Abstract
Background
The effect of various sedatives and anesthetics on vasopressor modulation of cerebral blood flow (CBF) in children is unclear. In adults, isoflurane has been described to decrease CBF to a lesser extent than fentanyl and midazolam. Most large animal models of neurocritical care use inhaled anesthetics for anesthesia. Investigations involving modulations of CBF would have improved translatability within a model that more closely approximates the current practice in the pediatric intensive care unit.
Methods
Fifteen (15) four-week-old piglets were given one of 2 anesthetic protocols: total IV anesthesia (TIVA) (midazolam 1 mg/kg/hr and fentanyl 100 mcg/kg/hr, N=8) or ISO (isoflurane 1.5–2% and fentanyl 100 mcg/kg/hr, N=7). Mean arterial blood pressure, intracranial pressure (ICP), CBF, and brain tissue oxygen tension were measured continuously as piglets were exposed to escalating doses of arginine vasopressin, norepinephrine (NE) and phenylephrine (PE).
Results
Baseline CBF was similar in two groups (ISO 38±10 vs. TIVA 35±26 ml/100gm/min) despite lower baseline cerebral perfusion pressure in the ISO group, 45±11 vs. 71±11 mmHg (p< 0.0005). Piglets in ISO group displayed increases in ICP with PE and NE (11±4 vs. 16±4 mmHg and 11±8 vs. 18±5 mmHg; p< 0.05), but in the TIVA group only exposure to PE resulted in increases in ICP when comparing maximal dose values to baseline data (11±4 vs. 15±5 mmHg; p < 0.05). Normalized CBF displayed statistically significant increases with regards to anesthetic group and vasopressor dose when piglets were exposed to NE and PE (p < 0.05), suggesting an impairment of autoregulation within ISO, but not TIVA.
Conclusion
The vasopressor effect on CBF was limited when using a narcotic-benzodiazepine-based anesthetic protocol compared to volatile anesthetics, consistent with a preservation of autoregulation. Selection of anesthetic drugs is critical to investigate mechanisms of cerebrovascular hemodynamics, and in translating critical care investigations between the laboratory and bedside.
Introduction
Traumatic brain injury (TBI) and stroke are common clinical conditions requiring admission to the intensive care unit, and are not uniquely adult diagnoses, with both being in the top ten causes of pediatric mortality.1 Postinjury management tasks the practitioner to limit secondary injury from imbalances in brain tissue metabolic supply and demand, excitotoxicity, systemic hypotension/hypertension, and systemic hypoxemia. Adequate cerebral blood flow (CBF) is commonly targeted as a theoretical point of manipulation, with too little CBF associated with ischemic injury and too much CBF associated with increased cerebral blood volume, vasogenic edema, and potential increases in intracranial pressure (ICP). Since CBF is difficult to measure on a continuous basis in the clinical setting, cerebral perfusion pressure (CPP) is commonly used as a surrogate. Although essential to the regulation of CBF, cerebrovascular resistance (CVR) regulation remains complex and poorly understood, even in the healthy brain. Regulation of CVR is heterogeneous and under local, not global control, with age and gender differences previously described.2–4 Furthermore, recent investigations have reported alterations in cerebrovascular function and physiology in critically ill patients without primary brain injury.5–8
While most IV sedative medications decrease cerebral metabolic rate (CMRO2) and CBFin a proportional amount,9 the inhaled anesthetics have a more varied response. A study of adult patients undergoing elective neurosurgical procedures demonstrated a decrease in CBF of 37% while sedated with fentanyl and midazolam, as compared to a 22% reduction when anesthetized with isoflurane (ISO), despite relative reductions of CMRO2 of 26% and 51% respectively.10 Additional studies have shown midazolam to be associated with as much a 71% decrease in CBF,9,11–15 although a single study did not see any difference in CBF with a midazolam infusion.16 Inhaled anesthetics such as ISO have been known to have more variable effects upon CBF, ranging from a 22% decrease to a 33% increase at 1.0 minimal alveolar concentration.10,17– 21 Unfortunately, only a small minority of these investigations have been performed in the pediatric population,19 and none obtained in patients in the pediatric intensive care unit (PICU).
