Abstract
Clinical trials demonstrate the regenerative potential of cardiac stem cell (CSC) therapy in the post-infarcted heart. Despite these encouraging preliminary clinical findings, the basic biology of these cells remains largely unexplored. The principal requirement for cell transplantation is to effectively prime them for survival within the unfavorable environment of the infarcted myocardium. In the adult mammalian heart, the β-O-linkage of N-acetylglucosamine (i.e., O-GlcNAc) to proteins is a unique post-translational modification that confers cardioprotection from various otherwise lethal stressors. It is not known whether this signaling system exists in cardiac stem cells. In the present study, we demonstrate that protein O-GlcNAcylation is an inducible stress response in adult murine Sca-1+/lin− CSCs and exerts an essential pro-survival role. Post-hypoxic CSCs responded by time-dependently increasing protein O-GlcNAcylation upon reoxygenation. We utilized pharmacological interventions for loss- and gain-of-function, i.e., enzymatic inhibition of OGT (adds the O-GlcNAc modification to proteins) by TT04, or inhibition of OGA (removes O-GlcNAc) by thiamet-G (ThG). Reduction in the O-GlcNAc signal (via TT04, or OGT gene deletion using Cre-mediated recombination) significantly sensitized CSCs to post-hypoxic injury, whereas augmenting O-GlcNAc levels (via ThG) enhanced cell survival. Diminished O-GlcNAc levels render CSCs more susceptible to the onset of post-hypoxic apoptotic processes via elevated PARP cleavage due to enhanced caspase-3/7 activation, whereas promoting O-GlcNAcylation can serve as a pre-emptive anti-apoptotic signal regulating the survival of CSCs. Thus, we report the primary demonstration of protein O-GlcNAcylation as an important pro-survival signal in CSCs, which could enhance CSC survival prior to in vivo autologous transfer.
Keywords: Adult stem cells, Cardiac, Cell biology, Hypoxia, Tissue-specific stem cells
Introduction
Over the past decade, several reports challenged the conventional wisdom that the adult mammalian heart is a quiescent, post-mitotic organ [1–3]. Several lines of evidence now firmly establish the reparative capacity of the myocardium under pathophysiological conditions. This conclusion was based both upon the identification and isolation of resident cardiac stem cells (CSCs) [4,5], and their therapeutic utility for autologous transplantation and functional improvement in the setting of acute and chronic myocardial infarction [6,7]. Such promising preclinical findings, though not without controversy, are driving rapid clinical translation [8].
The first wave of clinical application of cardiogenic cell types – skeletal myoblasts [9,10] and bone marrow-derived cells [11–13] – ushered in a phase of regenerative therapy driven now by mesenchymal (MSCs) [14,15] and cardiac stem cells (CSCs and CDCs) [8,16]. In most cases, excellent safety profiles have been demonstrated. Efficacy based on functional assessment (with ejection fraction as the primary end-point) is less inconsistent [9–13] and has shown promise in the more recent phase I clinical trials [8,16]. However, a significant impediment in optimizing stem cell therapy, irrespective of cell choice and its route of delivery, remains the issue of marginal cell retention and engraftment due to poor survival in the hostile milieu of the failing myocardium [17,18]. Enhancing or reinforcing the long-term persistence of CSCs could bolster their therapeutic efficacy.
Without question, understanding the basic biology of CSCs is essential for improving future, more effective iterations of cellular therapeutics in patients. In particular, better understanding of the mechanisms that control cell fate (survival and/or differentiation) could promote more effective cardiac repair [18]. Pro-survival signaling pathways represent potentially viable therapeutic targets in CSCs [7,19,20]. Unlike acute cardioprotective interventions where the ischemic insult happens prior to the experimental intervention, CSCs could be manipulated to enhance their survival in the remodeled, infarcted, or otherwise failing heart without limitation of clinical applicability. Here, we test the importance of a potentially novel cytoprotective signal in CSCs.
Numerous stress-inducible pathways have been identified in eukaryotes. In the adult mammalian heart, the glycosylation of proteins by β-O-linkage of N-acetylglucosamine (i.e., O-GlcNAc) is a unique post-translational modification that confers cardioprotection [21–28]. O-GlcNAc transferase (OGT - uridine diphospho-N-acetylglucosamine: peptide β-N-acetylglucosaminyl transferase) is the sole enzyme responsible for O-GlcNAcylating proteins, while O-GlcNAcase (OGA - O-β-N-acetylglucosaminidase) cleaves this post-translational modification. Because protein O-GlcNAcylation represents a metabolically-derived, apparently ubiquitous, and recruitable stress response in the cardiovascular system [29], it is rational to posit that such a signaling system might exist in more primitive cardiac cell types, such as CSCs. The objective of this study was to characterize the phenomenon of protein O-GlcNAcylation in CSCs and to assess its potential relevance as a necessary pro-survival signal during hypoxia-reoxygenation injury.
An unambiguous and definitive description of a resident cardiac stem cell remains elusive, owing to considerable overlap of surface molecular markers with hematopoietic and endothelial progenitor cells [30–33]. In general, adult cardiac stem cells are lineage-negative and express, uniquely or in combination, the stem cell antigens Sca-1, c-kit, and/or MDR-1; they are self-renewing, clonogenic and multipotent for the principle cell-types of the heart – cardiomyocytes, smooth muscle cells, and endothelial cells [4,32,34–36]. Because it is not known whether cardiac stem cells express a functional O-GlcNAcylating system, we utilized Sca-1+/lin− mouse CSCs to address this question and the possible functional role of O-GlcNAc levels in modulating CSC survival.
