Abstract
Background
Health-related quality of life (HRQOL) is diminished in depressed adult outpatients and especially impaired among depressed patients referred for ECT. We compare pretreatment HRQOL in ECT and non-ECT depressed patients from two large samples, and examined whether sustained remission in depressive symptoms after ECT is associated with normalization of HRQOL.
Methods
HRQOL was measured with the Medical Outcomes Study Short Form 36 (SF36) before ECT and 6 months after ECT in an effectiveness (n=286) and an efficacy (n=243) clinical trial.
Results
ECT patients had very low baseline SF36 scores. With one exception, SF36 subscale scores in both trials were significantly lower than those of depressed outpatients. A minority of patients in both trials entered and sustained remission over the 24 week timeframe. Among sustained remitters, average SF36 scores were no different from normative scores of the general adult population, except that in the effectiveness study ECT patients reported less Bodily Pain (p<0.05) and better Mental Health (p<0.05), while in the efficacy study ECT patients reported more difficulty with Role-Emotional. (p<0.01)
Limitations
Only a modest number of patients were observed in sustained remission.
Conclusions
HRQOL is very poor in patients referred for ECT. Depressed patients who experience sustained remission after ECT, however, can expect improvement in their quality of life that leaves many in a position indistinguishable from the general adult population.
Keywords: electroconvulsive therapy, depression, quality of life
INTRODUCTION
Major depressive disorder is associated with significant loss of health-related quality of life (HRQOL). (McCall et al., 1999a;Wells et al., 1989) Compared to other depressed inpatients, electroconvulsive therapy (ECT) patients had greater difficulty with instrumental activities of daily living (IADLs) and in filling their main social role. (McCall et al., 1999b)
Effective treatment of major depression is associated with improvements in HRQOL and function, although the time course for improvement in HRQOL lags behind symptom improvement. (Blazer, 1996;McCall et al., 2001;Mintz et al., 1992) Compared with depressed patients treated with medications, ECT patients have greater improvement in HRQOL and IADLs. Yet it is not known whether patients who achieve and maintain long-term improvement in depressive symptoms following ECT experience persistent deficits in HRQOL versus whether their HRQOL normalizes over time. In this report we compare in two large independent samples HRQOL across depressed patients scheduled for ECT, depressed patients not receiving ECT, and a general population sample. Among patients in both samples with sustained remission following ECT, we compare HRQOL at 6 months post-ECT against normative data to determine whether ECT patients achieve full recovery in HRQOL versus show persistent deficits.
METHOD
HRQOL
HRQOL was measured in 2 independent studies with the Medical Outcomes Study Short Form 36 (SF36). (Ware, Jr. et al., 1992;Ware et al., 2003) The first study was the ECT Services study (Services) and the second was the Optimization of ECT study (OPT); both are described in below and elsewhere.(Prudic et al., 2004;Sackeim et al., 2009) For both, the SF36 was measured at baseline prior to ECT, and again 6 months after acute ECT. SF36 data were scored in terms of 8 standard subscales: Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. The depressed outpatient sample (N=502) had a mean age of 41.6 years and were 75.8% female. Age-matched samples were selected from nonECT depressed outpatients and from a non-institutionalized, general population sample. (Ware, Kosinski, and Gandek, 2003)
The “Services” Study
Study Sites and Study Participation
The services study was conducted at 7 hospitals in the New York City metropolitan area. A clinical outcomes evaluator was assigned to each hospital and collected all the research information. After complete description of the study to the participants, written informed consent was obtained. The study was approved by the Institutional Review Board at each hospital and at the coordinating site.
Participants met the Diagnostic and Statistical Manual (DSM-IV)(American Psychiatric Association, 1994) criteria for a major depressive episode (unipolar or bipolar) or schizoaffective disorder, depressed, based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P). (First et al., 1996) Patients were excluded if they had received ECT in the past two months or had a modified Mini-Mental State Exam (mMMSE) score <15.(McDowell et al., 1997) There was no requirement for a minimum threshold of depression symptom severity. Patients were at least 18 years of age and provided informed consent.
Conduct of ECT and management of psychotropics
The conduct of ECT varied widely at the seven sites, and was performed according to the established patterns of ECT at each site. ECT administration varied by electrical waveform (brief pulse or sine wave), stimulating electrode placement, and electrical dose strategy. There were no restrictions on the use of psychotropics during and following the ECT course, with treatment dictated by practitioners’ clinical judgment.
