50.1 | 2.00, m | 46.1 | ||
| 18 | 0.98, s | 17.9 | 0.97, s | 18.9 | 0.99, s | 18.0 | ||
| 19 | 0.33, d (4.0), α | 29.8 | 0.32, d (4.0), α | 29.9 | 0.15, d (4.0), α | 27.1 | ||
| 0.55, d (4.0), β | 0.56, d (4.0), β | 0.40, d (4.0), β | ||||||
| 20 | 2.02, m | 42.1 | 2.18, m | 49.4 | 1.51b | 42.6 | ||
| 21 | 0.94, d (6.0) | 16.4 | 3.61, t, (8.6), α | 70.3 | 3.64, dd (11.0, 4.0), a | 62.4 | ||
| 4.07, t (8.6), β | 3.74, dd (11.0, 2.0), b | |||||||
| 22 | 4.10, brs | 81.1 | 3.69, t (7.0) | 83.1 | 1.98b | 31.7 | ||
| 2.14b | ||||||||
| 23 | 201.5 | 4.28, dd (8.8, 7.0) | 80.5 | 1.58b | 28.2 | |||
| 1.50b | ||||||||
| 24 | 6.13, s | 120.7 | 5.18, d (8.8) | 123.5 | 156.6 | |||
| 25 | 158.0 | 139.0 | 2.25, quin (6.8) | 33.8 | ||||
| 26 | 1.97, s | 28.0 | 1.77, s | 26.1 | 1.04, d (6.8) | 22.0 | ||
| 27 | 2.19, s | 21.4 | 1.75, s | 18.5 | 1.03, d (6.8) | 21.9 | ||
| 28 | 0.97, s | 25.4 | 0.97, s | 25.4 | 0.98, d (6.4) | 14.4 | ||
| 29 | 0.81, s | 14.0 | 0.80, s | 14.0 | ||||
| 30 | 0.88, s | 19.3 | 0.90, s | 19.3 | 0.91, s | 19.2 | ||
| 31 | 4.70, brs 4.74, brs |
106.3 | ||||||
1H NMR spectrum measured at 400 MHz, 13C NMR spectrum measured at 100 MHz; spectrum of compounds 5–7 were obtained in CDCl3 with TMS as internal standard, spectrum of 8 were obtained in methanol-d4. Assignments supported with 2D NMR spectra.
Overlapping signals.
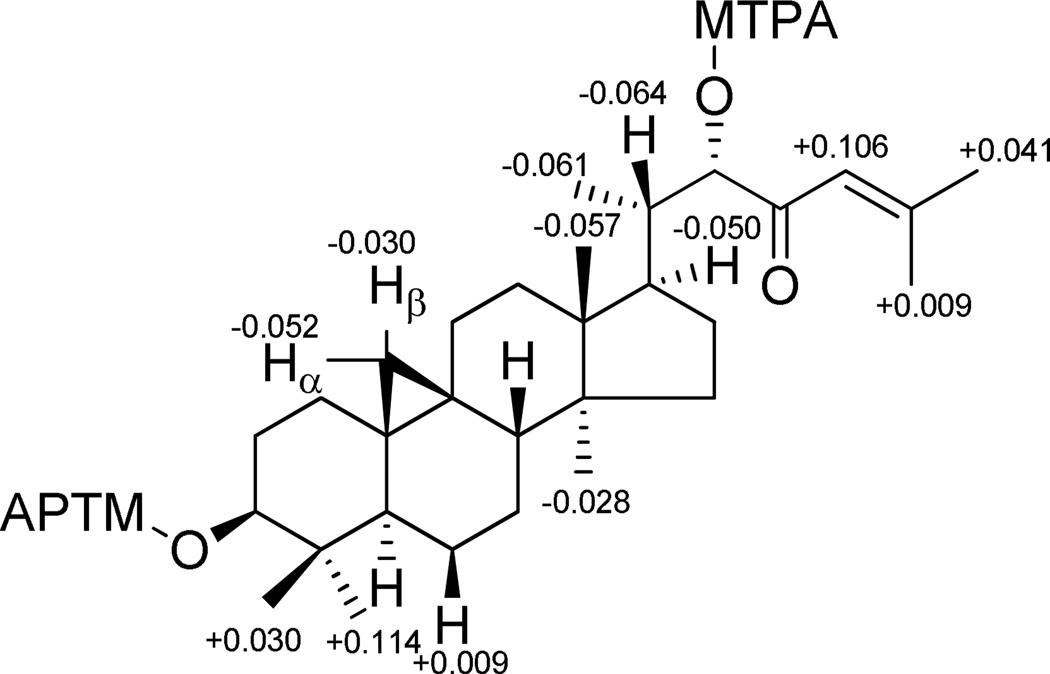
The absolute configurations of C-3 and C-22 in compound 5 were determined by the Mosher ester method. Treatment of 5 with (R)- and (S)-MTPA chloride gave the C-3 and C-22 (S)- and (R)-MTPA ester derivatives. Analysis of the 1H NMR chemical shift differences (ΔδS-R) between the (S)- and (R)-MTPA ester derivatives led to the assignment of the S-configuration both at C-3 and C-22 (Figure 2). Furthermore, in the NOESY spectrum, cross peaks of H3-28/H-19α and H-5, H-8/H3-18 and H-19β, as well as H-17/H3-30 provided evidence that the relative configurations of the remaining stereocenters of compound 5 are identical with those of previously reported related compounds.38,44–48 Hence, the structure of compound 5 was determined to be (3S,22S)-dihydroxycycloart-24-en-23-one, and this substance has been accorded the trivial name, perviridisinol A.
Figure 2.