Materials and Methods
Cell Culture and Flow Cytometric Analysis
CSCs were isolated from adult, male, wild-type mouse (C57BL6) heart cell outgrowth cultures subjected to sequential sorting for c-kit+/lin− markers using magnetic immunobeads [37]. The sorted cells were analyzed by flow cytometry at different passages (<P10 for the experiments outlined here) to ascertain the purity of the cultures. Harvested CSCs and cellular controls were blocked with FcBlock (0.005 mg/mL in PBS + 1% BSA) for 10 minutes at 4°C. CSCs and controls were stained with a cocktail of anti-mouse c-kit, Ly6AE (Sca-1), hematopoietic lineage cocktail, CD31, CD34, CD45 antibodies (BD BioSciences). Data were acquired on a LSRII flow cytometer (BD BioSciences) and analyzed using BD FACSDiva software. Unstained samples of all lines were used in setting discrimination gates. CSCs were cultured in DMEM/F12 containing leukemia inhibitory factor (1000 U/mL), basic fibroblast growth factor (20 ng/mL), epidermal growth factor (20 ng/mL), and 10% embryonic stem cell fetal bovine serum, as described [38].
Pharmacological Modulation of O-GlcNAcylation in CSCs
To inhibit OGT and, thereby, reduce O-GlcNAc levels, CSCs were pre-treated overnight (~16 h) with the OGT enzyme inhibitor TT04 [2H-1, 3-thiazine-6-carboxylic acid, 2-[(4-chlorophenyl) imino] tetrahydro-4-oxo-3-(2-tricyclo[3.3.1.13, 7] dec-1-ylethyl-)] (TimTec, Inc.) [22,39] at a final concentration of 0.001 mmol/L, prior to submitting them to hypoxia-reoxygenation injury. To augment O-GlcNAcylation of cellular proteins, CSCs were treated with thiamet-G (ThG; 0.025 mmol/L) (Cayman Chemical) [40], which inhibits OGA.
OGT Gene Deletion
CSCs carrying only loxP-flanked copies of the OGT gene were infected with replication-deficient adenovirus (Vector Biolabs) carrying the Cre recombinase gene (AdCreGFP) to knock out the OGT gene, or a control adenovirus (AdGFP), at 500 MOI for 72 h. Functional expression was ascertained by immunoblot analysis. CSCs were then subjected to hypoxia-reoxygenation for cytotoxicity assays.
In vitro Hypoxia-Reoxygenation Injury
To simulate ischemia-reperfusion, pre-treated CSCs were subjected to 3 or 6 h of hypoxia in Esumi lethal ischemia medium [22,23,28,41,42] for glucose and nutrient deprivation (containing 117 mmol/L NaCl, 12 mmol/L KCl, 0.9 mmol/L CaCl20.49 mmol/L MgCl24 mmol/L HEPES, 20 mmol/L sodium lactate, and 5.6 mmol/L L-glucose; pH 6.2) in sealed humidified hypoxic chambers (Billups-Rothenberg Inc.) flushed with 5% CO2 and 95% N2, for oxygen deprivation and maintained at 37°C. After the hypoxic period they were switched to Esumi control medium (containing 137 mmol/L NaCl, 3.8 mmol/L KCl, 0.9 mmol/L CaCl20.49 mmol/L MgCl24 mmol/L HEPES, and 5.6 mmol/L D-glucose; pH 7.4) and allowed to reoxygenate for 0, 1, or 3 h in the modular incubator. Similarly treated normoxic controls received Esumi control medium for the total period of 6 h and remained in the incubator during this period.
Protein Expression
Whole cell lysates were prepared using standard protocols for total cellular protein and 10–25 µg (as appropriate) was resolved by SDS-PAGE to immunoblot for detecting protein O-GlcNAcylation [23] or specific protein target, as indicated.
Cytotoxicity Assay
Cytotoxicity after hypoxia-reoxygenation of CSCs was quantitated by spectrophotometric determination of post-hypoxic (or normoxic) lactate dehydrogenase (LDH) activity released in the medium using a commercial kit (Roche Applied Science) and expressed relative to total LDH content [22,23,28,41].
Cell Viability Assay
CSCs were cultured in 96-well plates (0.02×105 cells/well) on day 1 and received ThG or TT04 pre-treatment on day 2 (for ~16 h). They were then subjected to hypoxia-reoxygenation (day 3) and the number of viable cells was determined colorimetrically at 490 nm after incubation with the MTS tetrazolium compound [CellTiter 96 AQueous One Solution Reagent (Promega)]. In similar additional experiments, differential uptake or exclusion of trypan blue was also used to determine the percentage of viable cells.
Confocal Microscopy
For assessment of cell death, CSCs cultured in 35 mm glass-bottom dishes and subjected to hypoxia-reoxygenation were loaded with the fluorescent DNA-binding dyes DAPI (5 µg/mL) and propidium iodide (PI; 5 µg/mL) during the last 30 minutes of reoxygenation. Stained nuclei were visualized with a 20x objective on a Nikon A1 Confocal Microscope using a 405 nm laser (for DAPI) and 561 nm laser (for PI). Data were analyzed using the NIS-Elements software (Nikon, Japan).