Classification of Clinical Outcomes
Depression severity was measured with the Hamilton Rating Scale for Depression (HRSD, 24-item). (Hamilton, 1960) Remission required a minimum reduction in HRSD scores of 60% and a postECT score of ≤10–12 days postECT. Patients were monitored every 4 weeks for 24 weeks following the ECT course. Relapse was ascribed when patients had ≥10-point increase in their HRSD score relative to immediately following ECT and a score of ≥16 at two consecutive interviews at least one week apart. Relapse was also ascribed when patients were hospitalized for treatment of depression, received another acute course of ECT, attempted suicide, or manifested psychotic symptoms. Patient were classified at the 24-week endpoint as ‘sustained remitters’ if they did not meet criteria for relapse at any time point during the 24-weeks following ECT.
The “OPT” Study
Study Sites and Study Participation
Three academic medical centers participated in the OPT study. Study subjects met the DSM IV criteria for major depressive episode (unipolar or bipolar). They also had a pretreatment HRSD-24 score of ≥21. Patients with a history of schizophrenia, schizoaffective disorder, non-mood disorder psychosis, neurological illness or insult, alcohol or drug abuse within the past year, ECT within the past 6 months, or severe medical illness that markedly increased the risks of ECT were excluded. After complete description of the study to the participants, written informed consent was obtained for all phases of the “OPT” study, and the Institutional Review Boards at each enrollment site and the coordinating site approved the study.
Conduct of ECT and management of psychotropics
The conduct of acute treatment: Phase 1
OPT patients were randomized to receive either RUL ECT at 6 times the seizure threshold (6×ST) or BL ECT at 1.5×ST, three times per week with a MECTA Spectrum device (MECTA Corp, Tualatin, OR) using standard brief pulse settings. Patients in either ECT group who did not show substantial improvement after 8 or more treatments were crossed over to high dosage (2.5×ST) BL ECT.
Patients were also randomized to receive blinded nortriptyline (NT), venlafaxine (VEN), or placebo (PL) starting the afternoon following the first ECT treatment, to achieve therapeutic blood levels (100–120 ng/ml) of NT or a minimum daily dose of 225 mg of VEN in all patients by the end of the ECT course. Patients and research staff were blinded to ECT and medication assignment except those involved in ECT administration were unmasked to ECT assignment.
Continuation Pharmacotherapy: Phase 2
Classification of Clinical Outcomes
Patients were classified as remitters if they had ≤60% reduction in HRSD scores relative to preECT baseline, with a maximum score of 10 both at an assessment within 2 days of ECT discontinuation. Patient who remained in remission for at least 1 week after ECT were offered participation in a randomized continuation trial (Phase 2).
In Phase 2, participants remained on either NT or VEN if they received that medication in Phase 1, while patients who received PL were randomized to NT or VEN. All patients also received open continuation treatment with lithium (Li). Blood samples were obtained to demonstrate a steady state NT blood level of 100–120 ng/ml and a steady state of Li level of 0.5–0.7 mEq/l. The dosing of VEN had a target of titrating up to 300 mg/d.
During the continuation phase, patients were followed until relapse or for six months. Patients were evaluated at weekly intervals for the first four weeks, and at two-week intervals for the remaining 20 weeks. As in the Services study, the criteria for relapse were a HRSD score > 16 that was maintained for at least one week (over two consecutive visits) and a mean absolute increase of ≥10 points at two consecutive visits relative to continuation trial baseline. Patients could also meet criteria for relapse if the patient was rated as considerably worse on the Clinical Global Impression at two consecutive visits over at least one week.
Performance on personal, retrograde memory function was assessed with Autobiographical Memory Inventory.(McElhiney et al., 1995) An inventory of personal memories was captured before ECT, and the percentage of those memories recalled at 24 weeks was then measured.
Statistics
Because the values of SF36 subscales vary with age and gender, ideally the data from each study would be individually matched by age and gender against published norms. Perfect matching was not possible, but good approximations were achieved. For the purposes of contrasting preECT HRQOL against nonECT depressed outpatients, we used the normative data for 502 depressed outpatients (mean age 41.6; 75.8% female). (Ware, Kosinski, and Gandek, 2003) The examination of whether sustained remission after ECT was associated with full recovery of HRQOL was conducted with different normative samples for the “Services” study versus the “OPT” study, since the baseline samples of participants from these studies had different mean ages. Means from the normative data were treated as fixed and means from the “Services” and “OPT” data were compared to them using one-sample, two-sided t-tests. Sample sizes at baseline were large enough to ensure the robustness of the t-test. Results on the smaller sample sizes in the follow-up data were confirmed using non-parametric tests. Models of scores on individual subscales were conducted with linear models.