ΔδS-R values of MTPA esters of 5
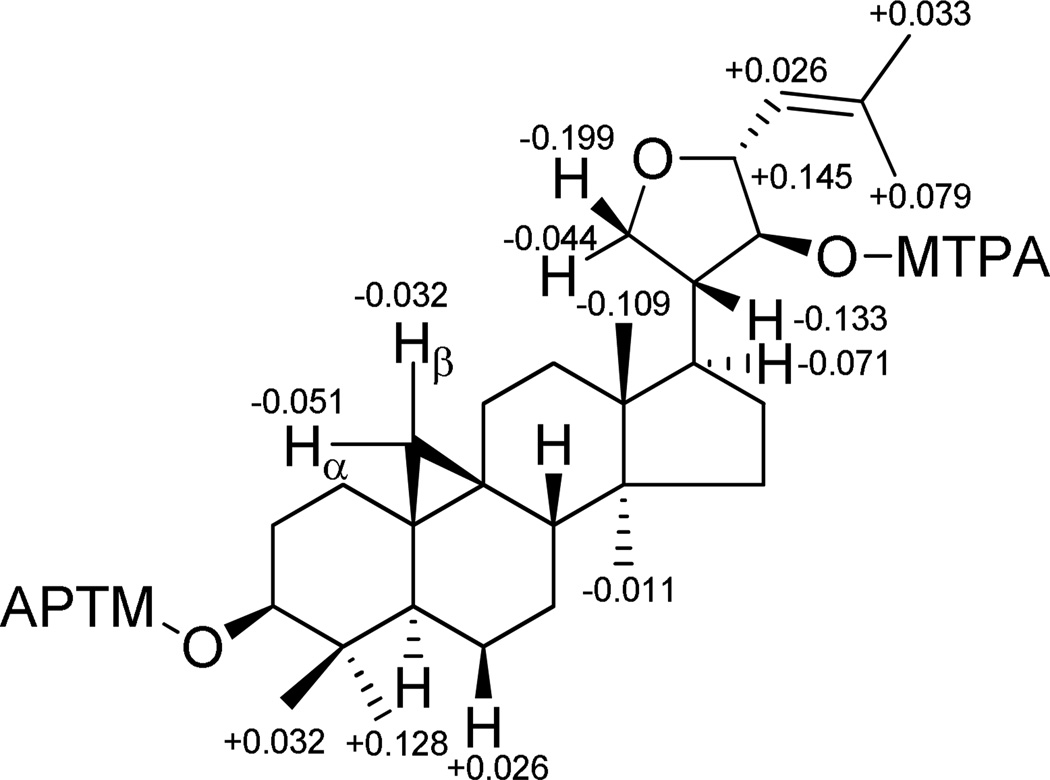
Compound 6 gave the same molecular formula as that of 5, C30H48O3, based on the [M + Na]+ ion peak at m/z 479.3496 in the HRESIMS. The 1H and 13C NMR spectra of 6 were closely comparable to those of compound 5, with the major differences occurring for signals in the side chain. On comparison of the 1H NMR data of these two compounds, the doublet of the secondary methyl group H3-21 at δH 0.94 in compound 5 was absent, while resonances of an oxygenated methylene appeared at δH 3.61 (1H, d, J = 8.6 Hz, H-21α) and 4.07 (1H, d, J = 8.6 Hz, H-21β). In addition, an extra oxygenated methine resonance appeared at δH 4.28 (1H, dd, J = 7.0 and 8.8 Hz, H-23), and showed COSY correlations with an olefinic signal at δH 5.18 (1H, d, J = 8.8 Hz, H-24) and an oxygenated methine at δH 3.69 (1H, t, J = 7.0 Hz, H-22), respectively. Correspondingly, in the 13C NMR spectrum of 6, instead of the carbon signals of the C-21 methyl group and the C-23 carbonyl group in 5, resonances for an oxygenated methylene at δC 70.3 (C-21) and an oxygenated methine at δC 80.5 (C-23) were observed (Table 3). From the NMR data, in combination with the molecular formula, an extra ring was required in addition to the tetracyclic ring system of a cycloartane triterpene skeleton. This analysis suggested that C-21 is connected with C-23 through an oxygen bridge to form a tetrahydrofuran ring in the side chain. This deduction was supported by HMBC correlations between H-21 with C-23, H-20 with H-21 and H-22, as well as H-23 and H-21β with C-22. The relative configurations of the stereocenters of the tetrahydrofuran ring were assigned as shown based on observed NOE effects between H-20/H-21β and H-23, H-17/H-21α and H-22, as well as H-24/H-22. The absolute configuration at both C-3 and C-22 were established as S by the Mosher ester procedure (Figure 3). Thus, the structure of compound 6 (perviridisinol B) was elucidated as 21,23R-epoxy-(3S,22S)-dihydroxycycloart-24-ene.
Figure 3.
ΔδS-R values of MTPA esters of 6
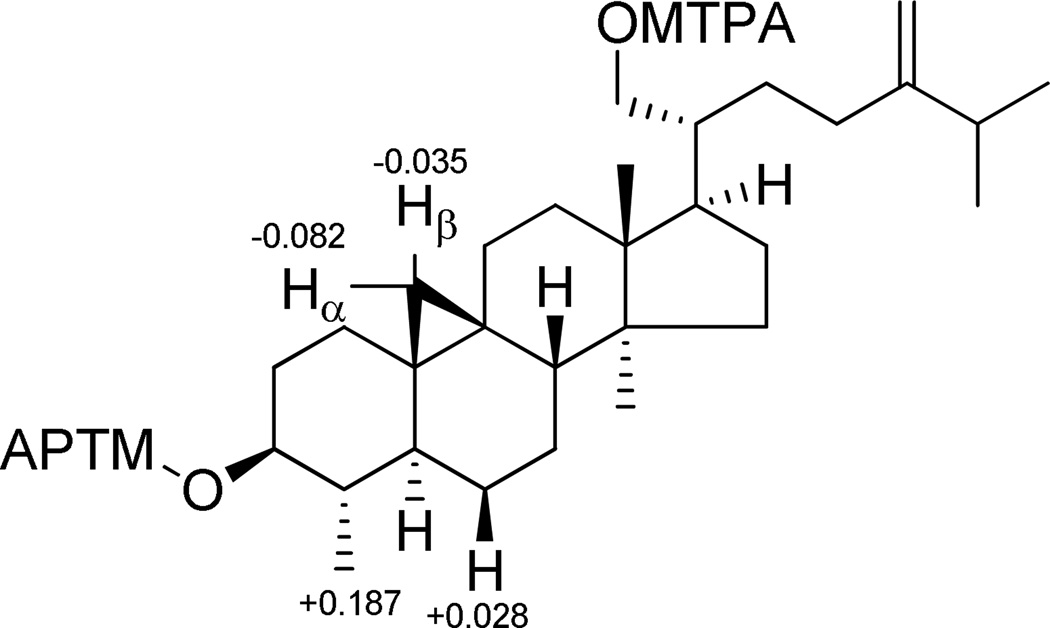
The HRESIMS of compound 7 gave a sodiated molecular ion peak at m/z 465.3715 [M + Na]+, consistent with a molecular formula of C30H50O2. The NMR data of 7 were similar to those of 24-methylenecycloartan-3β,21-diol, a known triterpene also isolated in the present study. In the low-field region of the 1H NMR spectrum, characteristic proton signals occurring as two singlets at δH 4.70 and 4.74 (each 1H, H-31), attributed to a terminal vinyl group, and well as two geminal protons at δH 3.64 (1H, dd, J = 11.0, 4.0 Hz, H-21a) and 3.74 (1H, dd, J = 11.0, 2.0 Hz, H-21b), corresponding to an oxygenated methylene, were recognized. In the high-field region of the 1H NMR spectrum, only five methyl groups were observed (Table 3). Besides signals for two methyl groups belonging to an isobutane moiety in the side chain (δH 1.04, 3H, d, J = 6.8 Hz, H-26; δH 1.03, 3H, d, J = 6.8 Hz, H-27) and two tertiary methyls (δH 0.91, 3H, s, H-30; δH 0.99, 3H, s, H-18) at the C/D ring junction, a secondary methyl group was apparent at δH 0.98 (3H, d, J = 6.4 Hz, H3-28). This methyl group signal displayed an HMBC correlation with the oxygenated methine carbon at δC 76.5 (C-3), and exhibited NOE associations with H-3 and H-19α. In the 13C NMR spectrum, instead of the quaternary carbon around δC 40.0 corresponding to C-4 found in 24-methylenecycloartan-3β,21-diol,37 a methine carbon appeared at δC 44.6, and showed an HMBC correlation with H3-28. All these observations suggested that C-29, the tertiary methyl group at C-4 in 24-methylenecycloartan-3β,21-diol, was absent in compound 7. This observation explained the different coupling pattern observed for H-3 (δH 3.22, 1H, ddd, J = 10.4, 9.0 and 4.5 Hz), when compared with H-3 (δH 3.28, 1H, dd, J = 10.8 and 4.4 Hz) of 3β-hydroxy cycloartane derivatives.38,44–48 The absolute configuration of C-3 was also determined as S by the Mosher ester procedure (Figure 4). A NOESY experiment revealed the consistent relative configuration of 7 with other cycloartane analogues isolated in this investigation. Thus, the structure of compound 7 (perviridisinol C) was determined as 3S,21-dihydroxy-24-methylene-29-norcycloartane.
Figure 4.
