Abstract
Previous data suggest women are at increased risk of death from aortic dissection. Therefore, we analyzed data from the GenTAC registry, the NIH-sponsored program that collects information about individuals with genetically-triggered thoracic aortic aneurysms and cardiovascular conditions. We performed cross-sectional analyses in adults with Marfan syndrome (MFS), familial thoracic aortic aneurysm or dissection (FTAAD), bicuspid aortic valve (BAV) with thoracic aortic aneurysm or dissection, and subjects under 50 years of age with thoracic aortic aneurysm or dissection (TAAD<50y). Women comprised 32% of 1449 subjects and were 21% of subjects with BAV, 34% with FTAAD, 22% with TAAD <50y, and 47% with MFS. Thoracic aortic dissections occurred with equal gender frequency yet women with BAV had more extensive dissections. Aortic size was smaller in women but was similar after controlling for BSA. Age at operation for aortic valve dysfunction, aneurysm or dissection did not differ by gender. Multivariate analysis (adjusting for age, BSA, hypertension, study site, diabetes, and subgroup diagnoses) showed that women had fewer total aortic surgeries (OR= 0.65, p < 0.01) and were less likely to receive angiotensin converting enzyme inhibitors (ACEi) (OR=0.68, p < 0.05). As in BAV, other genetically-triggered aortic diseases such as FTAAD and TAAD<50 are more common in males. In women, decreased prevalence of aortic operations and less treatment with ACEi may be due to their smaller absolute aortic diameters. Longitudinal studies are needed to determine if women are at higher risk for adverse events.
Keywords: Aorta, aneurysm, dissection, gender
INTRODUCTION
Women with cardiovascular disease face unique risks and outcomes when compared to men. For example, despite a lower incidence of coronary artery disease in young women versus young men, the clinical outcome of acute myocardial infarction among young females is worse [Vaccarino et al., 1999]. The International Registry of Acute Aortic Dissections (IRAD) identified significant gender-related differences in individuals with aortic dissection [Nienaber et al., 2004]. That study found that women are more likely to die following an acute dissection and to suffer more aorta-related complications than men. Davies et al. [2006] have suggested that female gender is an independent risk factor for adverse aortic events including dissection and rupture and death. In Marfan syndrome (MFS), early surgical replacement of the sinus of Valsalva has dramatically increased life expectancy [Silverman et al., 1995], yet whether women with MFS-related aortic disease are treated differently than men is not well studied. Furthermore, gender differences in other genetically-triggered or idiopathic aortic diseases including bicuspid aortic valve (BAV), familial thoracic aortic aneurysm and dissection (FTAAD) or individuals under 50 years of age with thoracic aortic aneurysm or dissection (TAAD<50) have received little attention.
The national registry of Genetically-Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) is a rich source of clinical data on individuals with known or presumed genetic conditions associated with aortic aneurysm and dissection [Eagle and GenTAC Consortium, 2009b; Kroner et al]. In this study we sought to determine if there were gender-based differences in the prevalence of aortic disease, its severity, and its treatment in the GenTAC cohort.
MATERIALS AND METHODS
The design and methods for the GenTAC Registry are described elsewhere [Eagle and GenTAC Consortium, 2009b]. Each of the five GenTAC Phase I sites obtained approval to conduct this study from their respective institutional review boards. Informed consent was obtained at the sites of enrollment.
Study Population
From November 1, 2007 to December 31, 2010, a total of 1449 adults with the conditions under study were enrolled into GenTAC, (including 458 with MFS, 495 with BAV, 219 with FTAAD and 277 with TAAD <50y) who comprise the population of this cross-sectional study [Kroner et al., 2011]. Subjects with Loeys-Dietz syndrome, Turner syndrome, Ehlers-Danlos syndrome, and Shprintzen-Goldberg syndrome were excluded due to small sample sizes. Description of dissection was based on the DeBakey classification [Debakey et al., 1965] where type I includes the ascending aorta, aortic arch and descending aorta, type II is confined to the ascending aorta, and type III is confined to the descending aorta. Dissections limited to the abdominal aorta were counted separately. Total aortic operations included the following: aortic valve repair, isolated aortic valve replacement, valve-replacing aortic root replacement, valve-sparring aortic root replacement, ascending aortic replacement, aortic arch replacement, coarctation repair, descending thoracic aortic replacement, and thoracoabdominal aortic replacement. On a subset of surgical patients, operative complications, age of first operation and, total number of operations were reviewed. Operative complications included: myocardial infarction, stroke, paraplegia/paralysis, prolonged intubation, acute renal failure, bleeding requiring operation, and vocal cord paralysis.
