Summary
Updating memories is critical for adaptive behaviors, but the rules and mechanisms governing that process are still not well defined. During a limited time window, the reactivation of consolidated aversive memories triggers memory lability and induces a plasticity-dependent reconsolidation process in the lateral amygdala (LA) [1–5]. However, whether new information is necessary for initiating reconsolidation is not known. Here we show that changing the temporal relationship between the conditioned (CS) and unconditioned (US) stimulus during reactivation is sufficient to trigger synaptic plasticity and reconsolidation of an aversive memory in the LA. These findings demonstrate that time is a core part of the CS-US association, and that new information must be presented during reactivation in order to trigger LA-dependent reconsolidation processes. In sum, this study provides new basic knowledge about the precise rules governing memory reconsolidation of aversive memories that might be used to treat traumatic memories.
Results and Discussion
Traumatic fear memories are strong and persistent and form the basis of several pathological disorders, including post-traumatic stress disorder (PTSD) and anxiety disorders. The search for procedures that may render these memories sensitive to pharmacological or behavioral treatments is thus critical. It is known that after memories have been consolidated into a long-term state they can enter a new labile state when reactivated prior to being reconsolidated. During this lability window, it is believed that memories are updated and new elements are incorporated [4]. However, the exact rules governing the updating processes during reconsolidation are not yet understood. One proposal emerging from the literature is that reconsolidation processes are initiated when additional learning is invoked during the reactivation procedure, forcing the original memory to be updated and reconsolidated [6, 7].
Pavlovian threat (fear) conditioning has been used extensively to study emotional learning and memory both in animals and humans [8], and the reconsolidation of auditory fear conditioning has been shown to be highly selective and dependent on plasticity mechanisms in the lateral amygdala (LA) [9, 10]. Interestingly, only weaker fear memories have been observed to be susceptible to reconsolidation; recently formed stronger fear memories appear to be less susceptible to reconsolidation interference even when using a CS-US trial for reactivation [5, 11]. This is potentially problematic for the use of reconsolidation blockade as part of a therapy for traumatic memories since these memories, by definition, involve strong aversive experiences.
In Pavlovian conditioning, the subject not only learns that the conditioned stimulus (CS) predicts the arrival of the unconditioned stimulus (US), but also when the US is expected to arrive [12]. Time is a critical element in associative learning [13, 14]. Here, we asked whether a change in CS-US time interval is necessary and sufficient to trigger an update of an aversive memory and its reconsolidation in an amygdala-dependent manner. To do so, we designed a temporal auditory fear conditioning protocol in which a 60s tone CS is associated with a footshock US delivered 30s after the tone onset (US@30; see supplemental figure 1). This design permits the presentation of a single training trial in a reactivation procedure that alters only the temporal relationship between the CS and US while equating the total number and duration of stimuli presented (context, CS and US).
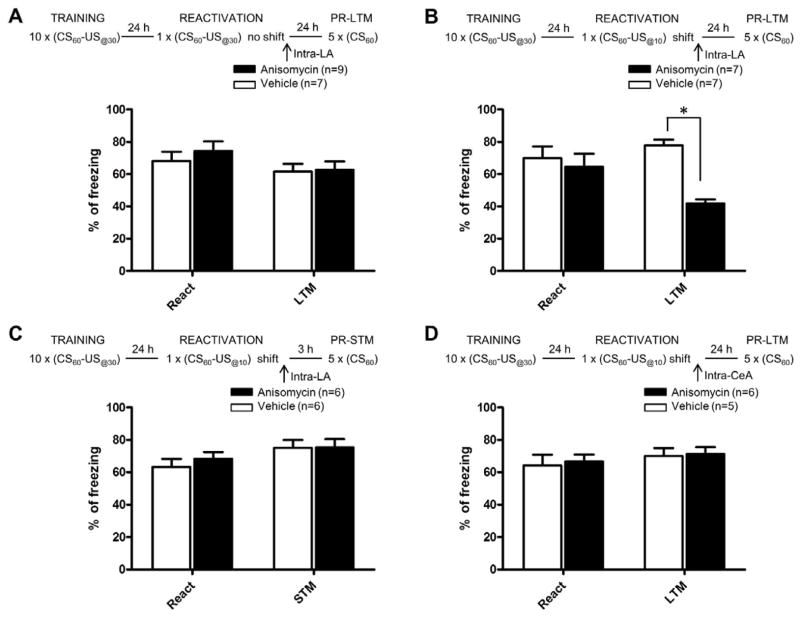
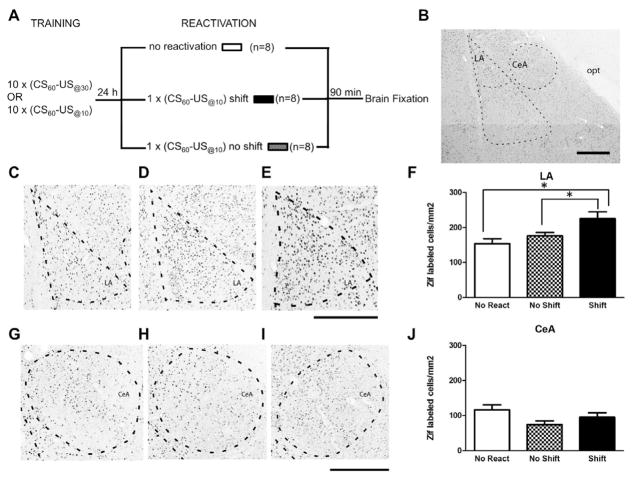