ΔδS-R values of MTPA esters of 7
The molecular formula of compound 8 was determined as C13H22O3 from the sodiated molecular ion peak at m/z 249.1459 [M + Na]+ (calcd 249.1467) in the HRESIMS. In the 1H NMR spectrum, proton signals of two tertiary alkyl methyls at δH 1.04 (3H, s, H-11) and 1.12 (3H, s, H-12), a methyl group located on a double bond at δH 2.07 (3H, d, J = 0.9 Hz, H-13), a methine group at δH 3.56 (1H, d, J = 10.4 and 5.4 Hz), as well as an oxygenated methylene group at δH 3.47 (2H, d, J = 5.4 Hz), were recognized (Table 3). Altogether, 13 carbon signals in the 13C NMR spectrum were sorted by their DEPT and HSQC data into three methyls, three alkyl methylenes, one alkyl methine, one quaternary carbon, an oxygenated methine, an oxygenated methylene, a trisubstituted double bond, and a carbonyl group. An α,β-unsaturated carbonyl moiety could be recognized based on the carbon signals of the carbonyl group at δC 201.3 (C-3) and of the double bond at δC 124.4 (C-4) and 168.7 (C-5). In the HMBC spectrum, proton signals of two geminal methyl goups, H3-11 and H3-12, showed strong correlations with a quaternary carbon at δC 36.3 (C-1), a methylene carbon adjacent to a carbonyl group at δC 47.0 (C-2), and a methine carbon at δC 51.3 (C-6), which was further found to correlate with H3-13, the proton signal of the methyl group on the double bond. In addition, the proton signal of the oxygenated methylene (H-10) correlated with the oxygenated methine (C-9) and an alkyl methylene (C-8). In the COSY spectrum, the alkyl methylenes, H-7 and H-8, correlated with one another, with H-7 also correlating with H-6. Based on the 1D- and 2D-NMR data analysis, the planar structure of compound 8 was elucidated as a C13 apocarotenoid derivative,49 9,10-dihydroxy-4-megastigmen-3-one.
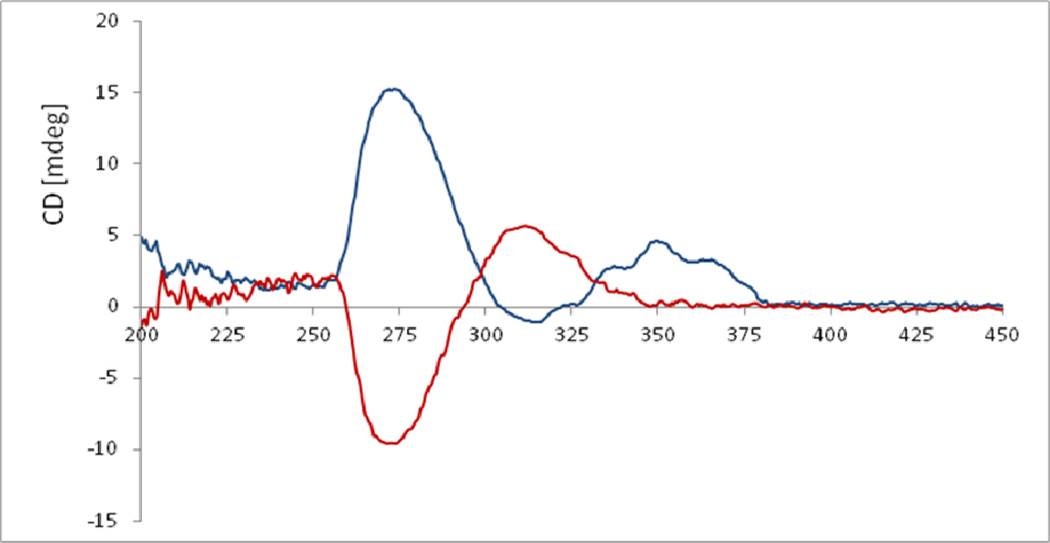
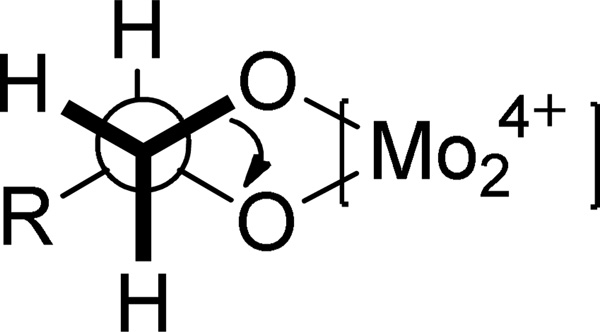
The absolute configuration of C-6 was determined as R based on the positive absorptions around 245 nm and 335 nm in the ECD spectrum (c 1.26×10−4 M, MeOH) of 8.49,50 As far as the acyclic 9,10-diol moiety is concerned, neither the Mosher ester procedure nor a regular ECD measurement could be used for the assignment of the absolute configuration of C-9. In this case, a practical and reliable method developed by Snatzke and Frelek was employed to solve the problem.51,52 After mixing compound 8 and dimolybdenum tetraacetate [Mo2(AcO)4] in DMSO, a ligand-metal complex possessing an suitable chromophoric group was formed, for which the induced circular dichroism spectrum (ICD) was recorded and analyzed. According to Snatzke’s theory, the absorption band around 310 nm (band IV) is one of these most reliably related to the absolute configuration of a diol derivative in the [Mo2(AcO)4]-induced CD (ICD) spectrum, which possesses the same sign of torsional angle of the O-C-C-O unit in the favored conformation. In the ICD spectrum of compound 8, the diagnostic Cotton effect around 310 nm was positive (Figure 5), which corresponds to a positive dihedral angle of the O-C-C-O moiety (Figure 6). Thus, the absolute configuration of C-9 of the 9,10-diol moiety in compound 8 was assigned as S.
Fig. 5.
( ) ECD spectrum of compound 8 in a DMSO solution.
) ECD spectrum of compound 8 in a DMSO solution.
( ) ECD spectrum of compound 8 in a DMSO solution of Mo2(OAc)4 (the inherent ECD was subtracted).
) ECD spectrum of compound 8 in a DMSO solution of Mo2(OAc)4 (the inherent ECD was subtracted).
Fig. 6.
The O-C-C-O dihedral in the compound 8-[Mo2]4+ complex
All isolates were evaluated for their cytotoxic activity against HT-29 human colon cancer cells. As shown in Table 4, cyclopenta[b]benzofuran derivatives were demonstrated as being the major cytotoxic substances from A. perviridis. Seven known rocaglaol derivatives (9–15) exhibited pronounced cytotoxicity against the HT-29 cell line, showing ED50 values ranging from 0.0007 to 0.056 µM, with rocaglaol (9) as the most potent compound. Four rocaglaol analogues, with the C-8b hydroxy group replaced by a methoxy group, including the new compounds 3 and 4, as well as two known compounds 8b-O-methylrocaglaol and methyl 8b-O-methylrocaglate (16), were found to be much less potently cytotoxic. This observation is consistent with the reported negative result observed for 8b-O-methylrocaglaol and methyl 8b-O-methylrocaglate (16), in the human monocytic leukemia cell lines, MONO-MAC-1 and MONO-MAC-6,39 and supported the conclusion that a free hydroxy group at C-8b is an essential feature of rocaglaol derivatives for the resulting cytotoxicity. The new cyclopenta[b]benzopyran derivative, perviridisin B (2), exhibited significant cytotoxicity (ED50 0.46 µM) against HT-29 cells, while its C-10 epimer, perviridisin A, was over 25 times less active in the same assay. In order to evaluate the selectivity of these potent cytotoxic agents isolated from A. perviridis for a tumorigenic cell line, compounds with ED50 values of less than 10 µM against HT-29 cells were further tested against the CCD-112CoN normal colon cell line. None of the compounds tested was found to show inhibitory activity against the employed normal cells at the relative high concentration of 50 µM. This preliminary selectivity testing result provided a favorable in vitro selectivity profile for any further development of these active flavaglines as oncology leads. In a previous mechanistic study, rocaglaol (9) was shown to cause G2/M-phase cell cycle arrest and to induce apoptosis of LNCaP human prostate cancer cells.53
Table 4.