Echocardiographic measurements
Reports of the most recent transthoracic echocardiogram were transmitted from GenTAC study sites at the time of enrollment and were entered into a database. Only data from subjects with native aortas were considered. The aortic annulus, sinuses of Valsalva (root), and ascending aorta were compared using absolute measurements. In order to account for differences in body size, values were indexed both as Z-scores and as observed/expected ratios. These values were determined from established formulas and include adjustments for age and body surface area (BSA) [Roman et al., 1989]. Aortic regurgitation and stenosis were evaluated by clinical report as absent, mild, moderate or severe.
Statistical Analysis
Data are described by means with standard deviations and frequencies with percentages. To investigate gender differences in demographic data and outcome measurements, bivariate chi-squared and t-tests for categorical and continuous variables, respectively, were used. Gender differences in demographics, general health, prescribed medications, surgical history, past dissections, and imaging data were examined by specific sub-group diagnoses and across the GenTAC study population. In order to account for multiple testing, and minimize the potential of falsely concluding a significant difference, the Holm-Bonferroni post-hoc test [Holm, 1979] was used for gender comparisons involving the total population. A multivariate logistic regression model, with gender as the outcome, was built to further analyze the gender comparisons by controlling for possible multicollinearity between each individual covariate's relationship with gender. All demographic, general health and drug history covariates were included in the logistic regression (except surgical, dissection and imaging data, which were excluded due to relatively low sample sizes), and odds ratios were calculated along with their 95% confidence intervals for each covariate. The model also controlled for history of hypertension, BSA, age at diagnosis, diabetes, study site and primary diagnosis regardless of whether these factors significantly differed by gender.
RESULTS
Demographics
Women comprised 457 (32%) of the total subjects (Table I). All groups had a statistically significant male predominance, except for MFS, where 217 of 458 (47%) were women. Women accounted for 105 of 495 (21%) with BAV, 75 of 219 (34%) with FTAAD, and 60 of 277 (22%) with TAAD <50y. There was no significant difference in age of enrollment by gender overall or within the diagnostic groups. There were also no gender differences among the diagnostic groups for race, ethnicity, or family history of disease. There was no gender difference in baseline coronary disease risk factors such as hypertension or diabetes (Table II). Although not statistically significant, men with BAV tended to report more atherosclerotic disease or myocardial infarction (8.8%) than women (5.1%, p = 0.10).
Table I.
Primary Diagnosis by Gender (n (%))
| Primary Diagnosis | Male (n = 992) | Female (n = 457) | Total (n = 1,449) | P-Value (Chisq) |
|---|---|---|---|---|
| MFS | 241 (52.6) | 217 (47.4) | 458 | 0.262 |
| BAV with TAAD | 390 (79) | 105 (21) | 495 | <0.0001 |
| FTAAD | 144 (65.8) | 75(34.3) | 219 | <0.0001 |
| TAAD. <50 | 217 (78.3) | 60 (21.7) | 277 | <0.0001 |
Abbreviations: Chisq = chi squared analyses, MFS = Marfan syndrome, BAV with TAAD= bicuspid aortic valve with thoracic aortic aneurysm or dissection, FTAAD = familial thoracic aortic aneurysm/dissection, TAAD<50 = other thoracic aortic aneurysm/dissection < 50 y.
Table II.