Vasopressors are commonly used in pediatric neurocritical care to modulate CPP, with the ultimate goal of increasing CBF. Phenylephrine (PE) is the most common vasopressor used in pediatric patients with TBI,22 because it selectively targets alpha receptors with pure systemic vasoconstriction. In mature animal models, increases in ICP have been observed with PE administration.23,24 Norepinephrine (NE) activates alpha receptors for peripheral vasoconstriction, but has additional activation of beta receptors for inotropy. It is gaining favor in adult neurocritical care as a preferred vasopressor, because it provides more predictable CPP augmentation when compared to dopamine.24,25 Arginine vasopressin (AVP), with its novel targeting of the endothelial receptor V1, is often used in catecholamine refractory states.26,27 In adults with TBI, AVP is the fourth most commonly used vasopressor.28 In swine TBI models, AVP was as effective as PE for CPP maintenance, yet AVP was associated with lower ICP and higher brain tissue oxygen tension (PbtO2).29
The objectives of this study were twofold: 1) To develop an anesthetic paradigm for large animal models that would improve the translatability of findings to the PICU. In animal models (both large and small) ISO is commonly used for anesthesia. It is our hope that using an animal model with an anesthetic paradigm clinically relevant to the PICU (in this case fentanyl and midazolam) will improve the chances of successfully translating new findings and therapies from the bench to the PICU bedside. 2) We believe there are several clinical scenarios where vasopressors would be used in pediatric patients without intracranial pathology such as sepsis, trauma without head injury, and acute respiratory distress syndrome. Some of these pediatric critically ill patients may require operative intervention and during that intervention may be transitioned to volatile anesthetics from their IV infusions. Using an immature large animal platform to model clinical practice in PICU patients requiring vasopressor therapy, we aimed to determine the cerebrovascular hemodynamic effects of different vasopressor drugs in piglets anesthetized with either fentanyl/midazolam or fentanyl/ISO.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Female, 4-week-old piglets (8–10 kg), which have been described to be anthropomorphically similar to a human toddler, were used for the study.30 Piglets were premedicated with intramuscular injection of ketamine (20 mg/kg) and xylazine (2 mg/kg) followed by 4% ISO and 100% FiO2 via snout mask, until abolishment of reflexive withdrawal to a pinch stimulus. Endotracheal intubation was followed by decrease in FiO2 to 21–30% and maintenance anesthesia was provided using 2% ISO. No neuromuscular blockade was administered for tracheal intubation or at any other point during the experiments. Two points of venous access were obtained via bilateral femoral cut-down. Additionally, the right femoral artery was cannulated for invasive blood pressure monitoring. Vital signs including heart rate, respiratory rate, mean arterial blood pressure (MAP) zeroed to the right atrium, arterial oxygen saturation (SaO2), end-tidal CO2, and rectal temperature were recorded for the duration of the experiment. A 20 ml/kg normal saline bolus was administered for replacement of preanesthetic dehydration, followed by a normal saline infusion at 4 ml/kg/hr. Mechanical ventilation was titrated with goals of normoxia (paO2 > 90 mmHg) and normocarbia (paCO2 38–45mmHg). The core body temperature goal of 37–38.5 degrees Celsius was accomplished with a conductive warming/cooling blanket system (Gaymar, Orchard Park, NY). With piglets in the prone position, three burr holes were drilled for placement of intracranial monitors in the frontal lobes as previously described.31 ICP was monitored via fiberoptic intraparenchymal pressure monitor (Integra, Plainsboro, NJ). PbtO2 of the left frontal lobe subcortical white matter was monitored with a Licox monitor (Integra, Plainsboro, NJ). CBF of the right frontal lobe subcortical white matter was measured continuously with a thermal diffusion intraparenchymal probe (Hemedex, Cambridge, MA).32,33
After all instrumentation was completed, piglets were transitioned to one of two different anesthetic plans. The first group, total IV anesthesia (TIVA), (n=8) had the ISO discontinued and flushed from the ventilator circuit. They were given a bolus of 1 mg/kg midazolam and 50 mcg/kg fentanyl and started on midazolam and fentanyl infusions (1 mg/kg/hr and 100 mcg/kg/hr, respectively).34 The second group, (ISO), (n=7), was continued on ISO at 1.5–2% maintenance, given a fentanyl bolus (50 mcg/kg) followed by a fentanyl infusion (100 mcg/kg/hr). The average latency between ketamine injection and baseline data procurement before first vasopressor was greater than 2 hrs; and between ISO discontinuation in TIVA and baseline data was more than 30 minutes with a gas flow rate of 5L/minute throughout the entire experiment.
Within each anesthetic group, each piglet was exposed to three vasopressors, AVP, NE, and PE, in random order, and doses doubled every 15 minutes until one of three conditions was met to prevent injury or early death to the animals: (1) reaching the highest dose described in large animal literature (AVP 0.08 units/kg/min,26 NE 1.5 mcg/kg/min,35 PE 25 mcg/kg/min26), (2) significant bradycardia or tachycardia (>200 or <40 bpm), or (3) systolic blood pressure exceeded 145 mmHg. Upon reaching any of these criteria, the vasopressor was discontinued, followed by a washout period exceeding 15 minutes, which was terminated when MAP values were stable for 5 minutes. Next, piglets were exposed to the second and subsequently third vasopressor in a similar fashion. After termination of the third vasopressor, the piglet was euthanized via pentobarbital overdose.
Physiological, arterial blood gas, and neuromonitoring variables data at baseline and at maximum vasopressor dose were analyzed within and across groups, using paired and unpaired Student’s t-tests as appropriate. The MAP, CBF, and PbtO2 data for each drug were compared across anesthetic groups and dose using repeated measures ANOVA tests and Tukey-Kramer tests were used for post hoc analysis with significance defined as p < 0.05.