Bioactivity Evaluation of Compounds Isolated from A. perviridisa
| compound | HT-29b | CCD112CoNlc | NF-κB (p65)d |
| 2 | 0.46 | >50 | 2.40 |
| 3 | 0.96 | >50 | >20 |
| 4 | 4.7 | >50 | >20 |
| 9 | 0.0007 | >50 | 0.005 |
| 10 | 0.012 | >50 | >20 |
| 11 | 0.0067 | >50 | 0.33 |
| 12 | 0.002 | >50 | 3.54 |
| 13 | 0.021 | >50 | >20 |
| 14e | 0.056 | >50 | - |
| 15 | 0.019 | >50 | >20 |
| 16 | 4.9 | >50 | >20 |
| 17 | >20 | - | 0.005 |
| paclitaxelf | 0.001 | 23.0 | - |
| rocaglamideg | 0.005 | >50 | 0.08 |
Results are expressed as ED50 values (µM).
Compounds 1, 5–8, 17, argenteanol, cabraleahydroxylactone, gigantamide A, 24-methylenecycloartan-3β,21-diol, 5,7,4′-tri-O-methylkaempferol, 8b-O-methylrocaglaol, and scopoletin, were inactive against HT-29 cells (ED50 > 10 µM).
Compounds with ED50 > 50 µM were considered inactive against CCD112CoNl cells.
Compounds 1, 3–8, 10, 13, 15, 16, argenteanol, cabraleahydroxylactone, gigantamide A, 24-methylenecycloartan-3β,21-diol, 5,7,4′-tri-O-methylkaempferol, 8b-O-methylrocaglaol, and scopoletin, were inactive against NF-κB (p65) assay (ED50 > 20 µM).
The NF-κB inhibitory activity for 14 was not tested due to the limited quantity obtained.
Used as a positive control substance for the cytotoxicity assay.
Used as a positive control substance for the NF-κB (p65) assay.
All the isolates were also evaluated for their NF-κB (p65) inhibitory activity in an enzyme-based ELISA assay. Rocaglaol (9) and the known sesquiterpene, 2-oxaisodauc-5-en-12-al (17), were extremely active, both with an ED50 value of 0.005 µM, more than 10 times more potent than the control compound, rocaglamide. For other rocaglate derivatives (10–16), a notable decrease of the NF-κB (p65) inhibitory activity was observed due to the substitution of a methoxycarbonyl or carboxamide at C-2 or/and the 4′-methoxy group being substituted by a 3′,4′-methylenedioxy in their structures, when compared with rocaglaol (9). Perviridisin B (2), a new compound with significant cytotoxicity against HT-29 cells, was also found to show moderate NF-κB inhibitory activity (ED50 = 2.4 µM) in the present investigation.
EXPERIMENTAL SECTION
General Experimental Procedures
Melting points were measured using a Fisher Scientific melting point apparatus and are uncorrected. Optical rotations were measured on a Perkin-Elmer 343 automatic polarimeter (PerkinElmer, Waltham, MA). UV spectra were run on a Hitachi U-2910 spectrophotometer (Hitachi, Tokyo, Japan). Electronic circular dichroism (ECD) spectra were recorded on a JASCO J-810 spectropolarimeter (JASCO Inc., Easton, MD). IR spectra were obtained on a Thermo Scientific Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA). NMR spectroscopic data were recorded at room temperature on Bruker Avance DRX-400 spectrometers (Bruker, Billerica, MA), using standard Bruker pulse sequences, and the data were processed using MestReNova 6.0 software (Mestrelab Research SL, Santiago de Compostela, Spain). High-resolution electrospray ionization mass spectra (HRESIMS) were performed on a Micromass Q-Tof™ II (Micromass, Wythenshawe, UK) mass spectrometer operated in the positive-ion mode, with NaI being used for mass calibration for a calibration range of m/z 100–2000. LC-MS experiments were performed on a liquid chromatographic/autosampler system that consisted of a Waters Alliance 2690 Separations Module (Waters, Milford, MA) and a Micromass LC-TOF™ II mass spectrometer (Micromass, Wythenshawe, UK) equipped with an orthogonal electrospray source (Z-spray). Column chromatography was carried out with silica gel (230–400 mesh; Sorbent Technologies, Atlanta, GA). Analytical TLC was conducted on precoated 250 µm thickness silica gel UV254 aluminum-backed plates (Sorbent Technologies). Waters Xbridge® (4.6 × 150 mm), semi-preparative (10 × 150 mm), and preparative (19 × 150 mm) C18 (5 µm) columns were used for analytical, semi-preparative, and preparative HPLC, respectively, as conducted on a Waters system comprised of a 600 controller, a 717 Plus autosampler, and a 2487 dual wavelength absorbance detector.
Plant Material
The roots and the combination of the fruits, leaves, and twigs of Aglaia perviridis were collected in Nui Chua National Park (11° 43' N; 109° 08' E; 730 m alt.), Ninh Thuan Province, Vietnam by D. D. S., T. N. N., and Vuong Tan Tu, in January, 2010, who also identified this plant, in cooperation with C. M. P.. A voucher specimen (original collection Soejarto et al.14595) has been deposited in the John G. Searle Herbarium of the Field Museum of Natural History (under accession number FM 2287877), Chicago, Illinois.
LC-MS Dereplication Procedure
LC-UV conditions
Sample concentration: 10 mg/mL MeOH solution; mobile phase: gradient elution of MeOH/H2O (0–10 min, from 62:38 to 70:30; 11–30 min, 100% MeOH); UV detection wavelength: 210 nm; flow rate: 0.75 mL/min. Injection volume: 45 µL for the 96-well plate with sample concentration of ca. 20 µg/mL, and 11.3 µL for the 96-well plate sample concentration of ca. 5 µg/mL, respectively.
Cytotoxicity assay screening
Fractions were collected into two 96-well plates (250 µL/well × 90 and negative control/well × 6) with sample concentrations of 20 µg/mL and 5 µg/mL, respectively, and was tested for the HT-29 cell growth inhibition activity, according to an established protocol.11
LC-MS conditions
HPLC conditions: mobile phase: a gradient elution of MeOH/H2O (0–10 min, from 62:38 to 70:30; 11–30 min, 100% MeOH); injection volume: 45 µL (10 mg/mL). The mobile phase flow rate was maintained at 0.75 mL/min and was split post column using a microsplitter valve (Upchurch Scientific, Oak Harbor, WA) to ca. 20 µL/min for introduction to the ESI source. Optimal ESI conditions: capillary voltage, 3000 V; source temperature, 110 °C; cone voltage, 55 V. Q1 was set to optimally pass ions from m/z 100–2000 and all ions transmitted into the pusher region of the TOF analyzer were scanned over m/z (100–1000 range) with a 1 sec integration time. Data were acquired in a continuum mode during the LC run. The result showed that the active fractions with cytotoxicity at the concentration of 20 µg/mL mainly included eleven m/z values of 507.2, 529.2, 493.2, 515.2, 471.2, 457.2, 521.2, 543.2, 535.2, 557.2, and 485.2, respectively.
Data analysis
A NAPRALERT database (http://www.napralert.org) search reported 656 compounds from the genus Aglaia, comprising flavaglines, terpenoids, flavonoids, and bisamides. The flavagline group is the major compound class of Aglaia species showing cytotoxic activity from the previous investigations.7,8,54–57 A combination analysis of the search results using each possible molecular formula with the substance role “occurrence” and the key word “Aglaia” in the SciFinder database (Chemical Abstracts Service) was carried out to check if the molecular weights from the active peaks match any known flavaglines isolated from the genus Aglaia.