patient demographics
| Marfan | BAV | FTAAD | TAAD<50 | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic Variable | Stat | Male (n=241) | Female (n=217) | p-value | Male (n=390) | Female (n=105) | p-value | Male (n=144) | Female (n=75) | p-value | Male (n=217) | Female (n=60) | p-value | Male (n=992) | Female (n=457) | All (n=1,449) | Adjusted p-value† |
| Mean Age at Enrollment | Mean (SD) | 40.0 (14.1) | 40.7 (14.1) | 0.6 | 50.9 (13.9) | 50.6 (13.2) | 0.85 | 49.1 (13.5) | 49.1 (15.6) | 0.98 | 42.9 (11.3) | 42.2 (12.0) | 0.7 | 46.2 (14.1) | 44.6 (14.6) | 45.7 | ns |
| Mean Age at Dx | mean (std) | 20.8 (15.1) | 19.1 (15.6) | 0.28 | 42.2 (18.3) | 42.8 (17.4) | 0.79 | 41.0 (15.3) | 43.1 (15.5) | 0.35 | 37.3 (11.2) | 35.6 (11.3) | 0.29 | 36.0 (17.9) | 30.9 (19.1) | 34.4 | 0.003 |
| Am. Indian | n(%) | 2 (0.8) | 1 (0.5) | 0.92 | 1 (0.3) | 1 (10) | 0.17 | 0 | 0 | 0.28 | 0 | 0 | 0.52 | 3 (0.3) | 2 (0.4) | 5 | |
| Asian | n(%) | 6 (2.5) | 4 (1.8) | 10 (2.6) | 2 (1.9) | 2 (1.4) | 0 | 10 (4.6) | 3 (5.0) | 28 (2.8) | 9 (2.0) | 37 | |||||
| Black | n(%) | 11 (4.6) | 12 (5.5) | 6 (1.5) | 3 (2.9) | 8 (4.8) | 1 | 26 (12.0) | 11 (18.3) | 51 (5.1) | 27 (5.9) | 78 | |||||
| Pac Island | n(%) | 0 | 0 | 0 | 1 (1.0) | 1 (0.7) | 2 (2.7) | 0 | 0 | 1 (0.1) | 3 (0.7) | 4 | ns | ||||
| White | n(%) | 217 (90.0) | 197 (90.8) | 365 (93.6) | 94 (89.5) | 127 (88.2) | 67 (89.3) | 167 (77.0) | 44 (73.3) | 876 (88.3) | 402 (88.0) | 1,278 | |||||
| Unknown | n(%) | 5 (2.1) | 3 (1.4) | 8 (2.1) | 4 (3.8) | 6 (4.2) | 5(6.7) | 14 (6.5) | 2 (3.3) | 33 (3.3) | 14 (3.1) | 47 | |||||
| Mean BSA (m2) | mean (std) | 2.22 (0.27) | 1.91 (0.21) | <0.001 | 2.06 (0.25) | 1.80 (0.21) | <0.001 | 2.09 (0.25) | 1.82 (0.23) | <0.001 | 2.12 (0.25) | 1.77 (0.19) | <0.001 | 2.12 (0.26) | 1.85 (0.22) | 2.03 | 0.003 |
| Hypertension (Yes) | n(%) | 23 (9.5) | 19 (8.8) | 0.77 | 99 (25.4) | 28 (26.7) | 0.79 | 29 (20.1) | 15(20.0) | 0.98 | 65 (30.0) | 23 (38.3) | 0.22 | 216 (21.8) | 85 (18.6) | 301 | ns |
| CVD/MI | n(%) | 5 (3.2) | 6 (3.9) | 0.76 | 30 (12.1) | 3 (4.8) | 0.1 | 9 (9.1) | 3 (7.3) | 0.72 | 10 (9.0) | 3 (8.1) | 0.87 | 54 (8.8) | 15 (5.1) | 69 | ns |
| Diabetes | n(%) | 12 (5.0) | 6 (2.8) | 0.18 | 20 (5.1) | 5 (4.8) | 0.98 | 7 (4.9) | 1 (13) | 0.3 | 12 (5.5) | 1 (1.7) | 0.38 | 51 (5.2) | 13 (2.8) | 64 | ns |
| Medication (any) | n(%) | 224 (93.0) | 185 (85.3) | 0.01 | 347 (89.0) | 91 (86.7) | 0.51 | 122 (84.7) | 61 (81.3) | 0.32 | 183 (84.3) | 52 (86.7) | 0.66 | 876 (88.3) | 389 (85.1) | 1,265 | ns |
| Beta-Blocker | n(%) | 208 (86.3) | 175 (80.7) | 0.1 | 283 (72.6) | 77 (73.3) | 0.87 | 110 (76.4) | 53 (70.7) | 0.36 | 158 (72.8) | 44 (73.3) | 0.94 | 759 (76.5) | 349 (76.4) | 1,108 | ns |
| Losartan | n(%) | 87 (36.1) | 69 (31.8) | 0.33 | 29 (7.4) | 8 (7.6) | 0.95 | 26 (18.1) | 16 (21.3) | 0.56 | 30 (13.8) | 6 (10.0) | 0.44 | 172 (17.3) | 99 (21.7) | 271 | ns |
| Other ARB | n(%) | 21 (8.7) | 10 (4.6) | 0.08 | 38 (9.7) | 16 (15.2) | 0.11 | 17 (11.8) | 9 (12.0) | 0.97 | 20 (9.2) | 4 (6.7) | 0.53 | 96 (9.9) | 39 (8.5) | 135 | ns |