RESULTS
The Services Study
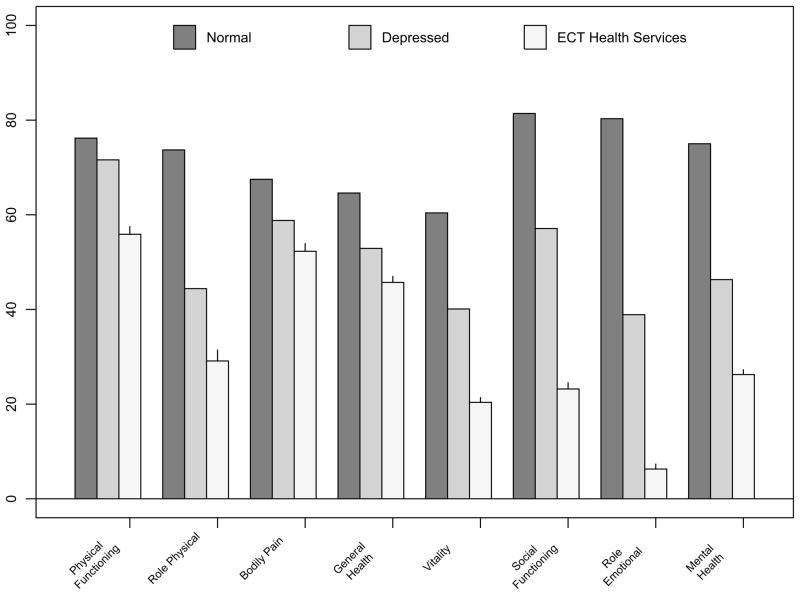
There were 347 participants in the “Services” study, 286 of whom contributed to the baseline HRQOL comparison between ECT patients and nonECT depressive controls. These 286 patients had a mean age of 55.6 years ±17.5, 62.4% were female and 13.6% non-white, with a baseline 24-item HRSD=31.2 ± 7.1. These participants received an average of 7.1 ± 3.1 ECT sessions, including 16% of whom received at least one sine-wave session, and 65.9% received at least one BL ECT session. Compared with the depressed outpatient controls, the ECT sample had significantly greater impairment on all 8 SF36 subscales (all p<0.0001)(Figure 1).
Figure 1.
Baseline SF-36 scores for Services-ECT patients (N=286, mean age 55.6 years) versus Outpatients with Depression (N=502) and Normals aged 55–64 (N=269)
Approximately one-half (46.7%) of the total sample met remission criteria at the conclusion of ECT. One hundred and forty-five remitted patients began the continuation phase follow-up period and were followed for the entire 24 weeks or until relapse. Of these, 55 completed the 24 weeks of follow-up without relapse. There were 38 patients who (1) initially remitted with ECT, (2) remained in remission for the entire period of follow up, and who (3) contributed SF36 scores both at baseline and at 24 weeks post ECT. These 38 patients had a mean age of 59.8 ± 19.7 years, 61.3% were female, and had a mean postECT HRSD score of 6.1 ± 2.5. HRSD scores at 24-week follow up were 4.1 ± 3.0. The adequacy of post-ECT antidepressant prophylaxis against relapse was uneven during the follow up period, with 53.3% of patients with sustained remission receiving adequate pharmacotherapy, and 49% receiving ≥1 outpatient continuation ECT session.
