Abstract
A field experiment was conducted in Bangladesh Agricultural University Farm to investigate the mitigating effects of soil amendments such as calcium carbide, calcium silicate, phosphogypsum, and biochar with urea fertilizer on global warming potentials (GWPs) of methane (CH4) and nitrous oxide (N2O) gases during rice cultivation under continuous and intermittent irrigations. Among the amendments phosphogypsum and silicate fertilizer, being potential source of electron acceptors, decreased maximum level of seasonal CH4 flux by 25–27 % and 32–38 % in continuous and intermittent irrigations, respectively. Biochar and calcium carbide amendments, acting as nitrification inhibitors, decreased N2O emissions by 36–40 % and 26–30 % under continuous and intermittent irrigations, respectively. The total GWP of CH4 and N2O gases were decreased by 7–27 % and 6–34 % with calcium carbide, phosphogypsum, and silicate fertilizer amendments under continuous and intermittent irrigations, respectively. However, biochar amendments increased overall GWP of CH4 and N2O gases.
Keywords: Global warming potential, CH4, N2O, Electron acceptors, Nitrification inhibitor, Rice paddy
Introduction
Greenhouse gas emissions from rice paddies have been accelerating the global warming, which is of great environmental concern. Among the greenhouse gases, methane (CH4) and nitrous oxide (N2O) are the most important due to their radiative effects as well as global warming potentials (GWPs) (IPCC 1995). CH4 and N2O gases are simultaneously emitted from rice fields due to their favorable production, consumption, and transport systems between the rice rhizospheric soil and the atmosphere. CH4 accounts for 15–20 % of the radiative forcing with a GWP of 25 times greater than carbon dioxide (CO2) on a mass basis (IPCC 2001), while N2O has a GWP 310 times more than CO2 and accounts for 6–8 % of the current global warming (Amon et al. 2002). N2O is naturally produced in soils through nitrification and denitrification (Davidson et al. 1986), which are significantly influenced by type of nitrogenous fertilizer, climate, and soil ecosystem (Akiyama et al. 2006; Synder et al. 2009). It has been reported that N2O is responsible for the destruction of stratospheric ozone layer (Graedel et al. 1992; Conrad 1996). In irrigated rice, gaseous losses of N may account for up to 48 % of the N applied (Reddy et al. 1980). Bangladesh is a low-lying deltaic country in South Asia, which has a subtropical monsoon climate, e.g., temperature 27–35 °C and high relative humidity 80–90 %. Rice is grown almost throughout the year, which may degrade the environment due to continuous emissions of CH4 and N2O gases from rice field. Total rice production in Bangladesh was 34.3 million tons in FY2008–09, where Boro rice contributed about 57 % (18.5 million tons), Transplanted Aman rice 33 %, and Aus rice contributed 10 % (Bangladesh Bureau of Statistics, 2009). Bangladesh will require more than 55.0 million tons of rice to meet the food demand of the expected total population (233.0 millions) by the year 2050. Therefore, rice cultivable area especially boro rice field must be expanded to fulfill the desired production target, which may significantly accelerate CH4 and N2O nitrous oxide gas emissions. Rice productivity and carbon storage in paddy soils mainly depend on field management practices such as tillage operations, crop residue management, soil amendments with desirable nutrients fertilizer applications, irrigation water, and drainage systems followed during rice cultivation, which ultimately will affect the rate of CO2, CH4, and N2O gas emissions from rice field (Doran and Smith 1987; Balesdent et al. 2000). Among the above options soil amendments such as silicate fertilization and phosphogypsum application along with nitrogenous fertilizer in rice farming significantly decreased seasonal CH4 flux by 16–20 % and increased rice productivity by 13–18 % in Korean paddy soil (Ali et al. 2008), whereas 12–21 % reduction in total seasonal CH4 flux and 5–18 % increase in rice grain yield was recorded through silicate fertilization with urea application in the upland rice paddy soils of Bangladesh (Ali et al. 2012). Another feasible soil amendment is biochar, a charcoal containing high levels of concentrated organic carbon, high porosity (Liang et al. 2006), and greater resistance to microbial degradation in soils (Cheng et al. 2008), which could be introduced in rice paddy soil for improving soil fertility and increasing rice productivity. Besides the potential for improving soil fertility and rice productivity biochar may contribute to mitigate greenhouse gas emissions and increase carbon sequestration in soil (Glaser et al. 2002; Lehmann and Rondon 2006; Dominic et al. 2010; Zhang et al. 2010; Bruun et al. 2011). Calcium carbide (CaC2), a nitrification inhibitor, could be added with the nitrogenous fertilizers in rice farming to minimize N2O emissions as well as to improve the nitrogen use efficiency (Bronson and Mosier 1991; Yaseen et al. 2006). Calcium carbide acts as a rich source of acetylene (C2H2) gas upon its reaction with water. Acetylene is an effective inhibitor of nitrification and denitrification (Aulakh et al. 2001).