| ACE-Inhibitor | n(%) | 49 (20.3) | 29 (13.4) | 0.05 | 143 (36.7) | 23 (21.9) | 0.005 | 41 (28.5) | 15 (20.0) | 0.17 | 69 (31.8) | 19 (31.7) | 0.99 | 302 (30.4) | 86 (18.8) | 388 | 0.003 |
| Verapamil/Diltiazem | n(%) | 31 (12.9) | 19 (8.8) | 0.16 | 13 (3.3) | 6 (5.7) | 0.26 | 9 (6.3) | 4 (5.3) | 0.79 | 4 (1.8) | 6 (10.0) | 0.003 | 57 (5.8) | 35 (7.7) | 92 | ns |
| Other Ca+ Channel | n(%) | 32 (13.3) | 15 (6.9) | 0.03 | 49 (12.6) | 16 (15.2) | 0.47 | 24 (16.7) | 12 (16.0) | 0.9 | 50 (23.0) | 9 (15.0) | 0.18 | 155 (15.6) | 52 (11.4) | 207 | ns |
| HMG-Coa | n(%) | 32 (13.3) | 21 (9.7) | 0.23 | 153 (39.2) | 33 (31.4) | 0.14 | 44 (30.6) | 22 (29.3) | 0.85 | 66 30.4) | 14 (23.3) | 0.28 | 295 (29.7) | 90 (19.7) | 385 | 0.003 |
Holm-Bonferroni test
Over 85% of men and women were on medications for cardiovascular disease (Table II). There was no difference by gender in overall medication usage; however, in univariate analyses women were less likely than men to take ACEi (19% vs. 30%) or HMG-CoA reductase inhibitor (statin) cholesterol lowering drugs (20% vs. 30%, both p <0.003). Within the MFS group, there was no gender-related difference in the use of beta-blocker or losartan; however, women with MFS were less likely to receive an ACE inhibitor (13% vs. 20%, p <0.05).
Echocardiographic data
After excluding those that had aortic operations, the diameter of the aortic annulus, sinuses of Valsalva and ascending aorta were examined for gender differences (Table III). Women demonstrated smaller absolute aortic measurements (p <0.003). However, after adjusting for body size and age there were no differences in Z score values or observed/predicted ratios of the aortic root between GenTAC men and women. Similarly, there were no gender differences in degree of aortic valve regurgitation or stenosis.
Table III.
Imaging data by gender
| Statistic | Male (n=992) | Female (n=457) | Total (n=1,449) | Adjusted p-value† | |
|---|---|---|---|---|---|
| Aortic Valve Annulus | n | 199 | 120 | 319 | |
| Native measurement (cm) | mean (std) | 2.47 (0.36) | 2.28 (0.32) | 2.40 (0.36) | 0.003 |
| Aortic Root | n | 336 | 184 | 520 | |
| Native measurement (cm) | mean (std) | 4.21 (0.54) | 3.83 (0.56) | 4.07 (0.57) | 0.003 |
| Z score | mean (std) | 3.25 (2.5) | 3.08 (3.25) | 3.19 (2.79) | ns |
| Indexed value (obs/pred) | mean (std) | 1.23 (0.15) | 1.21 (0.20) | 1.22 (0.17) | ns |
| Ascending Aorta | n | 248 | 136 | 384 | |
| Native measurement (cm) | mean (std) | 3.80 (0.80) | 3.42 (0.71) | 3.66 (0.79) | 0.003 |
| Aortic Regurgitation | n | 223 | 104 | 327 | |
| >= mild | n(%) | 130 (58.3) | 54 (51.9) | 184 (56.3) | ns |
| Aortic Stenosis | n | 84 | 34 | 118 | |
| >= mild | n(%) | 18 (21.4) | 7 (20.6) | 25 (21.2) | ns |
Holm-Bonferroni test
Aortic Dissection
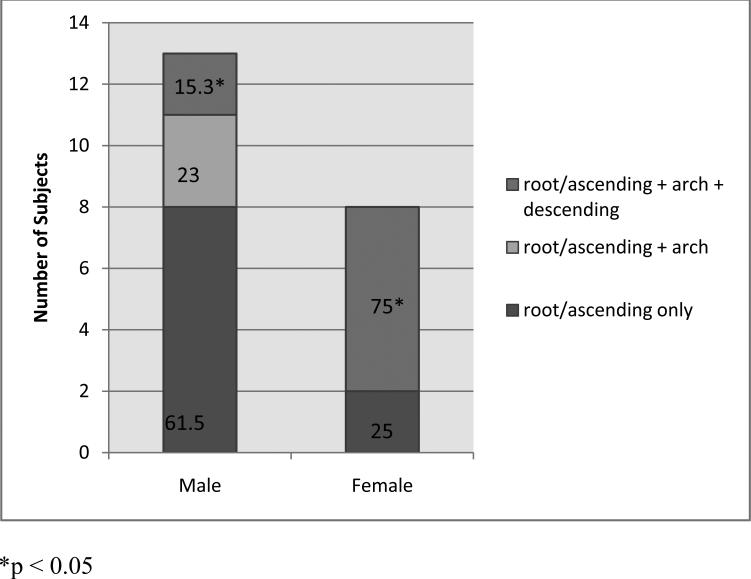