Results
Piglets in TIVA and ISO groups displayed similar baseline physiologic variables, except lower baseline MAP and CPP in the ISO group (Table 1). Although ICP was higher in the ISO group, it did not reach statistical significance (p = 0.06). There were no differences noted between anesthetic groups in the average maximal doses of each vasopressor (Table 2).
Table 1.
Baseline physiologic data of piglets
| TIVA | ISO | |
|---|---|---|
| Weight (Kg) | 9.0 ± 1.0 | 8.2 ± 0.9 |
|
|
||
| Rectal temperature (°C) | 36.7 ± 0.6 | 36.7 ± 0.5 |
|
|
||
| Heart Rate | 111 ± 19 | 108 ± 17 |
|
|
||
| Respiratory Rate | 26 ± 3 | 27± 2 |
|
|
||
| SaO2 (%) | 98 ± 1 | 98 ± 1 |
|
|
||
| MAP (mmHg) | 79 ± 10* | 55 ± 10 |
|
|
||
| ICP (mmHg) | 8 ± 3 | 11 ± 3 |
|
|
||
| CPP (mmHg) | 71 ± 11* | 45 ± 7 |
|
|
||
| CBF (ml/100gm/min) | 35 ± 27 | 38± 10 |
|
|
||
| PbtO2 (mmHg) | 10.8 ± 4.3 | 13.5 ± 4.7 |
|
|
||
| pH | 7.53 ± 0.04 | 7.53 ± 0.02 |
|
|
||
| pCO2 | 42.7 ± 3.1 | 40.0 ± 4.8 |
|
|
||
| paO2 | 137.0 ± 25.2 | 132.8 ± 25.4 |
|
|
||
| Lactate | 1.1 ± 0.3 | 1.6 ± 0.9 |
Values are mean ± standard deviation. Baseline measurements were performed in each animal before exposure to vasopressor drug. Total IV anesthesia (TIVA) = fentanyl and midazolam; ISO = fentanyl and isoflurane; SaO2 = arterial oxygen saturation; MAP = mean arterial blood pressure; ICP = intracranial pressure; CPP = cerebral perfusion pressure; CBF = cerebral blood flow; PbtO2 = brain tissue oxygen tension.
P< 0.001
Table 2.
Maximal dose of vasopressor at which termination criteria were met
| Vasopressor | TIVA | ISO |
|---|---|---|
| AVP (units/kg/min) | 0.08 ± 0.03 | 0.06 ± 0.02 |
|
|
||
| NE (mcg/kg/min) | 0.85 ± 0.44 | 0.54 ± 0.25 |
|
|
||
| PE (mcg/kg/min) | 6.7 ± 2.9 | 4.5 ± 1.1 |
Values are mean ± standard deviation. Total IV anesthesia (TIVA) = fentanyl and midazolam; ISO = fentanyl and isoflurane; AVP = vasopressin; NE = norepinephrine; PE = phenylephrine.
MAP
Initial MAP values, before initiation of the vasopressor (pre-vasopressor), were similar within each anesthetic group but initial MAP was lower in the ISO group compared to the TIVA group for all vasopressors (Table 3). Comparison of MAP values at the maximal dose of each vasopressor was similar between ISO and TIVA groups (Table 3). Additionally, all three vasopressors were able to achieve a statistically significant increase in MAP between baseline and maximal dose of vasopressor values, regardless of anesthesia used (Table 3). Two way ANOVA analysis investigating the effects of vasopressor dose and anesthetic group on MAP for each vasopressor revealed significant vasopressor dose effects for all vasopressors (p < 0.05), but anesthetic choice had no significant effect on MAP.
Table 3.
Physiologic response comparing characteristics at vasopressor initiation and maximal dose, within TIVA and ISO groups
| TIVA | ISO | ||||
|---|---|---|---|---|---|
|
| |||||
| Pre- vasopressor | Maximal Dose | Pre- vasopressor | Maximal Dose | ||
| AVP | MAP (mmHg) | 73 ± 121 | 90 ± 202 | 54 ± 7 | 83 ± 212 |
| ICP (mmHg) | 12 ± 6 | 13 ± 4 | 14 ± 5 | 13 ± 7 | |
| CPP (mmHg) | 61 ± 131 | 79 ± 192 | 41 ± 4 | 70 ± 232 | |
| PbtO2 (mmHg) | 15.6 ± 5.8 | 16.2 ± 6.8 | 16.1 ± 5.9 | 24.5 ± 11.6 | |
| CBF (mL/100gm/min) | 38± 20 | 37± 25 | 33± 9 | 38± 20 | |
| Temperature (°C) | 37.8±0.2 | 37.5±0.2 | 37.5±0.2 | 37.8±0.3 | |
|
| |||||
| NE | MAP (mmHg) | 77 ± 91 | 90 ± 82 | 55 ± 10 | 92 ± 142 |
| ICP (mmHg) | 14 ± 10 | 14 ± 4 | 11 ± 8 | 18 ± 52 | |
| CPP (mmHg) | 63 ± 121 | 75 ± 8 | 44 ± 14 | 74 ± 122 | |
| PbtO2 (mmHg) | 16.1 ± 5.9 | 22.2 ± 5.92 | 20.6 ± 9.9 | 36.3 ± 7.72,3 | |
| CBF (mL/100gm/min) | 30± 17 | 48± 15 | 29± 14 | 75± 222,3 | |
| Temperature (°C) | 37.9±0.1 | 38.1±0.2 | 37.8±0.2 | 38.0±0.1 | |
|
|
|||||
| PE | MAP (mmHg) | 71 ± 111 | 98 ± 232 | 59 ± 8 | 114 ± 82 |
| ICP (mmHg) | 11 ± 4 | 15 ± 52 | 11 ± 4 | 16 ± 42 | |
| CPP (mmHg) | 60 ± 10 | 83 ± 202 | 48 ± 10 | 98 ± 102 | |
| PbtO2 (mmHg) | 11.3 ± 3.41 | 18.8 ± 6.42 | 20.1 ± 8.6 | 38.1 ± 9.52,3 | |
| CBF (mL/100gm/min) | 41± 24 | 41± 23 | 35± 14 | 77± 252,3 | |
| Temperature (°C) | 37.6±0.3 | 38.0±0.3 | 37.5±0.3 | 37.8±0.2 | |
Values are mean ± standard deviation. Pre-vasopressor measurements were performed immediately before the initiation of the vasopressor drug infusion.