Apoptosis
Caspase-3/7 enzyme activity as an index of apoptosis was assayed in total cellular protein by incubation with a caspase-3/7 substrate containing the tetrapeptide sequence DEVD. Aminoluciferin, a substrate of luciferase, is produced in this reaction, generating a luminescent signal (Caspase-Glo 3/7 Assay kit; Promega) measured by a Modulus luminometer (Turner Biosystems) [28]. Bioluminescence was measured in relative luminescent units. Furthermore, to ascertain activation of this pathway, the main cleavage target of caspase-3, i.e., poly(ADP-ribose) polymerase-1 (PARP-1) was assessed by immunoblotting as described above.
Assessment of CSC Bioenergetics using the XF24 Extracellular Flux Analyzer
The bioenergetic response of CSCs was measured using the Seahorse Bioscience XF24 Flux Analyzer. XF methodology measures the two major energy producing pathways, oxidative phosphorylation and glycolysis. For mitochondrial respiration, XF analysis measures the oxygen consumption rate (OCR). For glycolytic flux, XF measures the extracellular acidification rate (ECAR). CSCs seeded at an initial density of 20,000 cells per well were pre-treated with TT04 (0.001 mmol/L) or thiamet-G (0.025 mmol/L), as indicated above. For XF experiments, the treatment medium was changed to 675 µL assay medium (unbuffered DMEM supplemented with 4 mmol/L glutamine, 5 mmol/L glucose, and 1 mmol/L pyruvate) containing the corresponding pharmacological treatment 1 h before assay. The XF24 automated protocol consisted of 10 min delay following microplate insertion, baseline OCR/ECAR measurements [3×(3 min mix, 2 min wait, 3 min measure)], followed by injection of Port A (oligomycin, 75 µL) and OCR/ECAR measurement (3 min mix, 2 min wait, 3 min measure), injection of Port B (FCCP, 83.3 µL) and OCR/ECAR measurement (3 min mix, 2 min wait, 3 min measure), and injection of Port C (antimycin A, 92.6 µL) and OCR/ECAR measurement (3 min mix, 2 min wait, 3 min measure). Stocks (1 mmol/L) of oligomycin (Sigma, 75351), FCCP (Sigma, C2920) and antimycin A (Sigma, A6874) were prepared in 100% DMSO (Sigma, 154938). Prior to assay, stocks were diluted in assay medium to yield 10 µg/ml oligomycin, 0.01 mmol/L FCCP, and 0.1 mmol/L antimycin A which, after injection, yielded final concentrations of 1 µg/ml oligomycin, 0.001 mmol/L FCCP, and 0.01 mmol/L antimycin A [43–46].
Statistical Analyses
Data are reported as mean±SEM, and were analyzed by unpaired t test or ANOVA with post hoc analysis (Bonferroni’s Multiple Comparison Test), as appropriate, using GraphPad Prism 5.0 software. Differences were accepted as significant when p<0.05.
Results
Characterization of CSCs derived from adult murine heart
After isolation and over progressive passages (<10), flow cytometric analyses revealed that the CSCs expressed the stem cell antigen 1 (Sca-1), averaging 79.54 ± 4.5%, but were negative for blood lineage markers, CD31, CD45, were CD34low and did not retain expression of c-kit (Figure 1) after passage. We did not detect c-kit in cultured murine CSCs, although c-kit expression is stable in human CSCs.
Figure 1.
Characterization of CSCs isolated from adult murine hearts. A-C) Flow cytometric analyses reveal abundant expression of Sca-1 (A), relative absence of lin (B), c-kit (A) and CD31 (C), and low expression of CD34 in CSC cultures (< passage ten). D) DIC image of CSCs in culture.
Protein O-GlcNAcylation is a stress-responsive signal in CSCs
CSCs were subjected to 1, 6 or 24 h of glucose deprivation following which, total cell protein was harvested and immunoblotted for analyzing changes in protein O-GlcNAcylation. Glucose-deprived CSCs responded by inducing a dynamic increase in O-GlcNAc levels at 24 h, and the specificity of the signal was validated using routine control measures (Figure 2A-C). On face value it may appear paradoxical that the absence of glucose to provide flux through the HBP would still lead to an increase in O-GlcNAc levels; however, this phenomenon occurs in other cell lines, perhaps via glycogen degradation [47]. Increased O-GlcNAcylation in the context of starvation demonstrates an inherently recruitable stress-induced signal that we report here for the first time to be extant in CSCs. Based on our previous work, we hypothesized that this survival response might represent a significant though latent mechanism for promoting CSC survival.
Figure 2.
Stress-induced O-GlcNAcylation of CSC proteins (n=5/group). A) Summary densitometric analysis of O-GlcNAc levels in glucose-deprived CSCs shows O-GlcNAcylation significantly increased at 24 h (#p<0.001 vs 0 h). B) A representative immunoblot is shown, using the primary antibody CTD110.6. Control measures were adopted to verify the observed O-GlcNAc signal on numerous proteins depicted by several immunopositive bands. The last lane (0+OGA; n=3/group) is the same sample loaded in lane 1 incubated in vitro with O-GlcNAcase (OGA, which cleaves O-GlcNAc) to confirm antibody specificity. C) The blot in B was coincubated with N-acetylglucosamine that competes for binding with CTD110.6.