Within the study population, 303 subjects survived an aortic dissection. Aortic dissection occurred with equal gender frequency in the entire population and in diagnostic subgroups. Irrespective of gender, history of dissection was more common in FTAAD (in 74 subjects, 34%) and TAAD<50y (88 subjects, 32%) than in MFS (108 subjects, 23%) or BAV (33 subjects, 7%). Age when a dissection operation occurred did not differ by gender (Table IV). Overall there were no gender differences in the percentages of subjects with history of DeBakey I (extending from the ascending to descending aorta), DeBakey II (confined to the ascending aorta), or DeBakey type III dissections (confined to the descending aorta). However, among those with BAV, history of DeBakey type I dissection was significantly more common in women than men (5.7% vs. 0.8%, p <0.005), (Table IV). Further, ascending aortic dissections were more extensive in women. Figure 1 shows that among the small number of survivors of BAV dissections involving the ascending aorta, 75% extended to the descending aorta in women, but only 15% extended that far in men (p<0.01).
Table IV.
Dissection Type by Primary Diagnosis and Gender
| Marfan | BAV | FTAAD | TAAD<50 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location/Type | Male (n=241) | Female (n= 217) | p-value | Male (n=390) | Female (n= 105) | p-value | Male (n=144) | Female (n= 75) | p-value | Male (n=217) | Female (n= 60) | p-value |
| Subjects with History of Dissection | 63 (26.1) | 45 (20.7) | 0.17 | 24 (6.1) | 9 (8.6) | 0.38 | 44(30.6) | 30 (40.0) | 0.16 | 69 (31.8) | 19 (31.7) | 0.98 |
| DeBakey (total subjects) | ||||||||||||
| I | 15 (6.2) | 10 (4.6) | 0.44 | 3 (0.8) | 6 (5.7) | 0.004 | 12 (8.3) | 3 (4.0) | 0.22 | 19 (8.8) | 3 (5.0) | 0.34 |
| II | 9 (3.7) | 6 (2.8) | 0.56 | 9 (2.3) | 2 (1.9) | 0.8 | 8 (5.6) | 5 (6.7) | 0.74 | 19 (8.8) | 7 (11.7) | 0.49 |
| III | 19 (7.9) | 11 (5.1) | 0.22 | 1 (0.3) | 0 | 6 (4.2) | 5 (6.7) | 0.42 | 8 (3.7) | 3 (5.0) | 0.64 | |
| Abdominal Only | 3 (1.2) | 0 | 0.25 | 0 | 1 (1.0) | 0.21 | 1 (0.7) | 3 (4.0) | 0.12 | 0 | 0 | |
| Age at Operation for Dissection | N = 30 | N = 24 | N = 12 | N = 8 | N = 23 | N = 11 | N = 40 | N = 9 | ||||
| 38.3 (10.6) | 41.4 (10.7) | 0.29 | 48.6 (13.1) | 46.1 (12.6) | 0.84 | 42.6 (10.7) | 46.2 (18.3) | 0.55 | 42.1 (6.9) | 40.4 (5.2) | 0.44 | |
Figure 1.
Dissection pattern among subjects with BAV. More extensive, dissections, were significantly more common in women compared to men (OR = 7.82, p < 0.01).
Operations
Table V summarizes the aggregate operative data. Aortic operations included those on the aortic valve, ascending aorta, descending aorta or combination of valve with ascending aortic replacements and are reported. Overall, fewer women had a history of any aortic operation (55% vs. 68%, p <0.001). This difference is largely demonstrated in the reduced percentage of operations in women with MFS (49% vs. 74%, p < 0.05), all diagnostic groups demonstrated a similar trend: BAV (69% vs 71%), FTAAD (52% vs 57%) and TAAD< 50 (52% vs. 57%). Examining the operative details, one exception occurred in women with TAAD <50, where more women than men reported an operation for thoracic aortic replacement (10% vs 3%). No gender-related differences were detected in age at first operation, numbers of operations or complications (data not shown).