We first verified that that intra-LA infusion of a protein synthesis inhibitor immediately after reactivation is insufficient to interfere with the reconsolidation of a recently formed strong fear memory [5]. Rats were given 10 CS-US@30 pairings followed 24 hrs later by a reactivation trial consisting of a single CS-US@30 pairing identical to the training condition. As expected, rats given intra-LA infusion of anisomycin following the reactivation trial showed equivalent levels of freezing during the reactivation trial (t(14)= 0.729, n.s.) and during the post-reactivation long-term memory test 24h later (PR-LTM, t(14 =0.135, n.s., figure 1A) relative to vehicle-infused controls. Thus, in agreement with Wang et al. [5], recently formed stronger fear memories are less susceptible to reconsolidation interference using a protein synthesis inhibitor. In contrast, when the CS-US time interval was reduced from 30 to 10s during the memory reactivation trial, rats infused with anisomycin showed significantly reduced freezing during the PR-LTM test relative to vehicle-infused controls (PR-LTM: t(12)=8.403, P<0.001; reactivation: t(12)= 0.488, n.s., figure 1B). This suggests that the detection of a difference in the CS-US interval between training and reactivation is sufficient to induce reconsolidation of a stronger aversive memory. Importantly, both anisomycin and vehicle groups showed equivalent levels of freezing during a test of post-reactivation short-term memory (PR-STM: t(10)=0.040, n.s.; reactivation: t(10)= 0.785, n.s., figure 1C), suggesting that the impairment observed during PR-LTM was due to the disruption of reconsolidation processes and not due to damage to the amygdala. Further, when anisomycin was infused into the central amygdala (CeA), no reconsolidation impairment was observed (PR-LTM: t(9)=0.271, n.s.; reactivation: t(9)=0.347, n.s., figure 1D). Thus, reconsolidation of a fear memory following a temporal shift depends upon protein synthesis in the LA, but not in the CeA, as previously reported using standard fear conditioning and reactivation procedures [15]. In agreement with this observation, we observed that a change in CS-US interval triggers plasticity mechanisms in the LA, but not the CeA, as measured by retrieval-induced expression of the immediate early gene (IEG) zif-268, a marker of synaptic plasticity that has been implicated in fear memory reconsolidation [16, 17] (figure 2). The number of zif-268 positive cells was significantly higher in the LA in rats reactivated with a change in the CS-US time interval relative to rats reactivated with the initial CS-US time interval and to those in the non-reactivated group (F(2,21)=6.011, P<0.01, figure 2C–F). No significant differences were observed between the three groups for the number of zif-268 positive cells in CeA (F(2,21)=2.892, n.s., figure 2G–J).
Figure 1. Changing the CS-US time interval during reactivation of strong aversive memories triggers a LA-dependent reconsolidation process.
Schematic of the experimental design and percentage of freezing (mean ± s.e.m.) to the CS during reactivation (React) and post-reactivation long-term memory test (PR-LTM) in rats infused with vehicle (white bars) or anisomycin (black bars). All four experiments consisted of training with 10 trials of 60s tone paired with a footshock US delivered 30s after tone onset (CS60 – US@30). Freezing during reactivation was equivalent between vehicle and anisomycin rats in all four experiments. (A) Rats reactivated with the same CS-US time interval as the one learned during training (CS60 – US@30) and given intra-LA anisomycin did not show an impairment of memory during PR-LTM. Rats infused with anisomycin in LA and reactivated with a CS-US interval shifted to 10s (CS60 – US@10) did show an impairment during PR-LTM (B) but not when memory was tested 3 hours (PR-STM) after reactivation (C). (D) The infusion of anisomycin in the central amygdala (CeA) did not induce an impairment of memory during LTM in the rats reactivated with a shifted CS-US interval. (*P<0.05).