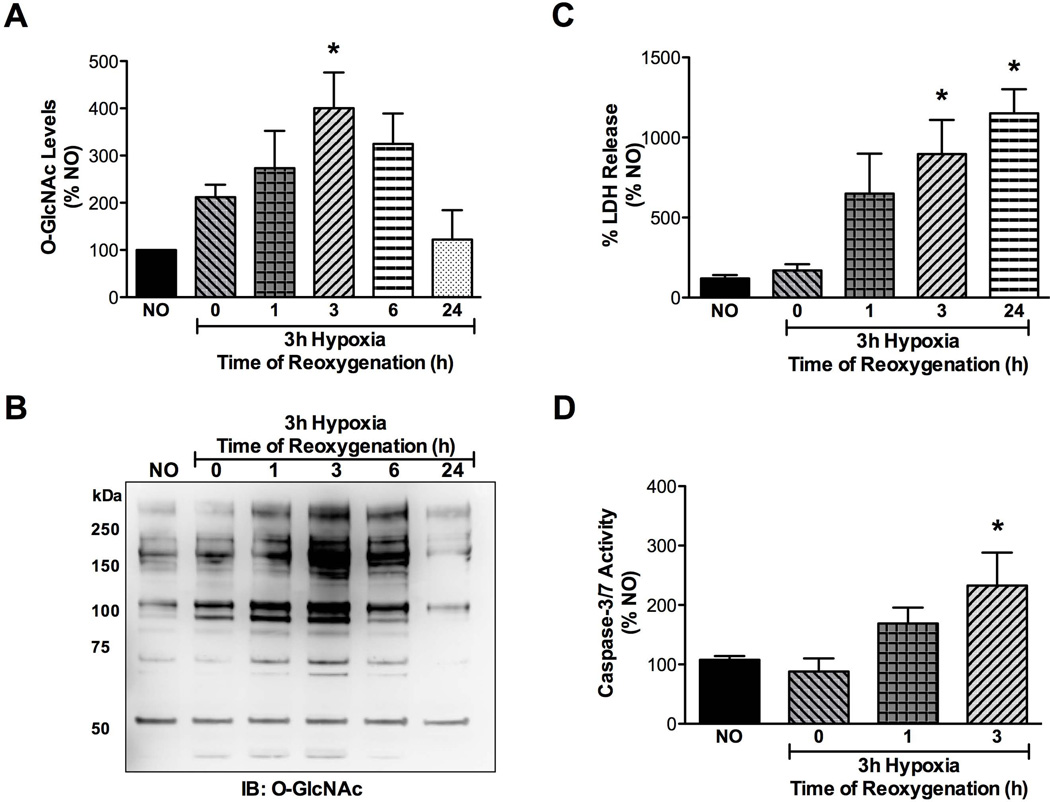
CSCs respond to hypoxia-reoxygenation injury by altering O-GlcNAc levels
In order to ascertain whether this might be true under the more lethal stress of hypoxia-reoxygenation, CSCs were subjected to hypoxia for 3h and reoxygenated for 0, 1, 3, 6 or 24 h, following which total proteins were analyzed by western blotting for effects on O-GlcNAc levels. As evidenced in Figure 3A-B, protein O-GlcNAcylation was dynamically altered upon reoxygenation. There was increased cell damage according to LDH activity (Figure 3C) and caspase-3/7 activation during 1 and 3 h of reoxygenation (Figure 3D), and these time points were selected for assessing drug treatments in further experiments. Taken together, it was apparent that CSCs have an innate capacity to respond to hypoxia-reoxygenation injury by activating the O-GlcNAc stress-signaling system. The existence of this basic biological pro-survival adaptation is demonstrated here in CSCs for the first time.
Figure 3.
Effects of hypoxia-reoxygenation (H/R) on CSCs (n=5/group). A,B) CSCs were subjected to 3 h hypoxia and reoxygenated for various durations. Densitometry of immunoblots demonstrated that O-GlcNAc levels peaked significantly (*p<0.02 vs normoxic control, NO; n=4/group) at 3 h of reoxygenation. This coincided with post-hypoxic cellular injury as assessed in C) significant LDH release (*p<0.02 vs normoxic control), and D) significant caspase-3/7 activation in CSCs (*p<0.02 vs normoxic control).