Table V.
Details of Aortic Operations by Gender and Condition
| Marfan | BAV | FTAAD | TAAD<50 | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n=241) | Female (n=217) | p-value | Male (n=390) | Female (n=105) | p-value | Male (n=144) | Female (n=75) | p-value | Male (n=217) | Female (n=60) | p-value | Male (n=992) | Female (n=457) | All (n=1,449) | Adjusted p-value†* | |
| History of aortic operation n (%) | 177 (73.4) | 107 (49.3) | <0.001 | 276 (70.8) | 73 (69.5) | 0.6 | 83 (57.6) | 39 (52.0) | 0.72 | 136 (62.7) | 34 (56.7) | 0.4 | 672 (67.7) | 253 (55.4) | 925 (63.8) | 0.001 |
| Aortic Valve Repair | 39 (16.2) | 14 (6.0) | <0.001 | 63 (16.2) | 12 (11.4) | 0.23 | 17 (11.8) | 12 (16.0) | 0.38 | 21 (9.7) | 6 (10.0) | 0.94 | 140 (14.1) | 43 (9.4) | 183(12.6) | ns* |
| Aortic Valve Replacement | 9 (3.7) | 6 (2.8) | 0.56 | 61 (15.6) | 16 (15.2) | 0.91 | 7 (4.9) | 2 (2.7) | 0.72 | 12 (5.5) | 2 (3.3) | 0.74 | 89 (9.0) | 26 (5.7) | 115 (7.9) | ns* |
| Aortic Root Replacement | 89 (36.9) | 46 (21.2) | <0.001 | 137 (35.1) | 33 (31.4) | 0.48 | 25 (19.1) | 11 (14.7) | 0.61 | 45 (20.7) | 12 (20.0) | 0.9 | 296 (29.8) | 102 (22.3) | 398 (27.5) | ns* |
| Valve-Sparing Aortic Root | 41 (17.0) | 25 (11.5) | 0.09 | 11 (2.8) | 7 (6.7) | 0.06 | 20 (13.9) | 9 (12.0) | 0.7 | 13 (6.0) | 6 (10.0) | 0.26 | 85 (8.6) | 47 (10.3) | 132 (9.1) | ns |
| AscAortic Replacement | 73 (30.3) | 38 (17.5) | 0.001 | 173 (44.4) | 56 (53.3) | 0.1 | 59 (41.0) | 27 (36.0) | 0.47 | 72 (33.2) | 18 (30.0) | 0.64 | 377 (38.0) | 139 (30.4) | 516 (35.6) | ns* |
| Aortic Arch Replacement | 30 (12.5) | 22 (10.1) | 0.43 | 117 (30.0) | 34 (32.4) | 0.63 | 25 (17.4) | 18 (24.0) | 0.24 | 43 (19.8) | 10 (16.7) | 0.58 | 215 (21.7) | 84 (18.7) | 299 (20.6) | ns |
| Desc. Thoracic Replace | 15 (6.2) | 12 (5.5) | 0.75 | 2 (0.5) | 2 (1.9) | 0.2 | 9 (6.3) | 5 (6.7) | 0.9 | 11 (5.1) | 6 (10.0) | 0.16 | 37 (3.7) | 25 (5.5) | 62 (4.3) | ns |
| Thorac. Aort. Replace | 34 (14.1) | 20 (9.2) | 0.1 | 1 (0.3) | 0 | 1 | 7 (4.9) | 8 (10.7) | 0.11 | 7 (3.2) | 6 (10.0) | 0.04 | 49 (4.9) | 34 (7.4) | 83 (5.7) | ns |
Holm-Bonferroni test
significant to < 0.01 without Holm-Bonferroni test
Multivariate analysis
The impact of univariate gender differences were examined by multivariate analysis. The multivariate model included other potential confounding variables such as site of enrollment, primary diagnosis, BSA, age at diagnosis, hypertension, and diabetes. The multivariate logistic regression estimates demonstrate, that overall, women had fewer aortic operations (OR= 0.65, p < 0.01) and less use of ACEi (OR= 0.68, p <0.05) compared to men (Table VI). Sensitivity analysis for the model demonstrated that missing values did not bias the odds ratio estimates (data not shown). Further regression analysis was performed to ensure that gender differences in ACEi use were not caused by gender differences in aortic regurgitation, age, left ventricular function, or other drug use.