AVP = arginine vasopressin; NE = norepinephrine; PE = Phenylephrine; Total IV anesthesia (TIVA) = fentanyl and midazolam infusion; ISO = fentanyl and isoflurane; MAP = mean arterial blood pressure; ICP = intracranial pressure; CPP = cerebral perfusion pressure; PbtO2 = brain tissue oxygen tension
p< 0.05 when comparing TIVA pre-vasopressor data to ISO pre-vasopressor data
p< 0.05 when comparing maximal dose response of vasopressor to pre-vasopressor data within group
p< 0.05 when comparing maximal dose response of vasopressor between TIVA and ISO
ICP
During exposure to AVP, no change in ICP was observed regardless of dose or anesthetic exposure (Table 3). In contrast, NE exposure in the ISO group produced increases in ICP that were not observed in the TIVA group. During PE administration, piglets in both anesthetic groups displayed similar statistically significant increases in ICP compared to baseline.
CPP
At the maximal dose of each vasopressor, CPP increased significantly above baseline for all groups (Table 3), except NE in the TIVA group (p= 0.068).
Brain Tissue Oxygen Tension
During the AVP portion of the experiment, piglets in both groups displayed no difference between PbtO2 at the maximal dose of the vasopressor when compared to the pre-AVP values within groups. Additionally, pre-AVP and max dose-AVP PbtO2 values themselves were similar between groups (Table 3). In both TIVA and ISO groups there was an increase in PbtO2 when exposed to NE (two-way ANOVA analysis, p < 0.05). TIVA and ISO piglets had similar pre-NE values, but PbtO2 was higher at maximal NE dose for ISO than for TIVA (Table 3) (p< 0.05). Pre-PE PbtO2 values were higher in the ISO group, as were PbtO2 values at the maximal dose of PE. (p< 0.05) On two-way ANOVA analysis of PE, both TIVA and ISO groups demonstrated a significant vasopressor dose effect on PbtO2 (p<0.05).
CBF and CVR
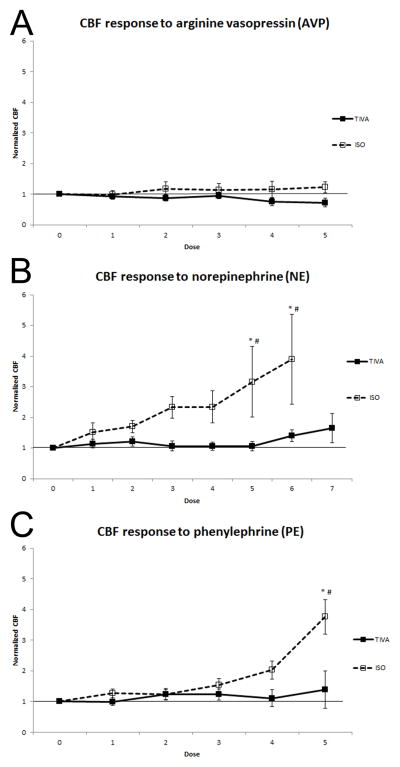
Normalized CBF (CBF at each dose divided by the pre-vasopressor CBF for each piglet) was evaluated via a two way ANOVA for each of the 3 vasopressors (Figures 1a, 1b, 1c) to evaluate the effects of vasopressor dose and anesthetic. Normalized CBF increased with escalating doses of NE and PE, but not AVP (p=0,02, p=0.03, p=0.93, respectively) regardless of anesthetic group. Additionally, increases in normalized CBF were greater for the ISO group than TIVA group when exposed to NE or PE, but not AVP (p=0.03, p=0.01, p=0.14, respectively). A group*dose effect was only statistically significant for PE (PE; p= 0.005, NE; p= 0.06, AVP; p= 0.55).
Figure 1.