Extraction and Isolation
The air-dried and finely ground combination of the leaves, twigs, and fruits of A. perviridis (880 g), was extracted by maceration in MeOH-H2O (95:5; 3 × 2 L) at room temperature for two days each. The solvent was evaporated under reduced pressure to yield 223 g of a crude extract, which was suspended in a MeOH-H2O (9:1) mixture and then extracted with hexanes (3 × 1 L) and CHCl3 (3 × 1 L), sequentially, to afford a CHCl3 -soluble extract (17 g). The CHCl3-soluble extract, with an IC50 value of 3.0 µg/mL against HT-29 human colon cancer cells, was separated by column chromatography over Si gel using a CH2Cl2-acetone gradient solvent system (30:1 to pure acetone). Of seven sub-fractions obtained, F1 and F5 were found to be the most active, with ED50 values of 0.3 and 0.8 µg/mL, respectively. Fraction F01 (1.1 g) was chromatographed over an open C18 column (2.2 × 20 cm) using MeOH-H2O mixtures (50:50 to 100% MeOH) for elution, to give 24 subfractions (F101–F124). Recrystallization of the yellow precipitates from F101 and F108 in MeOH afforded scopoletin (3.0 mg) and 5,7,4′-tri-O-methylkaempferol (5.0 mg), respectively. Compound 17 (2.0 mg) was obtained from subfractions F103, by column chromatography over silica gel with acetone-n-hexane (5:1 to 2:1). Subfraction F105 was fractionated over an open C18 column, eluted with MeOH-H2O (60:40 to 100% MeOH) to afford five subfractions (F1051–F1055). F1051 was purified by HPLC on a semi-preparative RP-18 column, using MeOH-H2O (0.1% formic acid) (55:45) as solvent system, to afford, in turn, 12 (3.0 mg), 10 (12.0 mg), 11 (5.0 mg), and 9 (4.0 mg). Subfraction F1052 was chromatographed by HPLC on a semi-preparative RP-18 column (CH3CN-H2O, 30:70), to give perviridisin A (1, 4.0 mg) and gigantamide (2.0 mg). Subfraction F1054 was passed over a semi-preparative RP-18 column (CH3CN-H2O, 30:70) by HPLC to yield (6R,9S)-9,10-dihydroxy-4-megastigmen-3-one (8, 2 mg). Subfraction F1055 was subjected to separation on a semi-preparative RP-18 column by HPLC, using CH3CN-H2O (30:70) as solvent system, to give a mixture of perviridisin B (2, 2.0 mg) and compound 13 (6.0 mg), which was resolved on the same HPLC column, using MeOH-H2O (50:50) for elution. Cabraleahydroxylactone (15 mg) was purified from subfraction F113 by chromatography over a silica gel column and eluted with CH2Cl2-acetone mixtures (20:1 to 2:1). F115 was chromatographed on a semi-preparative RP-18 column with a CH3CN-H2O (80:20) solvent system, to yield perviridisinol A (5, 7.0 mg). 24-Methylenecycloartan-3β, 21-diol (12.0 mg) was recrystallized from subfraction F119 using methanol as solvent. Perviridisinol C (7, 3.5 mg) was purified from subfraction F120 over a semi-preparative RP-18 column by HPLC, using CH3CN-H2O (50:50) as solvent system. Subfraction F122 was purified by HPLC using a semi-preparative RP-18 column (MeOH-H2O, 50:50), to afford argenteanol (23.0 mg) and perviridisinol B (6, 7.0 mg), respectively.
The roots of A. perviridis (370 g) were extracted and partitioned using the same procedure as described above to yield a CHCl3-soluble extract (3.0 g), which exhibited potent cytotoxicity against HT-29 cells (ED50 0.2 µg/mL). In order to decide whether or not to further pursue this lead, and thereby make the isolation procedure on this plant material more efficient, the CHCl3-soluble extract of the roots of A. perviridis was subjected to an LC-MS dereplication analysis. During this procedure, the effluent from the HPLC chromatography was split, with the two effluents analyzed by MS and screened using HT-29 cancer cells cultured in a 96-well plate, respectively. The results indicated the possible presence of four known cytotoxic rocaglate derivatives (9–12), which also occurred in a sample of a combination of the leaves, twigs, and fruits of A. perviridis, based on peaks at m/z 457, 515, 529 and 471, respectively. In addition, the putative elemental formula, C29H38O9, was consistent with the presence of a rare known rocaglate analogue that has not been found from A. perviridis previously, methyl 1-formyloxyrocaglate (15).39 Besides these known compounds, an unknown compound in a cytotoxic well corresponding to a possible molecular formula of C27H26O7 was evident from a sodiated ion peak at 485 amu. Accordingly, bioassay-guided fractionation was used to facilitate the isolation process from A. perviridis roots. This extract was fractionated over a Sephadex LH-20 column using MeOH to yield four fractions (F1′–F4′). The most active subfraction F4′ (700 mg, ED50 < 0.16 µg/mL) was subjected to separation over a preparative RP-18 HPLC column using a MeOH-H2O gradient solvent system (0–50 min 57:43; 50–80 min 70:30) for elution, to afford a mixture of 14 and 15, 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglaol (3, 4.0 mg), 16 (4.5 mg), methyl 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglate (4, 2.0 mg), and 8b-O-methyl rocaglaol (4.0 mg). The mixture of compounds 14 (0.8 mg) and 15 (1.5 mg) was further separated by HPLC on a semi-preparative RP-18 column (CH3CN-H2O, 50:50).
Perviridisin A (1): Colorless resin; [α]20D −22.0 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 218 (4.34), 272 (3.32) nm; ECD (c 4.64×10−5 M, MeOH) λmax (Δε) 230 (+6.72), 280 (+1.94) nm; IR (film) νmax 3420, 2937, 1662, 1618, 1592, 1516, 1457, 1438, 1252, 1216, 1201, 1149, 1098, 1031, 832, 752 cm−1; 1H NMR (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Table 1; HRESIMS m/z 669.2809 [M+Na]+ (calcd for C30H48O3Na, 669.2788).
Perviridisin B (2): Colorless resin; mp 270–272 °C; [α]20D +21.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 215 (4.32), 273 (3.18) nm; ECD (c 4.64×10−5 M, MeOH) λmax (Δε) 222 (−6.03), 280 (−3.51) nm; IR (film) νmax 3413, 2936, 1661, 1618, 1591, 1515, 1460, 1439, 1253, 1215, 1201, 1150, 1098, 1033, 833, 753 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 1; HRESIMS m/z 669.2763 [M+Na]+ (calcd for C36H42N2O10Na, 669.2788).
8b-O-Methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglaol (3): pale yellow amorphous powder; [α]20D −32.0 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 216 (4.26), 233 (3.97), 280 (3.53) nm; ECD (c 5.41×10−5 M, MeOH) λmax (Δε) 219 (−15.10), 280 (−2.16) nm; IR (film) νmax 3524, 2934, 1623, 1597, 1497, 1491, 1456, 1436, 1247, 1216, 1201, 1148, 1125, 1107, 1067, 1041, 1007, 936, 811, 756, 699 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 2; HRESIMS m/z 485.1582 [M+Na]+ (calcd for C27H26O7Na,485.1576).
Methyl 8b-O-methyl-4′-demethoxy-3′,4′-methylenedioxyrocaglate (4): pale yellow amorphous powder; [α]20D −7.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 216 (4.27), 233 (4.01), 280 (3.61) nm; nm; ECD (c 4.80×10−5 M, MeOH) λmax (Δε) 218 (−12.41), 239 (+1.04), 277 (−1.35) nm; IR (film) νmax 3502, 2932, 1746, 1624, 1598, 1500, 1456, 1437, 1242, 1203, 1149, 1124, 1107, 1067, 1041, 1007, 936, 813, 699 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 2; HRESIMS m/z 543.1638 [M+Na]+ (calcd for C29H28O9Na, 543.1631).