Intermittent irrigations in rice field are expected to decrease CH4 emission; however, there is a risk of increasing N2O emissions under well-drained conditions. Soil amendments such as silicate fertilizer and phosphogypsum with high content of electron acceptors along with intermittent irrigations may decrease CH4 emissions, while addition of nitrification inhibitors, e.g., calcium carbide and biochar with nitrogenous fertilizers may decrease N2O emissions via controlling the nitrification rate. In the present context of Bangladesh, inadequate information is available about the impacts of soil amendments under different irrigation water management practices on GWPs of CH4 and N2O gases. Therefore, this research experiment was undertaken to investigate the effects of the selected soil amendments on CH4 and N2O emissions from rice paddy field, and specifically to evaluate the feasibility of decreasing the total GWPs of CH4 and N2O gases under the continuous and intermittent irrigations.
Materials and Methods
Experimental Site, Soil Amendments, and Rice Cultivation
The experiment was carried out in an ideal paddy field, Bangladesh Agricultural University Farm, Mymensingh in 2010. The soil in the experimental site was silt loam, which belongs to Old Brahmaputra flood plains category. The organic matter content of the soil before experimentation was 39.6 ± 4.8 g kg−1 and other chemical properties were soil pH (1:5 with H2O) 6.2 ± 0.2, available P2O5: 68.9 ± 2.9 mg kg−1, available SiO2 82.6 ± 3.2 mg kg−1. Two irrigation water management systems such as continuous and intermittent irrigation practices were followed in this experiment. The experimental field was divided into three side by side blocks under each water management regime. Each block had five plots. The area of each unit plot was 100 m2. Five treatments such as urea alone (220 kg ha−1), urea plus calcium carbide (30 ppm), urea plus silicate fertilizer (500 kg ha−1), urea plus phosphogypsum (500 kg ha−1), and urea plus biochar (1 t ha−1) amendments were selected in this experiment. The experimental field was laid down in a randomized block design with triple replications. In total, there were 30 unit plots in our experiment; i.e., 2 water regimes * 5 treatments * 3 replications.
All the soil amendments except biochar were applied 2 days before rice transplanting in the field. The biochar was applied 1 week before final land preparation. The biochar used (<10 mm sized fraction) in this study was bagasse, a byproduct of sugarcane industry (pyrolysis at 400–500 °C for 2 h). For the field study, the biochar mass was ground to pass through a 2-mm sieve, and mixed thoroughly to obtain a powder consistency that would mix more uniformly with the soil. The contents of total organic carbon (TOC) and total nitrogen (TN) in biochar were 59.7 and 0.65 %, respectively, a total ash content of 20.8 %, and a pH (H2O) of 9.5. With respect to elemental analysis, the biochar contained 1.0 % Ca, 0.6 % Mg, 0.4 % Fe, and 2.6 % K. The contents of electron acceptors were 1.2, 3.0, 5.5, 4.9, and 4.0 % in urea, urea plus calcium carbide, urea plus calcium silicate, urea plus phosphogypsum, and urea plus biochar amendments, respectively (acid ammonium oxalate in darkness, citrate dithionate extraction method, Loeppert and Inskeep 1996; 2 M Na–acetate extraction method, Kumada and Asami 1958; Loeppert and Inskeep 1996). The basal chemical fertilizers were applied just before rice transplanting: 45 kg N ha−1 (urea), 90 kg P2O5 ha−1 (super phosphate), and 41 kg K2O ha−1 (potassium chloride). Second split of urea fertilizer (35 kg N ha−1) was applied at tiller initiation stage (around 3 weeks after rice transplanting) and the third split of fertilizer (30 kg N ha−1, 17 kg K2O ha−1) was applied at panicle initiation stage (around 6 weeks of rice transplanting).
Under the continuous irrigation system, water level in the rice field was kept at 5 cm depth. Under the intermittent irrigation system, rice field was irrigated during the final land preparation to rice transplanting time, active tillering stage, and flowering stage. The field was kept at moist conditions during the rest of the rice cultivation period. The rice cultivar used in this study was BRRI Dhan 29, indica type, semi dwarf herb with 10–14 tillers, duration 125–133 days. Twenty-one-day-old seedlings of rice cultivar BRRI Dhan 29 (indica type) were transplanted into field plots at a spacing 25 × 25 cm2 by the first week of January in 2010 and harvested in the middle of May in the same year.