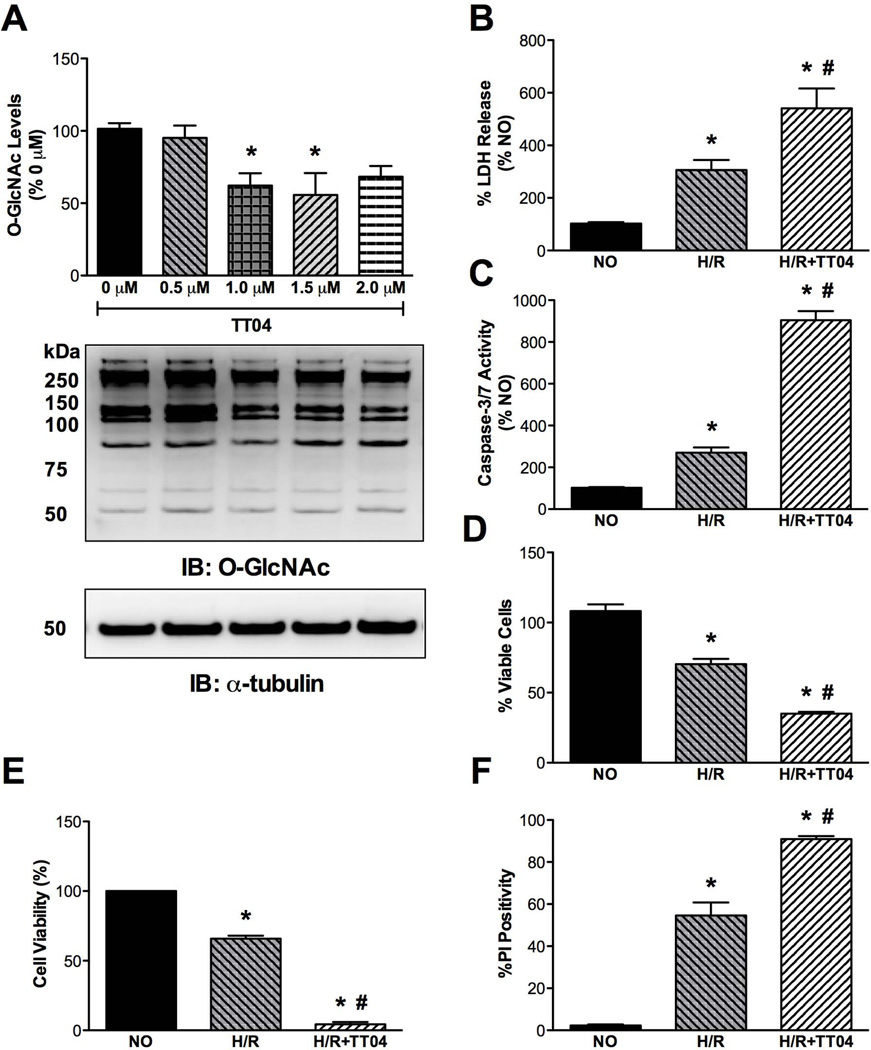
Protein O-GlcNAcylation can be pharmacologically manipulated in CSCs and inhibition of OGT sensitizes CSCs to post-hypoxic injury
To evaluate whether the endogenous response of O-GlcNAcylation imparted any effects on cell survival, we took a loss-of-function approach using the putative OGT inhibitor, TT04, at a concentration of 0.001 mmol/L (Figure 4A). This concentration was selected based on absence of cytotoxic effects the drug might have alone (data not shown). CSCs treated overnight (~16 h) with TT04 had decreased levels of O-GlcNAc-modified proteins, demonstrating that this was a viable route to examine the effects of OGT loss-of-function on hypoxia-reoxygenation injury in CSCs. Reduction in O-GlcNAc levels by pre-treatment (~16 h) of CSCs with the OGT inhibitor TT04 prior to submitting them to 3 h of hypoxia and 3 h reoxygenation exacerbated the extent of cell death. This was demonstrated by augmented LDH release (Figure 4B) and by enhancement of caspase-3/7 activities (Figure 4C), both of which were significantly greater than that observed with hypoxia-reoxygenation alone. We also assessed post-hypoxic cell viability and found it to be dramatically limited via OGT inhibition, as demonstrated by the MTS assay (Figure 4D) and the trypan blue exclusion assay (Figure 4E). The percentage of cells positive for propidium iodide (PI) was also significantly higher with TT04 treatment during hypoxia-reoxygenation (Figure 4F).
Figure 4.
Role of OGT inhibition on post-hypoxic CSC survival (n=5/group). A) Summary densitometric analysis of immunoblots (representative shown) demonstrating that TT04 (an OGT inhibitor) can reduce O-GlcNAc levels in CSCs by treatment with 0.001 mmol/L for ~16 h (*p<0.01 vs 0 mmol/L TT04). B) OGT inhibition significantly exacerbated post-hypoxic injury in CSCs, assessed by LDH activity (*p<0.01 vs normoxic control (NO); #p<0.001 vs H/R). C) Reduction in O-GlcNAc levels by TT04 also significantly increased caspase-3/7 activation following H/R (*p<0.01 vs NO; #p<0.001 vs H/R). D) OGT inhibition significantly exacerbated post-H/R CSC death, as determined by the MTS assay (*p<0.01 vs NO; #p<0.001 vs H/R), and E) as evidenced by trypan blue exclusion (*p<0.01 vs NO; #p<0.001 vs H/R; n=4/group). F) PI-indicated cell death was significantly higher following H/R due to OGT inhibition (*p<0.01 vs NO; #p<0.001 vs H/R).
CSCs expressing cre recombinase showed significant loss of OGT protein (Figure 5A) and reductions in O-GlcNAc levels, in the absence of cytotoxic effects (Figure 5B). The genetic loss of OGT recapitulated the pharmacologic studies of OGT inhibition, thereby assuaging any concerns regarding efficacy and specificity of the OGT inhibitor. To assess the effect of OGT gene deletion on post-hypoxic survival, CSCs were subjected to hypoxia-reoxygenation 72 h after treatment with AdCreGFP (500 MOI). A significant elevation of post-hypoxic LDH release was observed with AdCreGFP treatment (Figure 5C). Post-hypoxic cell viability was significantly reduced by AdCreGFP (Figure 5D). It was evident that limiting O-GlcNAcylation exacerbated cell death in CSCs. Thus, the endogenous O-GlcNAcylation in CSCs is important for cell survival. Nevertheless, the endogenous cytoprotective capacity could be further enhanced, as tested below.
Figure 5.