Perviridisinol A (5): White powder; mp 160–162 °C; [α]20D +76.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 205 (3.54), 241 (3.85) nm; IR (film) νmax 3446, 2936, 2869, 1699, 1676, 1618, 1457, 1379, 1217, 1100, 1025, 755 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 3; HRESIMS m/z 479.3502 [M + Na]+ (calcd for C30H48O3Na, 479.3501).
Perviridisinol B (6): white powder; mp 198–200 °C; [α]20D +2.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (3.78, end absorption) nm; IR (film) νmax 3395, 2930, 2866, 1699, 1457, 1378, 1215, 1097, 1023, 1006, 754 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 3; HRESIMS m/z 479.3496 [M + Na]+ (calcd for C30H48O3Na, 479.3501).
Perviridisinol C (7): white powder; mp 164–165 °C; [α]20D +29.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (3.40, end absorption) nm; IR (film) νmax 3285, 2961, 2923, 2868, 1453, 1377, 1041, 1006, 882, 757, 669 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Table 3; HRESIMS m/z 465.3715 [M + Na]+ (calcd for C30H50O2Na, 465.3709).
(6R,9S)-9,10-Dihydroxy-4-megastigmen-3-one (8): colorless gum; [α]20D +59.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 240 (3.81) nm; ECD (c 1.26×10−4 M, MeOH) λmax (Δε) 245 (+1.13), 335 (+0.35) nm; ECD (c 1.14×10−3 M, DMSO) λmax (Δε) 274 (+0.81), 350 (+0.21) nm; IR (film) νmax 3400, 2956, 2872, 1650, 1440, 1379, 1304, 1257, 1101, 1043, 871 cm−1; 1H NMR (400 MHz, methanol-d4) and 13C NMR (100 MHz, methanol-d4) data, see Table 3; HRESIMS m/z 249.1459 [M + Na]+ (calcd for C13H22O3Na, 249.1467).
Preparation of the (R) and (S)-MTPA Ester Derivatives of Compounds 5–7
The (R)- and the (S)-MTPA ester derivatives of compounds 5–7 were prepared in a manner described previously.58,59 In brief, two portions of each compound (1 mg) were added into two NMR tubes, and dried completely. Pyridine-d5 was added to both tubes (each 0.5 mL). Then, (S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl (MTPA) chloride (10 µL) or (R)-MTPA chloride (10 µL), was injected into the NMR tubes separately under a N2 gas protection and quickly mixed with the dissolved sample. The 1H NMR chemical shifts of the (R)- and the (S)-MTPA ester of 5–7 were recorded after the reaction were completed. COSY and NOESY experiments were used to establish the 1H NMR assignment, and only fully assigned signals used for the ΔδS-R calculation.
3,22-di-(R)-MTPA ester of perviridisinol A (5): 1H NMR data (400 MHz, pyridine-d5) δ 6.402 (1H, s, H-24), 5.638 (1H, d, J = 2.6 Hz, H-22), 4.991 (1H, dd, J = 11.7, 4.5 Hz, H-3), 2.430 (1H, m, H-20), 2.228 (3H, s, H-27), 2.206 (1H, m, H-17), 1.807 (3H, s, H-26), 1.209 (3H, d, J = 6.4 Hz, H-21), 1.016 (3H, s, H-18), 0.919 (3H, s, H-30), 0.890 (3H, s, H-28), 0.878 (3H, s, H-29), 0.621 (1H, m, H-6β), 0.450 (1H, d, J = 3.9 Hz, H-19β), 0.298 (1H, d, J = 3.9 Hz, H-19α).
3,22-di-(S)-MTPA ester of perviridisinol A (5): 1H NMR data (400 MHz, pyridine-d5) δ 6.508 (1H, s, H-24), 5.627 (1H, d, J = 2.4 Hz, H-22), 4.979 (1H, dd, J = 11.8, 4.3 Hz, H-3), 2.366 (1H, m, H-20), 2.237 (3H, s, H-27), 2.162 (1H, m, H-17), 1.848(3H, s, H-26), 1.148 (3H, d, J = 6.4 Hz, H-21), 1.004 (3H, s, H-28), 0.959 (3H, s, H-18), 0.908 (3H, s, H-29), 0.891 (3H, s, H-30), 0.630 (1H, m, H-6β), 0.420 (1H, d, J = 3.6 Hz, H-19β), 0.246 (1H, d, J = 3.6 Hz, H-19α).
3,22-di-(R)-MTPA ester of perviridisinol B (6): 1H NMR data (400 MHz, pyridine-d5) δ 5.647 (1H, d, J = 10.0 Hz, H-24), 5.524 (1H, t, J = 4.8 Hz, H-22), 5.000 (1H, dd, J = 11.1, 4.4 Hz, H-3), 4.758 (1H, dd, J = 8.7, 4.1 Hz, H-23), 4.206 (1H, t, J = 8.0 Hz, H-21β), 3.899 (1H, m, H-21α), 2.613 (1H, m, H-20), 2.179 (1H, m, H-17), 1.741 (3H, s, H-27), 1.667(3H, s, H-26), 0.970 (3H, s, H-18), 0.910 (3H, s, H-30), 0.891 (3H, s, H-28), 0.881 (3H, s, H-29), 0.641 (1H, m, H-6β), 0.476 (1H, d, J = 3.6 Hz, H-19β), 0.290 (1H, d, J = 3.7 Hz, H-19α).
3,22-di-(S)-MTPA ester of perviridisinol B (6): 1H NMR data (400 MHz, pyridine-d5) δ 5.671 (1H, d, J = 8.7 Hz, H-24), 5.492 (1H, t, J = 5.0 Hz, H-22), 4.990 (1H, dd, J = 11.7, 4.8 Hz, H-3), 4.902 (1H, dd, J = 8.8, 4.5 Hz, H-23), 4.186 (1H, t, J = 8.0 Hz, H-21β), 3.861 (1H, m, H-21α), 2.480 (1H, m, H-20), 2.105 (1H, m, H-17), 1.774 (3H, s, H-27), 1.746(3H, s, H-26), 1.011 (3H, s, H-28), 0.914 (3H, s, H-29), 0.899 (3H, s, H-30), 0.861 (3H, s, H-18), 0.667 (1H, m, H-6β), 0.445 (1H, d, J = 3.7 Hz, H-19β), 0.236 (1H, d, J = 4.0 Hz, H-19α).
3,21-di-(R)-MTPA ester of perviridisinol C (7): 1H NMR data (400 MHz, pyridine-d5) δ 4.949 (1H, td, J = 10.6, 4.5, H-3), 4.883 (1H, s, H-31b), 4.839 (1H, s, H-31a), 4.822 (1H, m, H-21β), 4.405 (1H, dd, J = 11.3, 5.1 Hz, H-21α), 2.238 (1H, m, H-25), 1.895 (1H, m, H-17), 1.830 (1H, m, H-20), 1.062 (3H, d, J = 6.7, H-26), 1.056 (3H, d, J = 6.9, H-27), 1.209 (3H, d, J = 6.4 Hz, H-21), 1.028 (3H, s, H-18), 0.899 (3H, s, H-30), 0.788 (3H, d, J = 6.3 Hz, H-28), 0.472 (1H, m, H-6β), 0.379 (1H, d, J = 3.4 Hz, H-19β), 0.162 (1H, d, J = 3.8 Hz, H-19α).
3,21-di-(S)-MTPA ester of perviridisinol C(7): 1H NMR data (400 MHz, pyridine-d5) δ 4.940 (1H, m, H-3), 4.900 (1H, s, H-31b), 4.846 (1H, s, H-31a), 4.692 (1H, dd, J = 11.3, 1.6 Hz, H-21β), 4.477 (1H, dd, J = 11.6, 3.9 Hz, H-21α), 2.259 (1H, m, H-25), 1.877 (1H, m, H-17), 1.803 (1H, m, H-20), 1.070 (3H, d, J = 6.7, H-26), 1.062 (3H, d, J = 7.0, H-27), 1.209 (3H, d, J = 6.4 Hz, H-21), 0.982 (3H, s, H-18), 0.975 (3H, d, J = 6.0 Hz, H-28), 0.823 (3H, s, H-30), 0.499 (1H, m, H-6β), 0.344 (1H, d, J = 3.7 Hz, H-19β), 0.080 (1H, d, J = 3.8 Hz, H-19α).