Gas Sampling and Analysis
A static chamber-GC method (Wang and Wang 2003; Zou et al. 2005a) was used to estimate CH4 and N2O emissions during rice cultivation. The air gas samples from the transparent glass chamber (diameter 60 cm, and height 110 cm) were collected by using 60-ml gas-tight syringes at 0-, 15-, and 30-min interval after chamber placement over the rice-planted plots. Gas samplings were carried out once on weekly basis during the rice cultivation. Gas samples were collected three times (8.00–12.00–16.00) in a day to get the average CH4 and N2O emissions during the cropping season. The surface area of each chamber was 0.25 m2 (0.5 × 0.5 m2). While gas sampling, the chamber was placed over six hills of rice vegetation. There were four holes at the bottom of each chamber through which water movement was controlled. Soil and air temperature inside the chamber was recorded for each set of emission measurements. Gas samples in the syringes were stored for analysis by GC in the Laboratory within a few hours. The mixing ratios of CH4 and N2O were simultaneously analyzed with a modified Gas chromatograph (Agilent 7890) equipped with a flame ionization detector (FID) and an electron capture detector (ECD; Wang and Wang 2003). A purified gas of nitrogen was used as the carrier gas for CH4, and a gas mixture of argon and methane (Ar–CH4) was used as the carrier gas for N2O. To remove CO2 and water vapor in the air samples entering the ECD detector, a filter column filled with ascarite was connected at the beginning of the separation column for N2O (Zheng et al. 2008). Nitrous oxide was separated by two stainless steel columns (column 1 with 1 m length and 2.2 mm i.d., column 2 with 3 m length and 2.2 mm i.d.) that were packed with 80–100 mesh porapack Q, and detected by the ECD. CH4 was detected by the FID. The oven was operated at 100 °C, the ECD at 300 °C, and the FID at 200 °C. Fluxes were determined from the slope of the mixing ratio change in three samples, taken at 0, 15, and 30 min after chamber closure. Sample sets were rejected unless they yielded a linear regression value of r2 greater than 0.90. Average fluxes and standard deviations of N2O were calculated from triplicate plots. Seasonal amounts of CH4 and N2O emissions were sequentially accumulated from the emissions between every two adjacent intervals of the measurements (Zou et al. 2005a, b).
Estimation of CH4 and N2O Emissions
CH4 and N2O emissions from paddy fields were calculated from the experimental data and estimated by the following equation at each growth stage (Rolston 1986)
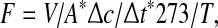 |
where F is the methane or nitrous oxide emission rate (mg m−2 h−1), V is the volume of chamber above soil (m3), A is the cross-section of chamber (m2), ∆C is the concentration difference between zero time and time t (mg m−3), and ∆t is the time duration between two sampling period (h), T (absolute temperature) = 273 + mean temperature in chamber (°C). The total CH4 or N2O emission from paddy fields was the summation of methane and nitrous oxide emissions in all growth stages of rice crop.
Estimation GWPs of CH4 and N2O
To estimate the GWP, CO2 is typically taken as the reference gas, and an increase or reduction in emission of CH4 and N2O is converted into “CO2-equivalents” by means of their GWPs. Recently, the net GWP has been estimated to complete understanding the agriculture impacts on radiative forcing (Frolking et al. 2004; Robertson and Grace 2004; Mosier et al. 2006). In this study, we used the IPCC factors to calculate the combined GWPs for 100 years (GWP = 25 × CH4 + 298 × N2O, kg CO2-equivalents ha−1) from CH4 and N2O under various agricultural practices. In addition, the greenhouse gas intensity (GHGI) was calculated by dividing GWP by grain yield for rice (Bhatia et al. 2005; Mosier et al. 2006).
Investigation of Rice Plant Growth and Yield Characteristics
Rice plant growth parameters such as plant height, tiller number, leaf area, leaf area index, shoot biomass, root volume, and porosity were investigated during the growing period. Yield components such as panicle number per plant, number of grains per panicle, ripened grains, 1000 grain weight, and harvest index were determined at the harvesting stage. Leaf area was measured by Leaf area meter (Li-3100, Li-COR, USA).