Sensitization of CSCs to post-hypoxic damage by OGT gene deletion (n=5/group). CSCs carrying the loxP-flanked OGT gene were infected with AdCreGFP (500 MOI) for 72h, resulting in A) significant loss of OGT expression (*p<0.001 vs AdGFP), B) significant reduction in protein O-GlcNAcylation (*p<0.001 vs AdGFP), C) significant aggravation of post-hypoxic cellular injury, assessed by LDH activity (*p<0.001 vs AdGFP), and D) significant loss of cell viability, according to MTS assay (*p<0.001 vs AdGFP; n=3/group).
Augmented protein O-GlcNAcylation protects CSCs from post-hypoxic injury
Gain-of-function for O-GlcNAcylation was achieved by treating CSCs overnight (~16 h) with thiamet-G (ThG: 0.025 mmol/L), which is a potent inhibitor of OGA (the enzyme that removes the O-GlcNAc modification from proteins). This resulted in elevated levels of O-GlcNAc as assessed by western blot (Figure 6A) that were increased over and above vehicle-treated CSCs following reoxygenation (Figure 6B). To ascertain whether CSCs could be rescued from post-hypoxic damage via reversing the loss of O-GlcNAc during hypoxia-reoxygenation, CSCs were pre-treated with ThG and it was found that elevated O-GlcNAc levels could improve cell survival with regards to reduction of LDH released into the medium (Figure 6C). A significant reduction in caspase-3/7 activities was observed with ThG (Figure 6D). The protective effects of enhanced O-GlcNAc signaling in CSCs were also demonstrated by a significant increase in post-hypoxic cell viability, according to the MTS assay (Figure 6E) and propidium iodide positivity (Figure 6F).
Figure 6.
Evaluation of the effects of augmented O-GlcNAcylation on post-hypoxic survival of CSCs (n=5/group, unless indicated otherwise). A) Thiamet-G, a potent OGA inhibitor, was used to significantly augment O-GlcNAc levels in CSCs (0.025 mmol/L, ~16 h treatment; *p<0.01 vs 0 mmol/L). B) O-GlcNAc levels remained significantly higher in ThG-treated CSCs following reoxygenation for 3 h (*p<0.001 vs normoxic control (NO); #p<0.01 vs H/R). C) Increased cell survival was evident following hypoxia-reoxygenation injury by augmenting O-GlcNAcylation in CSCs, as assessed by significantly lower LDH release (*p<0.001 vs NO; #p<0.001 vs H/R), D) significant reduction in caspase-3/7 activation (*p<0.001 vs NO; #p<0.001 vs H/R; n=3/group), E) significant recovery in post-hypoxic cell viability (*p<0.001 vs NO; #p<0.001 vs H/R), and F) significant protection from H/R-induced cell death determined by lowered PI positivity (*p<0.001 vs NO; #p<0.001 vs H/R).
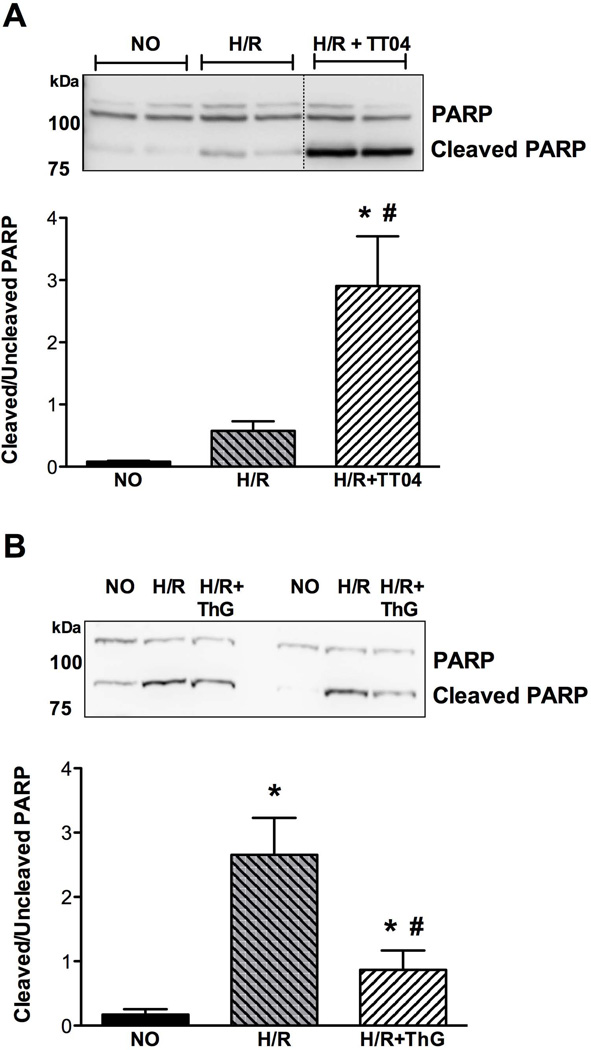
Inhibition of OGT potentiates apoptotic pathways following hypoxia-reoxygenation via augmenting the cleavage of PARP-1
Because caspase-3/7 activation observed in CSCs during hypoxia-reoxygenation appeared to be a critical mechanism for the ensuing cell death processes exacerbated by the inhibition of OGT, we were interested to verify whether this might further lead to the enhanced cleavage of the main downstream target of caspase-3, i.e., PARP-1. According to immunoblot analysis, the cleavage product of PARP-1 appeared when CSCs were reoxygenated, which was exacerbated by treatment with the OGT inhibitor, TT04 (Figure 7A). In short, the post-hypoxic activation of caspase-3/7 induced by lowered protein O-GlcNAcylation resulted in the downstream cleavage of full length PARP-1, sensitizing CSCs to apoptotic cell death. By increasing O-GlcNAcylation (via thiamet-G), PARP-1 cleavage was reduced, thereby demonstrating a robust pro-survival mechanism in CSCs (Figure 7B).