Table VI.
Associations with Female vs. Male Gender in Logistic Regression Analyse* (n = 1,296)
| Covariate | Odds Ratio (95% CI ) | Type 3 P-Value |
|---|---|---|
| Medications | ||
| ACE Inhibitor | 0.68 (0.47, 0.99) | 0.0452 |
| HMG-CoA reductase | 0.83 (0.56, 1.23) | 0.3749 |
| Losartan | 1.05 (0.71, 1.56) | 0.7808 |
| Calcium Channel Blocker | 0.90 (0.56, 1.43) | 0.6535 |
| History of Operation | 0.65 (0.48, 0.88) | 0.0065 |
Covariates of interest are those with a bivariate test p-value of less than 0.05 (see table 2). Imaging, dissection and some specific operation information were not included due to incomplete data. Model additionally controlled for history of hypertension, BSA, age at diagnosis, diabetes, primary diagnosis and study site.
DISCUSSION
The GenTAC registry recruits individuals with a variety of conditions known to be associated with genetic predisposition to aortic aneurysm and dissection. The overarching goal of the GenTAC project is to advance knowledge of the genetic triggers of aortic disease [Eagle and GenTAC Consortium, 2009b]. Therefore, our study explored the effect of gender as a fundamental genetic difference that runs through the most frequent diagnostic subgroups within the GenTAC cohort. With the exception of MFS, we found male predominance for the primary GenTAC diagnostic groups. In addition there were important gender differences in morbidity and medical/operative management. Women were less likely to undergo aortic surgery and women were less likely to be treated with ACE inhibitors.
The IRAD gender study provided worrisome data that women are at significantly higher risk for death after operation for dissection (OR 1.4, p <0.04). Unfortunately the GenTAC and IRAD data are not directly comparable in this regard because of significant differences in ascertainment between the two studies. IRAD is a longitudinal study that recruits individuals who have specifically suffered an acute aortic dissection and reports their outcome [Hagan et al., 2000]. GenTAC enrolls only living subjects and recruits either unoperated individuals or survivors of dissection or other aortic operations [Eagle and GenTAC Consortium, 2009a]. Only after longitudinal study of the GenTAC cohort will we be able to determine if female gender is a significant risk factor for death following thoracic aortic dissection as the IRAD study suggests.
The male predominance in BAV aortopathy is already well established.[Roberts, 1970] The present study supports and extends these observations by demonstrating a male predominance in two other diseases of the aorta, FTAAD and TAAD<50y. These striking gender differences in aortic diseases call out for novel hypotheses. Indeed, the possibility that some as yet undefined characteristic of the X chromosome protects women is suggested by the fact that women with a deficiency of the X chromosome (monosomy X or Turner syndrome) have significantly increased risk for BAV, aortic aneurysm and aortic dissection compared to 46,XX women [Carlson and Silberbach, 2009]. One hypothesis would be that there is a requirement for the full complement of a critical aorta-related product of a gene present in the pseudo-autosomal region (PAR1) of the second X chromosome. A second possibility would be that abnormal expression of autosomal genes associated with left heart obstruction or aortic aneurysm may be enhanced in the setting of missing or deficient X chromosomal factors. Alternatively, it is possible that there are missing X-related factors that increase susceptibility to testosterone during early developmental stages as suggested by Zhang et al. [2012]. Women with familial BAV may also lack X-related protective factors as in Turner syndrome since in our study, women with BAV have more extensive aortic dissection compared to men (DeBakey I). Studies of the biology of the X chromosome employing GenTAC DNA/tissue repositories and analyses of data from the GenTAC imaging core will permit exploration of some of these intriguing questions.
We believe that the predilection for aortic operations in males, particularly operations for aortic aneurysm in MFS men, may be related to their larger absolute aortic dimensions [Biaggi et al., 2009; Schvartzman et al., 2000]. In fact, we found that after adjusting aortic dimensions for values predicted for body size the gender difference disappeared. Therefore, men may benefit by being more likely to meet surgical criteria based on absolute size. Perhaps women face higher long-term morbidity risk because of the reluctance to medically treat or operate on the unindexed aorta. Women were also less likely to be treated with ACEi compared to men. This difference was not an effect of age, alternative drug choice, ventricular function, aortic function or hypertension. However, others have raised the concern that women being less likely to be treated with ACEi could be harmful in the setting of heart failure [Lenzen et al., 2008; Sheppard et al., 2005]. Whether treatment of more men than women with ACEi influences overall outcome in patients predisposed to aortic dissection requires long-term study of the GenTAC cohort.