Figure 2. Memory reactivation induces synaptic plasticity in LA only when the CS-US time interval is shifted.
(A) Schematic of the experimental design. (B) Photomicrograph showing the amygdala nuclei analyzed for Zif immunoreactivity. Photomicrographs of transverse Zif-stained sections from representative cases illustrating no reactivated animals (C), no shift (D) and shift animals (E) in the LA or in the CeA (G, H, I) at 2.8mm posterior to Bregma. The broken lines delineate the nuclei borders. Abbreviations: CeA, central nucleus of amygdala, LA, lateral nucleus of amygdala; opt, optic tract. Scale bar = 200 μm. (F) (J) represent the average of Zif-648 positive cells per square millimeter (mean ± s.e.m.) across three Anterior-Posterior levels. Only the rats whose memory was reactivated with a CS-US time interval different than the one used during training showed an increase in the expression of Zif-648 positive cells in the LA (F) but not in the CeA (J). (*P<0.05 Newman-Keuls post-hoc test)
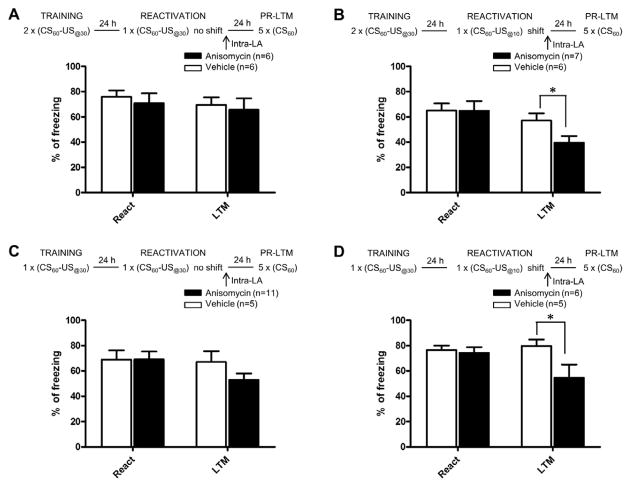
The aforementioned findings not only demonstrate that a change in the CS-US interval can return strong aversive memories to a labile state but also that the CS-US interval itself has been learned during the conditioning session. If a temporal discrepancy is the critical parameter that triggers reconsolidation, then increasing the CS-US interval should be equally effective as decreasing it. In our next experiment, we therefore trained rats with a 60s tone CS paired with a US-footshock delivered 10s after the tone onset. Twenty-four hours later, rats received intra-LA infusion of anisomycin or vehicle immediately after a CS-US reactivation trial with a US-footshock delivered 30s after tone onset. During the PR-LTM test, rats infused with anisomycin showed a significant decrease in the level of freezing compared to vehicle-infused rats (PR-LTM: t(13)=4.487, P<0.001; reactivation: t(13)=0.169, n.s., figure 3A). Therefore, a change in CS-US time interval, either shorter or longer, triggers an amygdala-dependent reconsolidation process. Remarkably, the temporal pattern of freezing during PR-LTM seemed to differ among the three experimental groups: no shift-US@30; shift-US@10; shift-US@30. Although no statistical comparison can be made between experimental groups because they belong to independent experiments, we can note (figure 3B–D) that while vehicle-treated shifted animals tend to express freezing at an equivalent level throughout the CS duration, the anisomycin-treated shifted rats show different pattern depending on their shifted conditions. In particular, anisomycin-treated rats shifted to a shorter CS-US interval show a higher level of freezing at the beginning of the CS and a progressive decay as time elapses during the CS, indicating a stronger contribution of the fear related to the new CS-US interval. Thus, although further experiments need to specifically address this issue, the present data suggest that when a change in the temporal parameters during reactivation is detected, the new temporal relationship between the CS and the US during reactivation is acquired in a single trial and possibly in an independent manner of the LA.
Figure 3. Time is a critical parameter of the CS-US association that triggers the update of strong fear memory.