Figure 7.
Potential mechanism of cell death regulated by O-GlcNAc in CSCs (n=4–5/group). A) Potentiation of apoptotic pathways is demonstrated by OGT inhibition following H/R. Post-hypoxic recovery (3 h reoxygenation) showed that reduction in O-GlcNAc levels significantly elevated PARP-1 cleavage (expressed as the ratio cleaved PARP/uncleaved PARP) (*p<0.05 vs normoxic control (NO); #p<0.01 vs H/R), whereas B) enhancing O-GlcNAcylation (via ThG-mediated OGA inhibition) mitigated post-hypoxic PARP1 cleavage (*p<0.05 vs NO; #p<0.01 vs H/R).
Bioenergetic profile of CSCs does not change by modulating O-GlcNAc levels
To directly assess the effects of pharmacological manipulation of O-GlcNAcylation on CSC bioenergetics, extracellular flux (XF) analysis was performed on CSCs treated with vehicle, thiamet-G or TT04. Bioenergetic profiles were generated by determining basal OCR, ATP-linked OCR (basal OCR - oligomycin OCR), proton leak (oligomycin OCR -antimycin A OCR), maximal OCR (FCCP OCR - antimycin A OCR), and non-mitochondrial OCR (antimycin A OCR). A decrease in O-GlcNAc signal (by TT04) did not result in alterations in OCR or ECAR values compared with vehicle (data not shown). Likewise, increased O-GlcNAcylation (ThG group) did not alter OCR or ECAR values compared with vehicle (data not shown). These data exclude one potential mechanism of cytoprotection.
Early Akt activation occurs in response to hypoxia-reoxygenation injury in CSCs
To assess whether Akt phosphorylation occurs during the early period of reoxygenation (at 30 min post-hypoxia) in CSCs, pAkt/total Akt ratios were determined by immunoblot (data not shown). Hypoxia-reoxygenation induced significant activation of Akt; this was not blocked by ThG pre-treatment; however, Akt phosphorylation was significantly enhanced by TT04 during reoxygenation. Thus, activation of Akt does not appear to be a mechanism of ThG-mediated cytoprotection.
Enhanced protein O-GlcNAcylation mitigates oxidative damage to CSCs
To assess whether reduction of oxidative stress might be a contributory mechanism involved in the protective effect of O-GlcNAcylation, CSCs were subjected to 150 min of hydrogen peroxide stress. Exposure to peroxide significantly enhanced cytotoxicity as determined by LDH release (see supplemental figure 1). It was evident that LDH release could be significantly suppressed by augmenting protein O-GlcNAcylation in CSCs using ThG.
Discussion
The present work establishes the existence of a stress-responsive glycosignaling molecular entity, i.e., O-GlcNAc, in adult cardiac stem cells. Because protein O-GlcNAcylation is associated with cytoprotection in differentiated cells, we examined whether manipulation of O-GlcNAc levels in CSCs would affect their survival under hypoxia-reoxygenation. We observed that injured CSCs respond to reoxygenation by inducing protein O-GlcNAcylation in a time-dependent manner. Blockade of OGT reduced O-GlcNAc levels and sensitized CSCs to reoxygenation injury, while OGA inhibition enhanced protein O-GlcNAcylation and markedly reduced post-hypoxic death.
O-GlcNAcylation of proteins is a stress-activated signal constituting a global cell survival response to diverse noxious stimuli [48–51]. Alterations in cardiac O-GlcNAc levels were found to occur under acutely stressful pathological conditions, using models of in vivo myocardial ischemia/reperfusion injury [26,52], ischemic preconditioning [21], and trauma-hemorrhagic shock [27,53], as well as upon in vitro exposure of cardiomyocytes to oxidative, hypoxic or ER stress [21,22,28]. Increasing glucosamine availability attenuates post-hypoxic injury in isolated perfused hearts [54,55] and neonatal rat cardiomyocytes [25], whereas inhibiting the rate-limiting enzyme GFAT negated the same [25]. We have previously validated the cytoprotective action of OGT [22] in the response of cardiomyocytes to acute oxidative/hypoxia-associated damage by employing a pharmacological inhibitor of OGT (TT04), genetic loss-of-function (translational silencing with RNAi or deletion through cre-lox recombination) and, conversely, adenoviral overexpression to enhance global O-GlcNAc levels [22]; similar findings were reported by other laboratories [26]. Such methods to manipulate OGA activity/expression demonstrated it to be a sensitizing stimulus for cellular injury in vitro [21,23,25] as well as during acute in vivo myocardial ischemia-reperfusion [21].
In terms of potential mechanistic insights, apoptosis is implicated in cardiovascular dysfunction caused by acute myocardial infarction and heart failure [56,57]. In the present study, we identified caspase activation as one (of likely multiple) potential target in cell death pathways. Caspase activation became even more pronounced when protein O-GlcNAcylation in CSCs was reduced. As a potential consequence of caspase activation, we observed enhanced cleavage of poly(ADP-ribose)polymerase-1 (PARP-1), a caspase substrate and mediator of cell death. Thus, O-GlcNAc may partially regulate cell-death by targeting molecular events such as caspase-mediated cleavage of PARP-1. In addition to attenuating apoptosis, our data indicate that O-GlcNAc could protect CSCs by limiting oxidative stress. Future studies will identify the specific targets of O-GlcNAc modification related to this process.