A) Normalized cerebral blood flow (CBF) response to vasopressin (AVP) with fentanyl/midazolam total IV anesthesia (TIVA) (solid line and filled squares) versus fentanyl/isoflurane (ISO) (dotted line and open squares). B) Normalized CBF response to norepinhephrine (NE) using TIVA (solid line and filled squares) versus ISO (dotted line and open squares). C) Normalized CBF response to phenylephrine (PE) with TIVA (solid line and filled squares) versus ISO (dotted line and open squares). * p < 0.05. N=8 for all data points unless otherwise noted (# N=6).
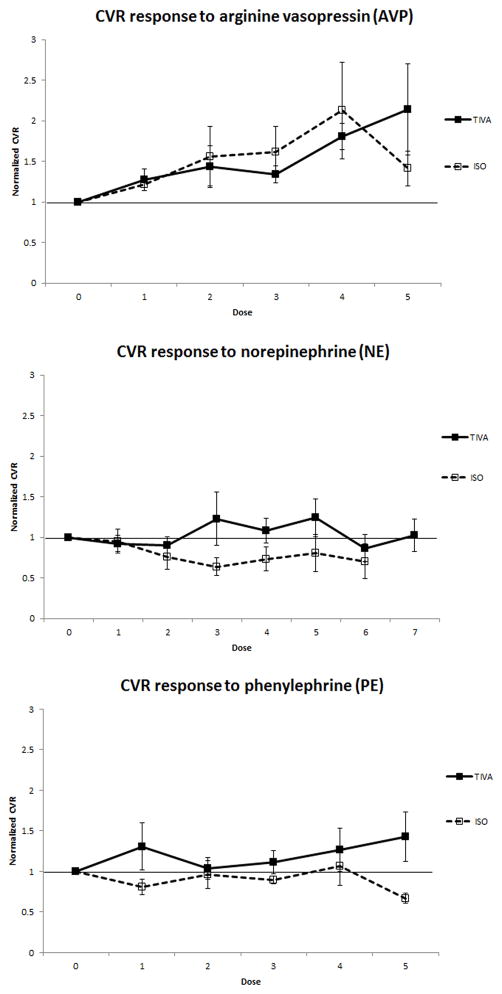
At each dose of each vasopressor, a CVR, defined as CPP/CBF, and normalized CVR (CVR at a given vasopressor dose divided by the baseline CVR before vasopressor initiation) was calculated.36 The normalized CVR was above 1 for all doses with AVP and PE with TIVA, indicating cerebrovascular vasoconstriction with a vasopressor-induced increase in MAP (Figure 2a, 2c). By contrast, the CVR was consistently below 1 for NE and PE with ISO (Figure 2b, 2c), indicating a vasodilatory pattern with vasopressor-augmented MAP. Normalized CVR while piglets were exposed to NE with TIVA were not exclusively above 1, but were consistently higher than piglets exposed to NE with ISO (Figure 2b), supporting similar findings to the other vasopressors.
Figure 2.
A) Normalized cerebrovascular resistance (CVR) response to vasopressin (AVP) under fentanyl/midazolam total IV anesthesia (TIVA) (solid line and filled squares) versus fentanyl/isoflurane (ISO) (dotted line and open squares). B) Normalized CVR response to norepinhephrine (NE) with TIVA (solid line and filled squares) versus ISO (dotted line and open squares). C) Normalized CVR response to phenylephrine (PE) with TIVA (solid line and filled squares) versus ISO (dotted line and open squares).
Discussion
In summary, animals anesthetized with ISO had significantly larger increases in CBF compared to TIVA when exposed to escalating doses of NE or PE despite similar MAP and CPP responses. We were able to calculate CVR and observe decreases in CVR with escalating doses of PE and NE solely in the ISO group. This provides some initial experimental evidence that the vasopressor effect on cerebrovascular hemodynamics and autoregulation is influenced by anesthetic choice.
We investigated changes in CBF within a range of MAP and CPP with 3 different vasopressors. All three vasopressors were effective at increasing MAP in both anesthetic groups, indicating that all three are acceptable choices as drugs of therapeutic hypertension. However, we noted interesting differences in ICP between the TIVA and ISO groups. Specifically, AVP did not show any changes in ICP in either anesthetic group. PE had a statistically significant increase in ICP in the TIVA group, which was even more pronounced in the ISO group. NE also demonstrated an increase in ICP only within the ISO group. With further increases in CBF due to exposure to NE and PE, the ISO group demonstrated statistically significant increases of ICP and alterations in CBF compared to TIVA. We speculate that the beta activity of NE, and the potential for smaller increases of central venous pressure,37 are possible explanations for the differences in ICP within the TIVA group, when comparing it to PE. Alternatively, the increased ICP observed with catecholamines may be attributable to effects on the blood-brain permeability by catecholamines and ISO.38,39 The maximal ICP increases observed were below clinical thresholds for intracranial hypertension (in a uninjured brain), but it is unclear if the magnitude of the ICP increases would be larger in the presence of intracranial pathology and future studies in a model of intracranial hypertension are warranted.