Investigation of Soil Properties
Soil redox potential (Eh) and soil pH were measured by Eh meter (PRN-41, DKK-TOA Corporation) and pH meter (Orion 3 star, Thermo electron corporation), respectively, during rice cultivation. At the harvesting stage, soil bulk density (BD) was analyzed using cores (volume 100 cm3, inner diameter 5 cm), filled with fresh moisture soils. The collected soil core samples were oven dried at 105 °C for 24 h and then measured the weight of dried core samples. Soil porosity was calculated using the BD and particle density (PD, 2.65 Mg m−3) according to the equation: porosity (%) = (1 − BD/PD) × 100. At harvesting stage, the chemical properties of the collected soil samples were analyzed for pH (1:5 with H2O), organic matter content (Wakley and Black method; Allison 1965), exchangeable Ca2+, Mg2+, and K+ (1 M NH4-acetate pH 7.0, AA, Shimazu 660), and available silicate content (1 M Na-acetate pH 4.0, UV spectrometer). The available phosphate content was determined using Lancaster method (RDA 1988). Ferrous iron and water-soluble iron concentrations in fresh soil samples were determined by 2 M Na-acetate extraction method (Modified from Kumada and Asami 1958) and distilled water method (Loeppert and Inskeep 1996), respectively. In the dried soil, the active iron and free iron concentrations were determined by modified acid ammonium oxalate in darkness and citrate dithionite bicarbonate dissolution procedures, respectively, (Loeppert and Inskeep 1996). The dissolved iron and manganese concentrations were quantified by Atomic absorption spectrophotometer (AA Shimadzu 660, Kyoto). The soil NH4+-N and NO3−-N contents were determined acidimetrically after extraction with 1 N KCl (Indophenol Blue Method and by Modified Griss-Ilosvay Method; Keeney and Nelson 1982).
Statistical Analysis
Statistical analyses were conducted using SAS software (SAS Institute 1990). Rice growth and yield parameters, soil properties, methane, and nitrous oxide emission data were subjected to the analysis of variance and regression. Fisher’s protected least significant difference (LSD) was calculated at the 0.05 probability level for making treatment mean comparisons.
Results
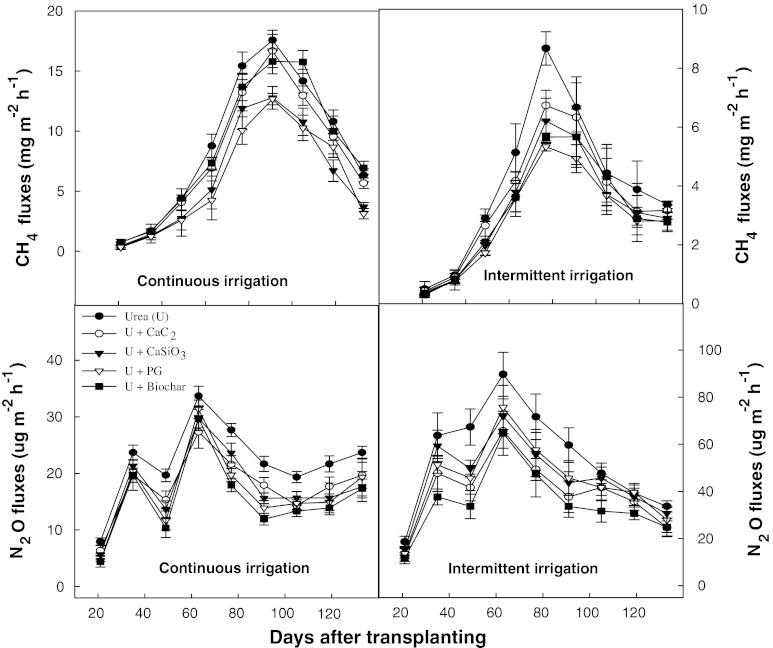
There were contrasting trends of CH4 and N2O emission rates under both continuous and intermittent irrigation systems (Fig. 1). Initially, CH4 emission rate was slow after rice saplings transplanting in the field until 36 DAT, then gradually increased onwards and sharply increased from panicle initiation stage (63 DAT), peaked (17.5 mg m−2 h−1) at heading stage (91 DAT) and finally dropped at rice harvesting stage. In contrast to CH4, N2O emissions were very low during the rice growing season. N2O fluxes showed first peak at active tillering stage (35–43 DAT) following second peak (35 μg m−2 h−1) at panicle initiation stage (63 DAT), probably due to splits application of urea which accelerated N mineralization rate, and finally increased N2O emission rate at the pre-harvesting stage (Fig. 1). Soil amendments such as phosphogypsum, silicate fertilizer, calcium carbide, and biochar in combination with urea were found very effective in controlling CH4 and N2O emission rates as compared to those of urea-treated rice planted plots (Fig. 1). Among the amendments silicate and phosphogypsum significantly decreased CH4 emission rate possibly due to their high content of ferric iron oxides and sulfate ions, being acted as electron acceptors, while N2O fluxes were decreased remarkably with biochar and calcium carbide amendments probably due to their effects as nitrification inhibitors. The contribution of the soil amendments such as silicate and phosphogypsum having electron acceptors were more pronounced in decreasing CH4 emission rates under the intermittent irrigation system as compared to continuously irrigated rice field. However, N2O emission rate significantly increased under the intermittent irrigation system probably due to increased N mineralization rate under moist dry condition. On the other hand, calcium carbide and biochar amendments were found effective in controlling N2O emission rate (Fig. 1).