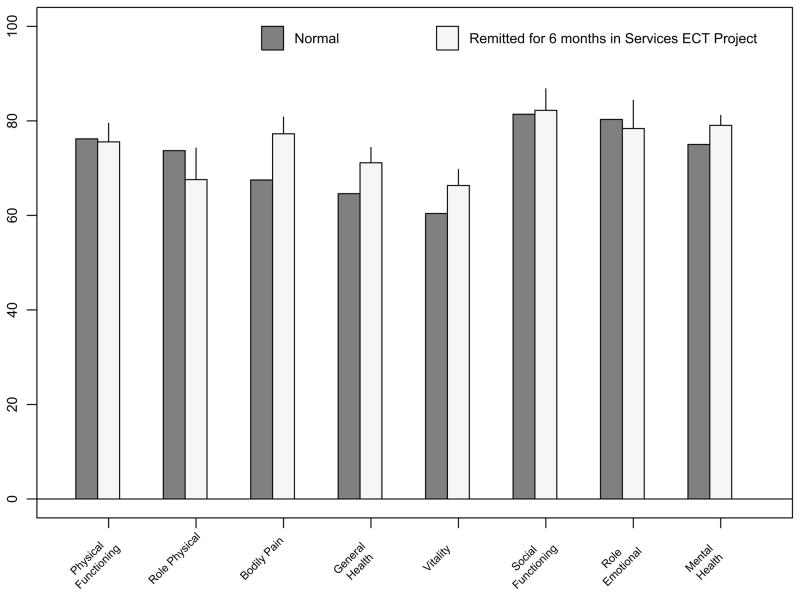
SF36 subscale scores did not significantly differ between sustained remitters and the normal comparison group taken from the general adult population, except for two subscales. Remitted ECT patients reported less Bodily Pain (p<0.05). For Mental Health, the mean among remitted ECT patients was higher; although the t-test the difference was not significant (p=0.07), the nonparametric p-value was 0.015(Figure 2).
Figure 2.
SF36 bar graph scores after 24 weeks of remission in Services ECT project (N=38, mean age 59.8), and Normals aged 55–64 (N=269)
The OPT Study
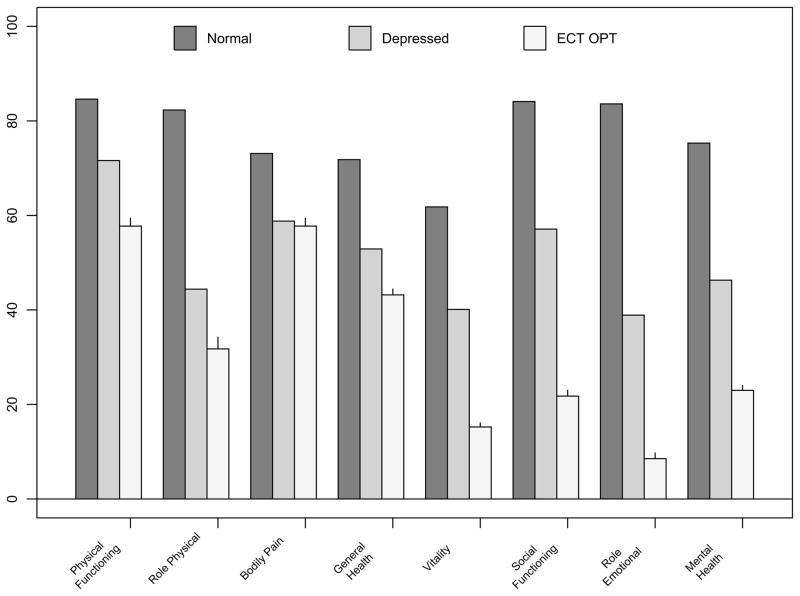
There were 319 participants enrolled in the “OPT” study, of which 243 contributed to the comparison of baseline HRQOL between ECT patients and the nonECT outpatient depressive comparison group. The OPT sample had a mean age of 47.7 ± 4.7 years, 61.3% were female, with a baseline 24-item HRSD=31.3 ± 6.4, and received an average of 8.1 ± 4.3 ECT sessions. All baseline SF36 subscale scores, except Bodily Pain, were significantly worse in the ECT sample (p<0.0001) than the depressed outpatient comparison group. (Figure 3) The lowest subscale scores were for Role Emotional, with 24 of 26 persons scoring ‘0’ prior to ECT.
Figure 3.
Baseline SF-36 bar graphs scores for OPT-ECT patients (N=243, mean age 47.7) versus Outpatients with Depression (N=502) and Normals aged 45–54 (N=338)
Roughly one-half (56.4%) of the total sample met remission criteria at the conclusion of ECT. One hundred and twenty-two remitted patients began the continuation phase follow-up, and 41 completed 24 weeks of follow-up without relapse. These patients were significantly older than the 61 patients who relapsed (mean age: 54.8 vs. 44.9 years, p= .0009). The adequacy of post-ECT continuation pharmacotherapy was good during the follow-up period, with mean VEN daily oral doses of 275.0 ± 43.8 mg, mean NT serum level of 108 ± 36.9 ng/ml, and mean serum lithium levels of 0.35 ± 0.15 mEq/L.