CBF was measured continuously using thermal diffusion methodology. The TIVA group showed no difference between baseline CBF and CBF at maximal dose for any of the 3 vasopressors. This is the normal physiologic response to increased MAP or CPP and argues for an intact pressure autoregulation within the TIVA group. For AVP and PE, normalized CVR was above 1 for all doses of vasopressors within TIVA, indicating vasoconstriction with increasing doses. NE-normalized CVR values were consistently higher within the TIVA group than in the ISO group, but not exclusively above 1. Similar to the TIVA group, AVP exposure in the ISO group did not show a statistically significant change in CBF, with normalized CVR above 1, supporting the presence of intact pressure autoregulation. In contrast, arterial blood pressure modulation with NE and PE in the ISO group increased CBF indicating loss of pressure autoregulation. This was further supported by normalized CVR values consistently below 1 indicating inappropriate decreases in CVR with increasing MAP and CPP. Alternative but less likely explanations for the observed increases in CBF in the ISO group include direct vasodilation of the cerebral vasculature by ISO or possibly NE, or increases in CMRO2 metabolic rate with increases in vasopressor dosing resulting in matched increases in CBF.
The implication of this lack of pressure autoregulation has clinical importance. There are several clinical scenarios where vasopressors would be used in PICU without intracranial pathology such as sepsis, trauma without head injury, and acute respiratory distress syndrome. Some of these pediatric critically ill patients may require operative intervention and during that intervention may be transitioned to volatile anesthetics from their IV infusions. This may lead to significant changes in CBF and cerebrovascular responses to vasopressor infusions and may be a consideration in anesthetic choice when such dysautoregulation with increased ICP could be deleterious.
There are several limitations to our experimental design which must be considered when translating our results to pediatric patients. First, intramuscular ketamine, which is not commonly used in the pediatric neurocritical care practice, was administered in both anesthetic groups to facilitate induction. Ketamine exposure was an average of 120 minutes before baseline data acquisition, at which time there was no evidence of increased ICP to raise concern for adverse effects of ketamine. Second, ISO was used in both groups, including TIVA, for induction and instrumentation. ISO exposure was limited in duration, flushed from the ventilator system to minimize continued exposure, and average latency between discontinuation of ISO and baseline data acquisition was more than 30 minutes. Planes of anesthesia may not be exactly analogous between the TIVA and ISO groups, which may limit the ability to draw a conclusion on the relative impact upon baseline CBF between anesthetics. However, both groups displayed a present reflexive withdrawal of the hind leg to painful stimuli, heart rate was similar between the groups, and no neuromuscular blockade was used throughout the studies suggesting similar anesthetic depths. Clinically this seems to be of limited concern, as dosing was targeted to anesthetic effect. Of note, this study does not absolve midazolam and fentanyl infusions from deleterious cerebral circulation effects. Rather, our intent was to model the current standard of care in the PICU and compare it to a common anesthetic used for PICU patients undergoing operative procedures. PbtO2 values were lower in the TIVA group which may be related to decreases in CMRO2, but its clinical consequence is unclear. Vasopressor pharmacodynamic data is limited in immature swine and we cannot exclude any residual effects from our studies. However, the washout period was not terminated until physiologic variables had returned to baseline. Baseline CPP was lower in the ISO group, which raises the concern of whether or not CPP was below the lower limit of autoregulation. Previous studies of immature swine have reported the lower limits of autoregulation at a much lower CPP than the ISO group.40 Last, AVP was used as the target of the V1 receptor, despite the endogenous vasopressin molecule for piglets being lysine vasopressin.29 AVP has been used in piglet models and is still an effective agent for achieving arterial hypertension.26,29,41,42
Within this immature large animal model, cerebral pressure autoregulation when challenged by vasopressor support (with PE or NE) was preserved using fentanyl and midazolam infusions, while it was adversely affected with ISO. Differences in cerebrovascular hemodynamic responses using IV versus volatile anesthetics have implications on choice of anesthetic and vasopressor for the care of the critically ill child requiring operative management, especially in those where impairment of cerebral autoregulation may be expected to have deleterious effects. Furthermore, selection of anesthetic drugs for large animal models is critical to investigate mechanisms of cerebrovascular hemodynamics, and in translating critical care investigations between the laboratory and bedside.
Acknowledgments
Funding: NIH K08NS064051, NIH U01NS069545, NIH R01NS39679
We thank Integra LifeSciences for providing intracranial pressure monitoring equipment and the Licox Brain Tissue Oxygen Monitoring System.
Footnotes
Reprints will not be available from the authors.
The authors declare no conflicts of interest.
This report was previously presented, in part, at the Society of Pediatric Anesthesia
DISCLOSURES:
Name: Benjamin Bruins, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Benjamin Bruins has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Todd J. Kilbaugh, MD
Contribution: This author helped design the study, analyze the data, and write the manuscript
Attestation: Todd J. Kilbaugh has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Susan S. Margulies, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Susan S. Margulies has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Stuart H. Friess, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Stuart H. Friess has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Contributor Information
Benjamin Bruins, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Todd J. Kilbaugh, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Susan S. Margulies, Department of Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania.