Study limitations
As a cross-sectional study, our observations represent a snapshot of the GenTAC cohort at different points within the natural (and unnatural) history of aortic disease. The analysis is exploratory, designed to be hypothesis generating rather than supporting. While men and women had equal access to our recruitment effort, we cannot exclude the possibility that more men were recruited or more women declined to participate at the GenTAC centers. Including study site in the multivariate analysis should have controlled for regional disparities in recruitment. Another possibility, suggested by results from IRAD, is that male predominance may have been enhanced by disproportional pre-enrollment mortality in women. We will address these potential limitations in future longitudinal analyses of the GenTAC population.
Conclusions
This cross-sectional analysis of a population at high risk for significant morbidity and mortality suggest multiple gender-based differences. In the GenTAC registry, MFS occurs with equal gender frequency; yet BAV with TAAD, FTAAD and TAAD< 50, affect fewer women. Women are less likely to have aortic operations. We speculate that fewer aortic operations are performed in women because of reluctance to operate or treat the smaller unindexed female aorta. Fewer women may be treated with ACEi. Longitudinal studies of the GenTAC registry will hopefully determine if these differences place women at undue risk.
ACKNOWLEDGMENTS
The GenTAC Registry has been supported by US Federal Government contracts HHSN268200648199C and HHSN268201000048C from the National Heart Lung and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Bethesda, MD).
REFERENCES
- Biaggi P, Matthews F, Braun J, Rousson V, Kaufmann PA, Jenni R. Gender, age, and body surface area are the major determinants of ascending aorta dimensions in subjects with apparently normal echocardiograms. J Am Soc Echocardiogr. 2009;22:720–725. doi: 10.1016/j.echo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Carlson M, Silberbach M. Dissection of the aorta in turner syndrome: Two cases and review of 85 cases in the literature. BMJ Case Rep. 20092009:bcr0620091998. doi: 10.1136/bcr.06.2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- DeBakey ME, Henly WS, Cooley DA, Morris GC, Jr, Crawford ED, Beall AC., Jr Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–149. [PubMed] [Google Scholar]
- Eagle KA, GenTAC Consortium Rationale and design of the national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC). Am Heart J. 2009a;157:319–326. doi: 10.1016/j.ahj.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle KA, GenTAC Consortium Rationale and design of the national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC). Am Heart J. 2009b;157:319–326. doi: 10.1016/j.ahj.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The international registry of acute aortic dissection (IRAD): New insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statisics. 1979:65–70. [Google Scholar]
- Kroner BL, Tolunay HE, Basson CT, Pyeritz RE, Holmes KW, Maslen CL, Milewicz DM, Lemaire SA, Hendershot T, Desvigne-Nickens P, Devereux RB, Dietz HC, Song HK, Ringer D, Mitchell M, Weinsaft JW, Ravekes W, Menashe V, Eagle KA. The national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC): Results from phase I and scientific opportunities in phase II. Am Heart J. 2011;162:627–632. e1. doi: 10.1016/j.ahj.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen MJ, Rosengren A, Scholte op Reimer WJ, Follath F, Boersma E, Simoons ML, Cleland JG, Komajda M. Management of patients with heart failure in clinical practice: Differences between men and women. Heart. 2008;94:e10. doi: 10.1136/hrt.2006.099523. [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, Bossone E, Fang J, Smith DE, Isselbacher EM, Pape LA, Eagle KA, International Registry of Acute Aortic Dissection Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- Roberts WC. The structure of the aortic valve in clinically isolated aortic stenosis: An autopsy study of 162 patients over 15 years of age. Circulation. 1970;42:91–97. doi: 10.1161/01.cir.42.1.91. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- Schvartzman PR, Fuchs FD, Mello AG, Coli M, Schvartzman M, Moreira LB. Normal values of echocardiographic measurements. A population-based study. Arq Bras Cardiol. 2000;75:107–114. doi: 10.1590/s0066-782x2000000800003. [DOI] [PubMed] [Google Scholar]
- Sheppard R, Behlouli H, Richard H, Pilote L. Effect of gender on treatment, resource utilization, and outcomes in congestive heart failure in quebec, canada. Am J Cardiol. 2005;95:955–959. doi: 10.1016/j.amjcard.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P. Life expectancy in the marfan syndrome. Am J Cardiol. 1995;75:157–160. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National registry of myocardial infarction 2 participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]