Determination of the Absolute Configuration of the Diol Moiety in Compound 8 by Snatzke’s Method
According to a published procedure,51,52 0.3 mg of compound 8 and 0.75 mg of Mo2(OAc)4 were dissolved in 1.0 mL dry DMSO to give a solution, with the ligand to metal molar ratio being around 1.0:1.2. The electronic transitions of the metal complex in DMSO was monitored by CD measurement immediately in the UV/vis region of 200 to 450 nm after mixing (recording a spectrum every 10 min), until a stationary induced circular dichrosim (ICD) spectrum was observed around 30 min later. The inherent ECD of compound 8 was subtracted to give a corrected ICD spectrum, and the characteristic absorption around 310 nm of the metal complex was used as key diagnostic information to analyze the absolute configuration of C-9 in compound 8.
Cytotoxicity Assays
All compounds isolated were evaluated against the HT-29 human colon cancer cell line, according to a previously described protocol.11 Compounds with a ED50 value less than 10 µM were further tested against the CCD-112CoN normal colon cell line, according to a publiched protocol.60
Enzyme-based ELISA NF-κB Assay
The enzyme-based ELISA NF-κB assay was carried out according to a published protocol.61,62 Rocaglamide was used as a positive control, with an ED50 value of 0.08 µM in this assay.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by grant P01 CA125066 (awarded to A. D. Kinghorn) from NCI, NIH. We are grateful to Mr. John Fowble, College of Pharmacy, The Ohio State University (OSU), and Dr. Chun-Hua Yuan, Campus Chemical Instrument Center, OSU, for facilitating the acquisition of the NMR spectra. We acknowledge Ms. Nan Kleinholz, Mr. Mark Apsega and Dr. Kari Green-Church, Campus Chemical Instrument Center, OSU, for the mass spectrometric measurements. The plant material was collected under the terms and conditions of a Memorandum of Agreement between the University of Illinois at Chicago and the Institute of Ecology and Biological Resources (IEBR) of the Vietnam Academy of Science and Technology, Hanoi, Vietnam. Thanks are expressed to the Director of Nui Chua National Park for permission, and to the Director of IEBR for overseeing the field operation in the collection of the plant.
Footnotes
ASSOCIATED CONTENT
Supporting Information. 1H, 13C and 2D NMR spectra of compounds 1–8, 1H NMR of (R)- and (S)-MTPA esters of 5–7. This material is available free-of-charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Pannell CM. A Taxonomic Monograph of the Genus Aglaia Lour (Meliaceae) Kew, Richmond, Surrey, UK: Kew Bulletin Additional Series XVI; HMSO; 1992. [Google Scholar]
- 2.Proksch P, Edrada RA, Ebel R, Bohnenstengel FI, Nugroho BW. Curr. Org. Chem. 2001;5:923–938. [Google Scholar]
- 3.Kim S, Salim A, Swanson SM, Kinghorn AD. Anti-Cancer Agents Med. Chem. 2006;6:319–345. doi: 10.2174/187152006777698123. [DOI] [PubMed] [Google Scholar]
- 4.Ebada SS, Lajkiewicz N, Porco JA, Jr, Li-Weber M, Proksch P. In: Progress in the Chemistry of Organic Natural Products. Kinghorn AD, Falk H, Kobayashi J, editors. Vol 94. Vienna: Springer-Verlag; 2011. pp. 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro N, Thuaud F, Nebigil C, Désaubry L. Bioorg. Med. Chem. 2012;20:1857–1864. doi: 10.1016/j.bmc.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Su B-N, Chai H, Mi Q, Riswan S, Kardono LBS, Afriastini JJ, Santarsiero BD, Mesecar AD, Farnsworth NR, Cordell GA, Swanson SM, Kinghorn AD. Bioorg. Med. Chem. 2006;14:960–972. doi: 10.1016/j.bmc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Cui B, Chai H, Santisuk T, Reutrakul V, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Tetrahedron. 1997;53:17625–17632. [Google Scholar]
- 8.Lee SK, Cui B, Mehta RR, Kinghorn AD, Pezzuto JM. Chem.-Biol. Interact. 1998;115:215–228. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 9.Hwang BY, Su BN, Chai H-B, Mi Q, Kardono LBS, Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, Fairchild CR, Vite GD, Rose WC, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J. Org. Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. ibid., 2004, 69, 6156. [DOI] [PubMed] [Google Scholar]
- 10.Salim AA, Chai H-B, Richman I, Riswan S, Kardono LBS, Farnsworth NR, Carcache-Blanco EJ, Kinghorn AD. Tetrahedron. 2007;63:7926–7934. doi: 10.1016/j.tet.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan L, Kardono LBS, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. J. Nat. Prod. 2010;73:1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salim AA, Pawlus AD, Chai H-B, Farnsworth NR, Kinghorn AD, Carcache-Blanco EJ. Bioorg. Med. Chem. Lett. 2007;17:109–112. doi: 10.1016/j.bmcl.2006.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivero-Cruz JF, Chai H-B, Kardono LBS, Setyowati FM, Afriatini JJ, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J. Nat. Prod. 2004;67:343–347. doi: 10.1021/np0304417. [DOI] [PubMed] [Google Scholar]
- 14.King ML, Chiang C-C, Ling H-C, Fujita E, Occhiai M, McPhail AT. J. Chem. Soc., Chem. Commun. 1982:1150–1151. [Google Scholar]
- 15.Meurer-Grimes BM, Yu J, Vairo GL. 6710075 B2. U.S. patent. 2004
- 16.Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SMH, Wendel HG, Brem B, Greger H, Lowe SW, Porco JA, Jr, Pelletier J. J. Clin. Invest. 2008;118:1–11. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, Davis ME, Rozewski DM, Johnson AJ, Su BN, Goettl VM, Heerema NA, Lin TS, Lehman A, Zhang XL, Jarjoura D, Newman DJ, Byrd JC, Kinghorn AD, Grever MR. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alinari L, Prince CJ, Edwards RB, Towns W, Mani R, Lehman A, Zhang X, Jarjoura D, Pan L, Kinghorn AD, Grever MR, Baiocchi RA, Lucas DM. Clin. Cancer Res. 2012;18:4600–4611. doi: 10.1158/1078-0432.CCR-12-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pannell CM. Kew Bull. 2004;59:87–94. [Google Scholar]
- 20.Gerard B, Cencic R, Pelletier J, Porco JA. Angew. Chem. Int. Ed. 2007;46:7831–7834. doi: 10.1002/anie.200702707. [DOI] [PubMed] [Google Scholar]