Stuart H. Friess, Department of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis, Missouri.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. The Journal of head trauma rehabilitation. 2006;21:544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–34. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wintermark M, Lepori D, Cotting J, Roulet E, van Melle G, Meuli R, Maeder P, Regli L, Verdun FR, Deonna T, Schnyder P, Gudinchet F. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113:1642–52. doi: 10.1542/peds.113.6.1642. [DOI] [PubMed] [Google Scholar]
- 4.Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–8. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–6. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 6.Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, David M, Werner C, Engelhard K. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16:R181. doi: 10.1186/cc11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, Mormann F, Weide J, Fliessbach K, Hoeft A, Jessen F, Putensen C, Heneka MT. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. Journal of neurology, neurosurgery, and psychiatry. 2012 doi: 10.1136/jnnp-2012-302883. [DOI] [PubMed] [Google Scholar]
- 8.Rosengarten B, Krekel D, Kuhnert S, Schulz R. Early neurovascular uncoupling in the brain during community acquired pneumonia. Crit Care. 2012;16:R64. doi: 10.1186/cc11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff J. Cerebrovascular and metabolic effects of midazolam and flumazenil. Acta Anaesthesiol Scand Suppl. 1990;92:75–7. doi: 10.1111/j.1399-6576.1990.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 10.Olsen KS, Henriksen L, Dige-Petersen H, Chraemmer-Jorgensen B, Rosenorn J. Effect of ketanserin on global cerebral blood flow and cerebral oxygen metabolism during midazolam-fentanyl or isoflurane anaesthesia. Br J Anaesth. 1992;69:263–8. doi: 10.1093/bja/69.3.263. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa Y, Iwasaki K, Aoki K, Gokan D, Hirose N, Kato J, Ogawa S. The different effects of midazolam and propofol sedation on dynamic cerebral autoregulation. Anesth Analg. 111:1279–84. doi: 10.1213/ANE.0b013e3181f42fc0. [DOI] [PubMed] [Google Scholar]
- 12.Cheng MA, Hoffman WE, Baughman VL, Albrecht RF. The effects of midazolam and sufentanil sedation on middle cerebral artery blood flow velocity in awake patients. J Neurosurg Anesthesiol. 1993;5:232–6. doi: 10.1097/00008506-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman WE, Miletich DJ, Albrecht RF. The effects of midazolam on cerebral blood flow and oxygen consumption and its interaction with nitrous oxide. Anesth Analg. 1986;65:729–33. [PubMed] [Google Scholar]
- 14.Forster A, Juge O, Morel D. Effects of midazolam on cerebral blood flow in human volunteers. Anesthesiology. 1982;56:453–5. doi: 10.1097/00000542-198206000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Nugent M, Artru AA, Michenfelder JD. Cerebral metabolic, vascular and protective effects of midazolam maleate: comparison to diazepam. Anesthesiology. 1982;56:172–6. doi: 10.1097/00000542-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen L, Cold GE, Holdgard HO, Johansen UT, Jensen S. The effects of midazolam on cerebral blood flow and oxygen consumption. Interaction with nitrous oxide in patients undergoing craniotomy for supratentorial cerebral tumours. Anaesthesia. 1990;45:1016–9. doi: 10.1111/j.1365-2044.1990.tb14877.x. [DOI] [PubMed] [Google Scholar]
- 17.Cucchiara RF, Theye RA, Michenfelder JD. The effects of isoflurane on canine cerebral metabolism and blood flow. Anesthesiology. 1974;40:571–4. doi: 10.1097/00000542-197406000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Todd MM, Weeks J. Comparative effects of propofol, pentobarbital, and isoflurane on cerebral blood flow and blood volume. J Neurosurg Anesthesiol. 1996;8:296–303. doi: 10.1097/00008506-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Smith JH, Karsli C, Lagace A, Luginbuehl I, Barlow R, Bissonnette B. Cerebral blood flow velocity increases when propofol is changed to desflurane, but not when isoflurane is changed to desflurane in children. Acta Anaesthesiol Scand. 2005;49:23–7. doi: 10.1111/j.1399-6576.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 20.Kochs E, Hoffman WE, Werner C, Albrecht RF, Schulte am Esch J. Cerebral blood flow velocity in relation to cerebral blood flow, cerebral metabolic rate for oxygen, and electroencephalogram analysis during isoflurane anesthesia in dogs. Anesth Analg. 1993;76:1222–6. doi: 10.1213/00000539-199376060-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gelman S, Fowler KC, Smith LR. Regional blood flow during isoflurane and halothane anesthesia. Anesth Analg. 1984;63:557–65. [PubMed] [Google Scholar]