Schematic of the experimental design and (A) percentage of freezing (mean ± s.e.m.) to the CS during reactivation (React) with a longer CS-US interval (US@30s) than the one learned during training (US@10s) and during post-reactivation long-term memory test (PR-LTM) in rats infused in the LA with vehicle (white bars) or anisomycin (black bars). Freezing during reactivation was equivalent between groups. Rats given intra-LA anisomycin showed an impairment of memory during PR-LTM. (B, C, D) Temporal pattern of freezing during PR-LTM tests (mean ± s.e.m. in 3s bins), for rats non-shifted (B), shifted with a US delivered later (C) or shifted with a US delivered earlier (D) during reactivation. In all experiments, there was a significant effect of time (B:F(19,280)=4.62; C:F(19,260)=2.91; D:F(19,240)=4.45; # P<0.0001). Only when a change in CS-US interval was imposed during reactivation, the anisomycin produced a significant reduction of freezing (B:F(1,280)=2.24, n.s.; C:F(1,260)=234.7 and D:F(1,240)=1019.6; *P<0.0001). When anisomycin was infused after reactivation with a shifted CS-US time interval from 30 to 10s, the temporal pattern of freezing was different from vehicle controls (D: time x group interaction F(19,240)=3.50; + P<0.0001).
We next asked whether a strong fear memory established with fewer training trials is susceptible to reconsolidation interference with anisomycin in the absence of new information. Rats were conditioned with two pairings of the CS with the US@30 followed by reactivation with a single CS-US trial with no change in the CS-US interval. Both anisomycin and vehicle-infused rats showed similar levels of freezing during reactivation (t(10)=0.537, n.s.) and during PR-LTM (t(10)=0.365, n.s., figure 4A). In contrast, when the CS-US interval was reduced to 10s during the reactivation trial, anisomycin treated animals showed impaired PR-LTM compared to vehicle rats (PR-LTM: t(11)=2.231, P<0.05; reactivation: t(11)=0.031, n.s., figure 4B). Similar findings were observed when a single CS-US pairing was used during training (no-shift condition: PR-LTM: t(14)=1.523, n.s.; reactivation: t(14)=0.019, n.s., figure 4C; shift condition: PR-LTM (t(9)=2.294, P<0.05; reactivation: t(9)=0.443, n.s., figure 4D). Thus, fear memories appear to require new information during reactivation for the memory to become destabilized. Further, these findings suggest that the CS-US time interval in auditory fear conditioning, like the CS-US association, can be learned in a single trial; the learning of the timing (see figure 3), thus, does not depend on the prior learning of the association. These findings extend those of a previous study [18] by showing that an interval as short as 30s can be learned after a single CS-US training trial in an auditory fear conditioning paradigm, and that the precision of the US timing at the outset of learning is at least 20s, as a difference between 10 and 30s was detected. This adds empirical support to the concept of temporal maps as pre-requisite for associative learning [14]
Figure 4. The CS-US time interval is learned at the same time as the CS-US association.
Schematic of the experimental design and percentage of freezing (mean ± s.e.m.) to the CS during reactivation (React) and post-reactivation long-term memory test (PR-LTM) in rats infused in the LA with vehicle (white bars) or anisomycin (black bars). Freezing during reactivation was equivalent between vehicle and anisomycin rats in all experiments. (A, B) Rats trained with 2 CS-US pairings, (A) reactivated with the same CS-US interval than the one learned during training (US@30) and given intra-LA anisomycin did not show an impairment of memory during PR-LTM; in contrast (B), when memory was reactivated with a different CS-US time interval (US@10), anisomycin infused rats showed an impairment of memory. (C, D) The same effect was observed after one-trial training. *P<0.05.
Conclusions
In sum, our results demonstrate that when a change in the temporal relationship between CS and US is detected, an update of the previously acquired aversive memory and its reconsolidation is triggered in an amygdala-dependent manner. Our results also demonstrate unequivocally, and for the first time with amygdala dependent memories, that when the association is well learned, changing the temporal association architecture is sufficient to trigger reconsolidation even of recently acquired strong aversive memories [5]. In contrast, if nothing novel is added and no additional learning is elicited, the aversive memory trace is not rendered labile [6, 7, 19]. In contrast to the findings of Duvarci & Nader [11], in our study the freezing level reached its maximum after a single training trial, indicating that the CS-US association was fully learned; the additional CS-US trial during reactivation with no change in the temporal structure was therefore not sufficient to trigger additional learning, as in Wang et al. [5]. Given that learning the time interval and learning the association may be tightly intertwined, and as time is a critical element of the US expectation [20, 21], changing the temporal relationship between CS and US appears to elicit an update of temporal expectancy rules (e.g. modifying the previously consolidated temporal association), and therefore may be the most powerful tool to trigger reconsolidation.
Our results also demonstrate that changes in the temporal relationship between CS and US trigger synaptic plasticity and reconsolidation processes in the LA. Neurophysiological studies in human and non-human primates, as well as in rodents, have suggested that the amygdala may be involved in temporally-based prediction error detection, both in appetitive and aversive Pavlovian situations. In effect, the amygdala shows anticipatory neurophysiological activity [22, 23], as well as reactivity to surprising temporal irregularities or unexpected events [24, 25]. Whether temporal processing (timing, CS-US interval storage, and comparison between experienced and expected US value) is computed in the LA is not known. Our results strongly suggest that aversive prediction error detection - whether processed in part in the amygdala itself or only transmitted from upstream neural structures - is a fundamental mechanism in triggering reconsolidation of aversive memories in the amygdala. Collectively, our findings provide precise boundary rules for effective destabilization of strong aversive memories.