A principal requirement of any cell-based therapy for repairing the damaged myocardium with functionally competent cells may include elevated endurance capabilities [18]. To withstand pathological stress (e.g. hypoxia, oxidants, inflammation) in the post-infarct myocardium, adoptively transferred cells must be appropriately ‘primed’ to minimize loss due to cell death. As demonstrated here, targeting O-GlcNAcylation represents a promising approach for simple, but highly protective, modification of CSCs to promote retention of transplanted CSCs. Of course, it is likely that survival of CSCs in situ is subject to further regulation by various paracrine signals. Transplantation of O-GlcNAc-enhanced CSCs in an animal model of myocardial infarction is required to clearly demonstrate the full translational capacity of enhanced O-GlcNAcylation in CSCs; such studies are the focus of ongoing work in our laboratory.
In summary, this study demonstrates that the O-GlcNAcylation system is present in adult cardiac stem cells and is a determinant of cell viability. Because protein O-GlcNAcylation represents a robust pro-survival signal during hypoxia-reoxygenation injury in CSCs, our findings clearly signify a potential mechanism for fine-tuning cellular therapeutics for myocardial infarction.
Supplementary Material
Supplemental Figure 1. Augmented protein O-GlcNAcylation may also regulate cell survival by limiting oxidative stress-induced death. Significant oxidative damage to CSCs occurred upon 150 min exposure to 250 µmol/L hydrogen peroxide as assessed by LDH release (*p<0.05 vs 0 µmol/L; n=4/group), whereas increasing O-GlcNAc levels (ThG-mediated OGA inhibition) significantly promoted CSC survival (#p<0.01 vs 250 µmol/L; n=4/group).
Acknowledgements
This work was supported by grants from the NIH (R01 HL083320, R01 HL094419, P20 RR024489, P01 HL078825, R37 HL055757).
References
- 1.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Leri A, Finato N, et al. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. New Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 6.Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer KM, Cottage CT, Wu W, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Dib N, Michler RE, Pagani FD, et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 10.Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 11.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 12.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 14.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 18.Mohsin S, Siddiqi S, Collins B, et al. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gude N, Muraski J, Rubio M, et al. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 20.D'Amario D, Cabral-Da-Silva MC, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Jones SP, Zachara NE, Ngoh GA, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 22.Ngoh GA, Watson LJ, Facundo HT, et al. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngoh GA, Facundo HT, Hamid T, et al. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson LJ, Facundo HT, Ngoh GA, et al. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178–C187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 26.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294:C1509–C1520. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou L, Yang S, Champattanachai V, et al. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am J Physiol Heart Circ Physiol. 2009;296:H515–H523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–H1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngoh GA, Facundo HT, Zafir A, et al. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 31.Linke A, Muller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anversa P, Kajstura J, Leri A, et al. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 33.Di Nardo P, Forte G, Ahluwalia A, et al. Cardiac progenitor cells: potency and control. J Cell Physiol. 2010;224:590–600. doi: 10.1002/jcp.22165. [DOI] [PubMed] [Google Scholar]
- 34.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 36.Pfister O, Mouquet F, Jain M, et al. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Guo Y, Ou Q, et al. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fransioli J, Bailey B, Gude NA, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–14589. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 40.Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 41.Ngoh GA, Watson LJ, Facundo HT, et al. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brar BK, Stephanou A, Wagstaff MJ, et al. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- 43.Hill BG, Dranka BP, Zou L, et al. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sansbury BE, Jones SP, Riggs DW, et al. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact. 2011;191:288–295. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sansbury BE, Riggs DW, Brainard RE, et al. Responses of hypertrophied myocytes to reactive species: implications for glycolysis and electrophile metabolism. Biochem J. 2011;435:519–528. doi: 10.1042/BJ20101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Readnower RD, Brainard RE, Hill BG, et al. Standardized Bioenergetic Profiling of Adult Mouse Cardiomyocytes. Physiol Genomics. 2012 doi: 10.1152/physiolgenomics.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang JG, Park SY, Ji S, et al. O-GlcNAc protein modification in cancer cells increases in response to glucose deprivation through glycogen degradation. J Biol Chem. 2009;284:34777–34784. doi: 10.1074/jbc.M109.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zachara NE, O'Donnell N, Cheung WD, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 49.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Kazemi Z, Chang H, Haserodt S, et al. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem. 2010;285:39096–39107. doi: 10.1074/jbc.M110.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zachara NE. The roles of O-linked beta-N-acetylglucosamine in cardiovascular physiology and disease. Am J Physiol Heart Circ Physiol. 2012;302:H1905–H1918. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Not LG, Brocks CA, Vamhidy L, et al. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med. 2010;38:562–571. doi: 10.1097/CCM.0b013e3181cb10b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fulop N, Zhang Z, Marchase RB, et al. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2227–H2236. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Augmented protein O-GlcNAcylation may also regulate cell survival by limiting oxidative stress-induced death. Significant oxidative damage to CSCs occurred upon 150 min exposure to 250 µmol/L hydrogen peroxide as assessed by LDH release (*p<0.05 vs 0 µmol/L; n=4/group), whereas increasing O-GlcNAc levels (ThG-mediated OGA inhibition) significantly promoted CSC survival (#p<0.01 vs 250 µmol/L; n=4/group).