- 21.El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA. Angew. Chem. Int. Ed. 2007;46:7835–7838. doi: 10.1002/anie.200702700. [DOI] [PubMed] [Google Scholar]
- 22.Adams TE, El Sous M, Hawkins BC, Hirner S, Holloway G, Khoo ML, Owen DJ, Savage GP, Scammells PJ, Rizzacasa MA. J. Am. Chem. Soc. 2009;131:1607–1616. doi: 10.1021/ja808402e. [DOI] [PubMed] [Google Scholar]
- 23.Cencic R, Carrier M, Galicia-Va´zquez G, Bordeleau M-E, Sukarieh R, Bourdeau A, Brem B, Teodoro JG, Greger H, Tremblay ML, Porco JA, Jr, Pelletier J. PLoS ONE. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doroshow JT. NCI Cancer Bull. 2009;20:4. [Google Scholar]
- 25.Wu Z, Raven PH, Hong D. Flora of China. Vol. 11. St. Louis: Missouri Botanical Garden Press; 2008. p. 121. [Google Scholar]
- 26.Yang S-M, Fu W-W, Wang D-X, Tan Ch.-H, Zhu D-Y. J. Asian Nat. Prod. Res. 2008;10:459–462. doi: 10.1080/10286020801948367. [DOI] [PubMed] [Google Scholar]
- 27.Yang S-M, Tan Ch.-H, Luo H-F, Wang D-X, Zhu D-Y. Helv. Chim. Acta. 2008;91:333–337. [Google Scholar]
- 28.Chin Y-W, Chae H-S, Lee J, Bach TT, Ahn K-S, Lee H-K, Joung H, Oh S-R. Bull. Korean Chem. Soc. 2010;31:2665–2667. [Google Scholar]
- 29.Zhang L, Zhang J-H, Yang S-M, Tan Ch-H, Luo H-F, Zhu D-Y. J. Asian Nat. Prod. Res. 2010;12:215–219. doi: 10.1080/10286020903565226. [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi F, Satasook C, Isman MB, Towers GHN. Phytochemistry. 1993;32:307–310. [Google Scholar]
- 31.Dumontet V, Thoison O, Omobuwajo OR, Martin M-T, Perromat G, Chiaroni A, Riche C, Pais M, Sevenet T, Hadi AH. Tetrahedron. 1996;52:6931–6942. [Google Scholar]
- 32.Duong TN, Edrada R, Ebel R, Wray V, Frank W, Duong AT, Lin WH, Proksch P. J. Nat. Prod. 2007;70:1640–1643. doi: 10.1021/np070184w. [DOI] [PubMed] [Google Scholar]
- 33.Jares EA, Pomilio AB. J. High Resol. Chromatogr. 1989;12:565–568. [Google Scholar]
- 34.Mehta G, Krishnamurthy N, Karra SR. J. Chem. Soc. Chem. Commun. 1989;18:1299–1300. [Google Scholar]
- 35.Jerezano A, Jimenez F, del Carmen Cruz M, Montiel LE, Delgado F, Tamariz J. Helv. Chim. Acta. 2011;94:185–198. [Google Scholar]
- 36.Smith JA, Maloney DJ, Hecht SM, Lannigan DA. Bioorg. Med. Chem. 2007;15:5018–5034. doi: 10.1016/j.bmc.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 37.Arthur HR, Ko PDS, Cheung HT. Phytochemistry. 1974;13:2551–2557. [Google Scholar]
- 38.Omobuwajo OR, Martin MT, Perromat G, Sevenet T, Awang K, Pais M. Phytochemistry. 1996;41:1325–1328. doi: 10.1016/0031-9422(95)00745-8. [DOI] [PubMed] [Google Scholar]
- 39.Bohnenstengel FI, Steube KG, Meyer C, Quentmeier H, Nugroho BW, Proksch PZ. Naturforsch. 1999 doi: 10.1515/znc-1999-1212. 54c, 1075–1083. [DOI] [PubMed] [Google Scholar]
- 40.Hiort J, Chaidir, Bohnenstengel FI, Nugroho BW, Schneider C, Wray V, Witte L, Hung PD, Kiet LC, Proksch P. J. Nat. Prod. 1999;62:1632–1635. [Google Scholar]
- 41.Dumontet V, Thoison O, Omobuwajo OR, Martin M-T, Perromat G, Chiaroni A, Riche C, Pais M, Sevenet T, Hadi AH. Tetrahedron. 1996;52:6931–6942. [Google Scholar]
- 42.Xu YJ, Wu XH, Tan BK, Lai YH, Vittal JJ, Imiyabir Z, Madani L, Khozirah KS, Goh SH. J. Nat. Prod. 2000;63:473–476. doi: 10.1021/np990454d. [DOI] [PubMed] [Google Scholar]
- 43.Chaidir, Lin WH, Ebel R, Edrada RA, Wray V, Nimtz M, Sumaryono W, Proksch P. J. Nat. Prod. 2001;64:1216–1220. doi: 10.1021/np0102354. [DOI] [PubMed] [Google Scholar]
- 44.Inada A, Murayta H, Inatomi Y, Nakanishi T, Darnaedi D. J. Nat. Prod. 1995;58:1143–1146. [Google Scholar]
- 45.Inada A, Sorano T, Murata H, Inatomi Y, Darnaedi D, Nakanishi T. Chem. Pharm. Bull. 2001;49:1226–1228. doi: 10.1248/cpb.49.1226. [DOI] [PubMed] [Google Scholar]
- 46.Mohamad K, Martin MT, Leroy E, Tempete C, Sevenet T, Awang K, Pais M. J. Nat. Prod. 1997;60:81–85. doi: 10.1021/np960594c. [DOI] [PubMed] [Google Scholar]
- 47.Weber S, Puripattanavong J, Brecht V, Frahm AW. J. Nat. Prod. 2000;63:636–642. doi: 10.1021/np9905923. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y-J, Imiyabir Z, Lai Y-H, Vittal JJ, Goh S-H. ACGC Chem. Res. Commun. 2001;13:37–41. [Google Scholar]
- 49.DellaGreca M, Di MC, Zarrelli A, D'Abrosca B. J. Nat. Prod. 2004;67:1492–1495. doi: 10.1021/np049857q. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q-P, Zhang B-F, Chou G-X, Wang Z-T. Helv. Chim. Acta. 2011;94:1130–1138. [Google Scholar]
- 51.Frelek J, Ikekawa N, Takatsuto S, Snatzke G. Chirality. 1997;9:578–582. [Google Scholar]
- 52.Di Bari L, Pescitelli G, Pratelli C, Pini D, Salvadori P. J. Org. Chem. 2001;66:4819–4825. doi: 10.1021/jo010136v. [DOI] [PubMed] [Google Scholar]
- 53.Mi Q, Su BN, Chai H, Cordell GA, Farnsworth NR, Kinghorn AD, Swanson SM. Anticancer Res. 2006;26:947–952. [PubMed] [Google Scholar]
- 54.Muellner AN, Samuel R, Chase MW, Pannell CM, Greger H. Am. J. Bot. 2005;92:534–543. doi: 10.3732/ajb.92.3.534. [DOI] [PubMed] [Google Scholar]
- 55.Hausott B, Greger H, Marian B. Int. J. Cancer. 2004;109:933–940. doi: 10.1002/ijc.20033. [DOI] [PubMed] [Google Scholar]
- 56.Wang SK, Duh CY. Planta Med. 2001;67:555–557. doi: 10.1055/s-2001-16471. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro N, Thuaud F, Nebigil C, Desaubry L. Bioorg. Med. Chem. 2012;20:1857–1864. doi: 10.1016/j.bmc.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 58.Rieser MJ, Hui YH, Rupprecht JK, Kozlowski JF, Wood KV, McLaughlin JL, Hanson PR, Zhuang Z, Hoye TR. J. Am. Chem. Soc. 1992;114:10203–10213. [Google Scholar]
- 59.Su B-N, Park EJ, Mbwambo ZH, Santarsiero BD, Mesecar AD, Fong HHS, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 2002;65:1278–1282. doi: 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- 60.Still PC, Yi B, González-Cestari TF, Pan L, Pavlovicz R, Chai H-B, Ninh TN, Soejarto DD, McKay DB, Kinghorn AD. J. Nat. Prod. 2012;76 doi: 10.1021/np3007414. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Nucleic Acids Res. 2001;29:e21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng Y, Balunas MJ, Kim J-A, Lantvit DD, Chin Y-W, Chai H-B, Sugiarso S, Kardono LBS, Fong HHS, Pezzuto JM, Swanson SM, Carcache-Blanco EJ, Kinghorn AD. J. Nat. Prod. 2009;72:1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.