Experimental Procedures
Behavioral Experiments
Adult Sprague Dawley rats provided by Hiltop Laboratories (Scottdale, PA, US) and weighing 250–300g at the beginning of the experiments were used. All procedures were in accordance with the NIH Guide for the Care and Use of Experimental Animals, and were approved by the New York University Animal Care and Use Committee.
After recovering from surgical implantation of cannulae (see supplemental information for details and supplemental figures 2 and 3 for cannulae placements) rats were fear conditioned with a novel protocol that allowed us to change the time of arrival of the US. The CS was a 60-sec, 5 kHz, 80 dB SPL sine wave tone. The US (1s, 1mA footshock) was delivered 30 (CS60-US@30) or 10 (CS60-US@10) seconds after the onset of the tone depending on the experiment (see supplemental figure 1). Memory reactivation session took place 24 h after fear conditioning by presenting one reinforced trial. The US was delivered either at the same time after the tone onset as during conditioning (no shift groups) or at a different time (shift groups). Immediately after, the rats received an infusion of anysomicin or vehicle in the LA or in the CeA depending on the experiment. The memory retention tests were performed either 3 hours (PR-STM) or 24 hours (PR-LTM) after reactivation and involved 5 tone alone presentations in a modified context (see supplemental figure 4 for contextual freezing). Freezing was used to measure the conditional emotional fear response.
Immunohistochemistry
In separate groups of animals, ninety minutes after the reactivation session, brains were taken, cut and processed for Zif-268 immunohistochemistry (see supplemental information).
Statistical Analysis
ANOVA and post-hoc tests were performed using GraphPad Prism 5.0 software. The significance level was set at α=5%.
Supplementary Material
Highlights.
Fear reconsolidation is only triggered when memory is updated with new information.
Time is a core-part of the Pavlovian CS-US association.
Reactivation-synaptic plasticity occurs only when error prediction is detected.
Acknowledgments
We thank Claudia Farb for her excellent assistance with histology and Begoña Brotons for her help in the design of the graphical abstract. R.M. was recipient of the grants: CAPES (# 2350/09-2) and FAPESP (#11/08575-7, 12/06825-9) from the government of Brazil. V.D. was supported by ANR grants. V.D. and J.E.L. were recipients of collaborative grants from CNRS and from Partner University Fund.
Footnotes
Supplemental Information includes 4 figures, and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 2.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;7:5–12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudai Y. Predicting not to predict too much: how the cellular machinery of memory anticipates the uncertain future. Phil Trans R Soc B. 2009;364:1255–1262. doi: 10.1098/rstb.2008.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, de Oliveira Alvares HL, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- 6.Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol Learn Mem. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 10.Díaz-Mataix L, Debiec J, LeDoux JE, Doyère V. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci. 2011;31:9538–9543. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlov IP. Conditional Reflexes. New York: Dover Publications; 1927/1960. [Google Scholar]
- 13.Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balsam PD, Drew MR, Gallistel CR. Time and Associative Learning. Comp Cogn Behav Rev. 2010;5:1–22. doi: 10.3819/ccbr.2010.50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debiec J, Díaz-Mataix L, Bush DE, Doyère V, LeDoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13:536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2010;18:24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;11:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M, Schlesinger LS, Sorenson CA. Temporal specificity of fear conditioning: effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J Exp Psychol Anim Behav Process. 1989;15:295–310. [PubMed] [Google Scholar]
- 19.Finnie PS, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev. 2012;36:1667–1707. doi: 10.1016/j.neubiorev.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 20.McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci. 2011;34:283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychol Rev. 1981;88:135–170. [PubMed] [Google Scholar]; Moses SN, Houck JM, Martin T, Hanlon FM, Ryan JD, Thoma RJ, Weisend MP, Jackson EM, Pekkonen E, Tesche CD. Dynamic neural activity recorded from human amygdala during fear conditioning using magnetoencephalography. Brain Res Bull. 2007;71:452–460. doi: 10.1016/j.brainresbull.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur J Neurosci. 2007;26:2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- 24.Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Lüthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metereau E, Dreher JC. Cereb Cortex. 2012. Cerebral Correlates of Salient Prediction Error for Different Rewards and Punishments. Published online Feb 23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.