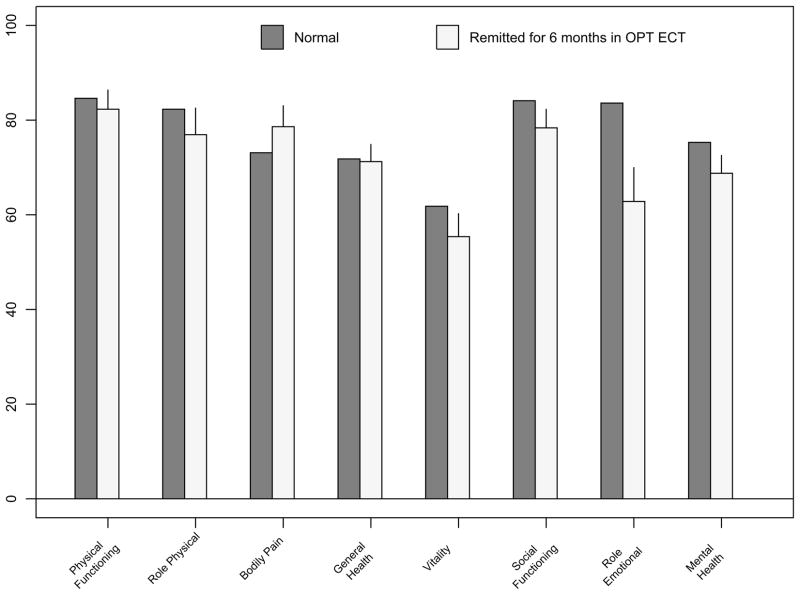
There were 26 patients who (1) initially remitted with ECT, (2) remained in remission for the entire 24 weeks of follow up, and who (3) contributed SF36 scores both at baseline and at 24 weeks post ECT. These 26 had a mean age of 53.0 ± 15.3 years and 50.0% were female. These 26 patients entered the continuation phase with a HRSD of 5.7 ± 2.5, and finished the 24 weeks continuation with a HRSD of 6.6 ± 4.1 (range 1–15). Sustained remitters and the sample from the general adult population did not significantly differ in SF36 subscale scores, except that remitted ECT patients had significantly greater impairment in Role Emotional (RE)(p<0.01). (Figure 4) Five of these 26 patients had RE scores of “0” at 24 weeks of follow-up, despite meeting remission criteria. A regression model for RE score at 24 weeks for these patients, including age, gender, baseline RE score, 24-week HRSD score, and autobiographical memory score, was significant (F=5.7; df=6,15; p<0.005). Within this model, significant predictors were 24-week HRSD (p<0.005), and baseline RE (p<0.05). Four of the 5 patients with RE score of “0” at 24 weeks also had RE scores of “0” at baseline. Age, gender, and autobiographical memory score were not significant predictors of 24 week RE score.
Figure 4.
SF36 bar graph scores after 24 weeks of remission in OPT ECT (N=26, mean age 52.6) and Normals aged 45 54 (N=338)
DISCUSSION
Despite broad differences in treatment settings, eligibility criteria, and treatment provision, an effectiveness and efficacy study yielded strikingly similar results regarding HRQOL. In both studies, HRQOL as captured by SF36 subscales was markedly impaired in depressed inpatients prior to beginning ECT, and substantially worse than that seen in depressed outpatients who are not receiving ECT. Following the rationale that health care resources should be allocated in relation to the magnitude of expected improvement in quality of life, improvements from very low baseline levels of HRQOL before ECT may help to justify the expense of this treatment. (Russell et al., 1996) It also may be that the treatment is more acceptable to patients precisely because of their profound suffering and debility.