- 22.Di Gennaro JL, Mack CD, Malakouti A, Zimmerman JJ, Armstead W, Vavilala MS. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 32:420–30. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherian L, Chacko G, Goodman JC, Robertson CS. Cerebral hemodynamic effects of phenylephrine and L-arginine after cortical impact injury. Crit Care Med. 1999;27:2512–7. doi: 10.1097/00003246-199911000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Pfister D, Strebel SP, Steiner LA. Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur J Anaesthesiol Suppl. 2008;42:98–103. doi: 10.1017/S0265021507003407. [DOI] [PubMed] [Google Scholar]
- 25.Steiner LA, Johnston AJ, Czosnyka M, Chatfield DA, Salvador R, Coles JP, Gupta AK, Pickard JD, Menon DK. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit Care Med. 2004;32:1049–54. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 26.Feinstein AJ, Patel MB, Sanui M, Cohn SM, Majetschak M, Proctor KG. Resuscitation with pressors after traumatic brain injury. J Am Coll Surg. 2005;201:536–45. doi: 10.1016/j.jamcollsurg.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Tsuneyoshi I, Yamada H, Kakihana Y, Nakamura M, Nakano Y, Boyle WA., 3rd Hemodynamic and metabolic effects of low-dose vasopressin infusions in vasodilatory septic shock. Crit Care Med. 2001;29:487–93. doi: 10.1097/00003246-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang J, Souter MJ, Chesnut RM, Vavilala MS. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 15:46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudkiewicz M, Proctor KG. Tissue oxygenation during management of cerebral perfusion pressure with phenylephrine or vasopressin. Crit Care Med. 2008;36:2641–50. doi: 10.1097/CCM.0b013e3181847af3. [DOI] [PubMed] [Google Scholar]
- 30.Missios S, Harris BT, Dodge CP, Simoni MK, Costine BA, Lee YL, Quebada PB, Hillier SC, Adams LB, Duhaime AC. Scaled cortical impact in immature swine: effect of age and gender on lesion volume. J Neurotrauma. 2009;26:1943–51. doi: 10.1089/neu.2009.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friess SH, Ralston J, Eucker SA, Helfaer MA, Smith C, Margulies SS. Neurocritical care monitoring correlates with neuropathology in a swine model of pediatric traumatic brain injury. Neurosurgery. 69:1139–47. doi: 10.1227/NEU.0b013e3182284aa1. discussion 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajkoczy P, Roth H, Horn P, Lucke T, Thome C, Hubner U, Martin GT, Zappletal C, Klar E, Schilling L, Schmiedek P. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000;93:265–74. doi: 10.3171/jns.2000.93.2.0265. [DOI] [PubMed] [Google Scholar]
- 33.Clausen T, Scharf A, Menzel M, Soukup J, Holz C, Rieger A, Hanisch F, Brath E, Nemeth N, Miko I, Vajkoczy P, Radke J, Henze D. Influence of moderate and profound hyperventilation on cerebral blood flow, oxygenation and metabolism. Brain Res. 2004;1019:113–23. doi: 10.1016/j.brainres.2004.05.099. [DOI] [PubMed] [Google Scholar]
- 34.Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. Boca Raton: CRC Press; 2007. [Google Scholar]
- 35.Nachar RA, Booth EA, Friedlich P, Borzage M, Soleymani S, Wider MD, Seri I. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr Res. 70:473–9. doi: 10.1203/PDR.0b013e31822e178e. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal G, Sanchez-Mejia RO, Phan N, Hemphill JC, 3rd, Martin C, Manley GT. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J Neurosurg. 2011;114:62–70. doi: 10.3171/2010.6.JNS091360. [DOI] [PubMed] [Google Scholar]
- 37.Nouira S, Elatrous S, Dimassi S, Besbes L, Boukef R, Mohamed B, Abroug F. Effects of norepinephrine on static and dynamic preload indicators in experimental hemorrhagic shock. Crit Care Med. 2005;33:2339–43. doi: 10.1097/01.ccm.0000182801.48137.13. [DOI] [PubMed] [Google Scholar]
- 38.Myburgh JA, Upton RN, Grant C, Martinez A. The cerebrovascular effects of adrenaline, noradrenaline and dopamine infusions under propofol and isoflurane anaesthesia in sheep. Anaesth Intensive Care. 2002;30:725–33. doi: 10.1177/0310057X0203000602. [DOI] [PubMed] [Google Scholar]
- 39.Chi OZ, Anwar M, Sinha AK, Wei HM, Klein SL, Weiss HR. Effects of isoflurane on transport across the blood-brain barrier. Anesthesiology. 1992;76:426–31. doi: 10.1097/00000542-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, Smielewski P, Shaffner DH. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108:1278–83. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 41.Muller S, How OJ, Hermansen SE, Stenberg TA, Sager G, Myrmel T. Vasopressin impairs brain, heart and kidney perfusion: an experimental study in pigs after transient myocardial ischemia. Crit Care. 2008;12:R20. doi: 10.1186/cc6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon F, Giudici R, Scheuerle A, Groger M, Asfar P, Vogt JA, Wachter U, Ploner F, Georgieff M, Moller P, Laporte R, Radermacher P, Calzia E, Hauser B. Comparison of cardiac, hepatic, and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: a randomized controlled trial. Crit Care. 2009;13:R113. doi: 10.1186/cc7959. [DOI] [PMC free article] [PubMed] [Google Scholar]