The second major finding is that among patients who experience sustained symptom remission following ECT administration, HRQOL improves to the point that treated patients are nearly indistinguishable from the non-institutionalized, general adult population. The only differences observed between the patients in sustained remission and the general population sample were an advantage for both Bodily Pain and Mental Health in the ECT patients in the Services study and a disadvantage in Role Emotional (RE) in the ECT patients in the OPT study. If, however, the finding of lower RE scores in ECT patients in the OPT study is an accurate depiction of remitted ECT patients’ HRQOL, then it suggests that 6 months after ECT, some remitted patients may still be less than optimally effective in their work and other regular daily activities due to emotional problems. Lack of full normalization in HRQOL could be a result of many factors, including persistent or residual effects of depressive illness, iatrogenic effects of treatment, persistent social disadvantage, or general medical conditions, some of which might have emerged after the conclusion of ECT. Our regression models of the 24-week RE scores suggests that persistent problems in this domain reflect the severity of difficulties in the domain at baseline, before ECT, as well as the presence of residual symptoms of depression into remission. Problems with autobiographical memory were not significantly related to persistent deficits in RE scores. Nonetheless, despite these possibilities, sustained remission with ECT led to a near normalization of HRQOL in most domains. Thus, ECT appears to convey substantial improvement in HRQOL among patients who have sustained depression symptom remission
This report has several strengths and limitations. A major strength was the availability of data from two distinct types of ECT trials – an effectiveness and efficacy study. Both studies provided large numbers of patients for examination of baseline HRQOL. An important limitation is the relatively small number of patients who had both sustained remission after ECT and complete data for the SF36 at baseline and 24 weeks later, with the two studies contributing a total of only 64 persons for these analyses. Although unlikely, there is the theoretical possibility that the findings were biased to the extent that study attrition among remitted ECT patients may have been higher for those with lower HRQOL. A second limitation was the difficulty in identifying suitable normative values against which to compare the ECT groups. For the purposes of the baseline comparisons, the comparison group of depressed outpatients was younger (mean=41.6 years) than either the baseline age of the Services study (55 years) or the OPT study (49 years). As SF36 subscale scores decline slowly over mid to late life, (Ware, Kosinski, and Gandek, 2003) the use of a younger depressed outpatient groups as a comparison may have slightly exaggerated the differences between ECT and non-ECT patients. The age-matching between the sustained remitters and the general adult population norms narrowed the age differences. Also, the difference in baseline mean ages between the two studies led us to choose different normative samples for the general adult population for the purpose of comparison. The normative data for the 45–54 year old norms were similar to the norms for the non-institutionalized general adult population norms of all adults in the US, with subscale differences of no more than 1–2 points between the entire population and the 45–54 year-old norms. However, the norms for Physical Function, Role-Physical, Bodily Pain, and General Health were 5–10 points lower for each subscale in the 55–64 year olds.
Additionally, this study did not establish conclusively that patients with sustained remission following ECT return to premorbid levels of HRQOL. Such a demonstration would require assessment of HRQOL prior to the onset of the depressive episode to establish that there is return to baseline values rather than comparison with age-matched general population controls. Such a design poses great logistical challenges, especially in view of the highly variable duration of depression episodes and the low rate of ECT use in the community.
In summary, HRQOL is profoundly impaired in patients scheduled to receive ECT. We suspect that impaired HRQOL contributes to referral decisions for ECT. ECT leads to short-term improvement in HRQOL for most patients, (McCall WV et al., 2011;McCall et al., 2004;McCall et al., 2006) and sustained remission after ECT produces further improvement in HRQOL with near-normalization of HRQOL values. Unfortunately, not all patients benefit from ECT and depressive relapse is common after acute response, leading to a loss of improvement in HRQOL.(McCall WV, Rosenquist PB, Kimball, Haskett R, Isenberg, Prudic, Lasater, and Sackeim, 2011;McCall, Prudic, Olfson, and Sackeim, 2006) Only a minority of patients achieves sustained remission following ECT. When this is accomplished, there appears to be full normalization of HRQOL 6 months after ECT. Thus, the principal clinical challenge in achieving fullest possible normalization of HRQOL in ECT patients is increasing the remission rate and decreasing the relapse rate.
Acknowledgments
Role of the Funding Sources: NIMH. R01MH59069:01-05 was awarded to Dr Prudic to support the “Services” study, while the OPT study was supported in part by NIMH RO1 MH35636 (Dr Sackeim), RO1 MH61609 (Dr Sackeim), RO1 MH61594 (Dr McCall), MO1 RR07122 (Dr. McCall), RO1 MH61621 (Dr Isenberg), and RO1 MH61591 (Dr Haskett) from the US Public Health Service, Rockville, MD; a grant from Wyeth Pharmaceuticals for the purchase of the medications used in this study, and the loaning of ECT devices from MECTA Corp.
Footnotes
The clinical trials identifier for the OPT study was NCT00045916. http://clinicaltrials.gov/ct2/search
Conflict of Interest: Dr McCall’s present work in ECT is supported by NIMH 1U01MH086127-01
Dr Prudic’s present work is supported by NIMH 1U01MH084241, NARSAD, and Alzheimer’s AssociationIIRG-09-131861
Dr. McCall has been a scientific advisor for Sunovion and Astra Zeneca within the last 24 months
Dr Reboussin has no disclosures
Dr Prudic has no disclosures
Dr Haskett has no disclosures
Dr Isenberg is a full-time employee of WellPoint. Points of view expressed in the paper do not necessarily reflect the official position of WellPoint.
Dr Olfson has no disclosures
Dr Rosenquist has no disclosures
Dr Sackeim is a consultant for the following: Cervel Neurotech, Inc.; Cyberonics Inc.; Eli Lilly; Magstim Limited; MECTA Corporation; Neuronetics Inc.; NeuroPace Inc.; Novartis Inc., Pfizer Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 1994. [Google Scholar]
- 2.Blazer DG. Severe episode of depression in late life: the long road to recovery. Am J Psychiatry. 1996;153(12):1620–1623. doi: 10.1176/ajp.153.12.1620. [DOI] [PubMed] [Google Scholar]
- 3.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (with Psychotic Screen) (SCID-I/P) New York: 1996. [Google Scholar]
- 4.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCall WV, Rosenquist PB, Kimball J, Haskett R, Isenberg K, Prudic J, Lasater B, Sackeim HA. Health-related quality of life in a clinical trial of ECT followed by continuation pharmacotherapy: effects immediately after ECT and at 24 weeks. J ECT. 2011;27:97–102. doi: 10.1097/YCT.0b013e318205c7d7. [DOI] [PubMed] [Google Scholar]
- 6.McCall WV, Cohen W, Reboussin B, Lawton P. Effects of mood and age on quality of life in depressed inpatients. J Affect Disord. 1999a;55(2–3):107–114. doi: 10.1016/s0165-0327(98)00204-3. [DOI] [PubMed] [Google Scholar]
- 7.McCall WV, Cohen W, Reboussin B, Lawton P. Pretreatment differences in specific symptoms and quality of life among depressed inpatients who do and do not receive electroconvulsive therapy: a hypothesis regarding why the elderly are more likely to receive ECT. J ECT. 1999b;15(3):193–201. [PubMed] [Google Scholar]
- 8.McCall WV, Reboussin BA, Cohen W, Lawton P. Electroconvulsive therapy is associated with superior symptomatic and functional change in depressed patients after psychiatric hospitalization. J Affect Disord. 2001;63(1–3):17–25. doi: 10.1016/s0165-0327(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 9.McCall W, Dunn A, Rosenquist PB. Quality of life and function after ECT. British Journal of Psychiatry. 2004;(185):405–409. doi: 10.1192/bjp.185.5.405. [DOI] [PubMed] [Google Scholar]
- 10.McCall WV, Prudic J, Olfson M, Sackeim HA. Health-related quality of life following ECT in a large community sample. Journal of Affective Disorders. 2006;90:269–274. doi: 10.1016/j.jad.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: The Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 12.McElhiney MC, Moody BJ, Steif B, Prudic J, Devanand DP, Nobler MS, Sackeim HA. Autobiographical memory and mood; effects of electroconvulsive therapy. Neuropsychology. 1995;9(501):517. [Google Scholar]
- 13.Mintz J, Mintz LI, Arruda MJ, Hwang SS. Treatments of depression and the functional capacity to work. Arch Gen Psychiatry. 1992;49(10):761–768. doi: 10.1001/archpsyc.1992.01820100005001. [DOI] [PubMed] [Google Scholar]
- 14.Prudic J, Olfson M, Marcus S, Fuller R, Sackeim HA. Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry. 2004;55(3):301–312. doi: 10.1016/j.biopsych.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Russell L, Gold M, Siegel J, et al. The role of cost-effectiveness analysis in health and medicine: Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 16.Sackeim HA, Dillingham E, Prudic J, Cooper T, McCall WV, Rosenquist P, Isenberg K, Garcia K, Mulsant BH, Haskett RF. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes. Archives of General Psychiatry. 2009;66:729–737. doi: 10.1001/archgenpsychiatry.2009.75. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 18.Ware J, Kosinski M, Gandek B. Manual & Interpretation Guide. QualityMetric Incorporated; Lincoln, RI: 2003. SF-36 Health Survey. [Google Scholar]
- 19.Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262(7):914–919. [PubMed] [Google Scholar]