Fig. 1.
Trends of CH4 and N2O fluxes from continuous and intermittent irrigations with soil amendments during rice cultivation (error bars Standard deviation among the mean values)
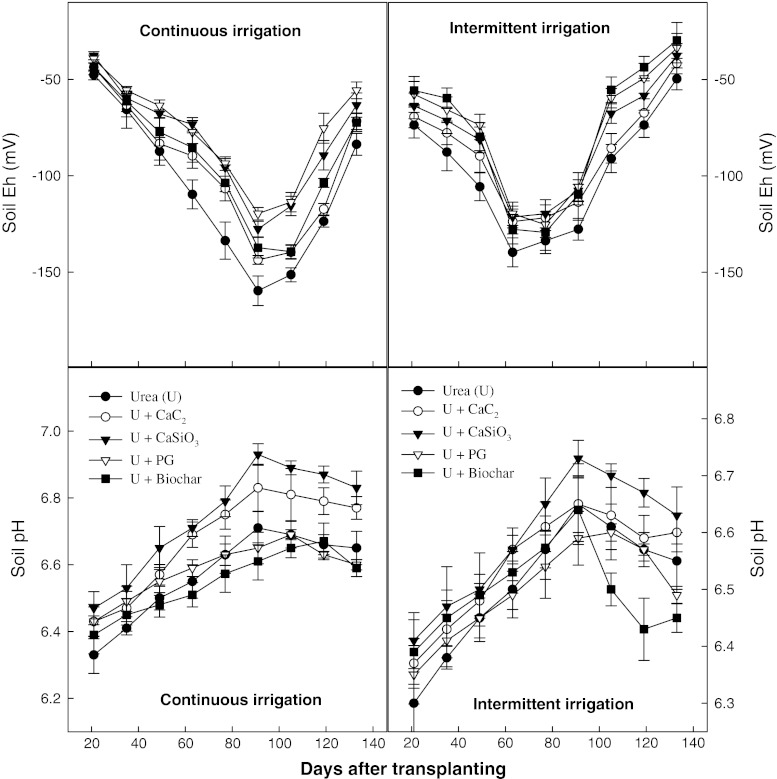
The applied soil amendments contributed to improve the soil redox potential status (Eh) of rice paddy soils and soil pH under both irrigation systems (Fig. 2). Soil pH gradually increased with the application of soil amendments in both irrigation systems, probably due to the release of alkaline materials such as Ca, Mg, and K ions in soil. However, soil redox potential (Eh) value decreased sharply after 35 DAT under continuous irrigation system mainly due to the development of anaerobic conditions and rapid decomposition of soil organic matter. The intense reductive condition, e.g., soil Eh value −100 to −150 mV was observed during flowering to heading stage (77–95 DAT), which caused significant amount of CH4 emissions (Fig. 2). Soil amendments with intermittent irrigations significantly improved the soil redox potential status and soil porosity, which ultimately decreased CH4 emissions during the entire rice growing period.
Fig. 2.
Trends of soil pH and soil Eh under continuous and intermittent irrigations with soil amendments during rice cultivation (error bars Standard deviation among the mean values)
The total seasonal CH4 flux from the control plots were 124.0 and 90.0 kg CH4 ha−1 from continuous and intermittent irrigated rice fields, respectively. Soil amendments, except biochar, significantly decreased total seasonal CH4 emissions under both irrigation systems. Under continuous irrigation, the total seasonal CH4 emissions were decreased by 5, 25, and 27 % with calcium carbide, calcium silicate, and phosphogypsum amendments, respectively, whereas under intermittent irrigations CH4 emissions were decreased by 5, 32, and 38 % with calcium carbide, calcium silicate, and phosphogypsum amendments, respectively. However, biochar amendments increased CH4 emissions by 5 and 6.0 % under continuous and intermittent irrigated conditions, respectively (Table 1).
Table 1.
Rice grain yield, seasonal CH4 and N2O emissions, and their GWPs with soil amendments and irrigation regimes
| Irrigation system (A) | Soil amendments (B) | No. of panicles (m−2) | Grain yield (kg ha−1) | CH4 emission | N2O emission | Total GWP (Mg CO2 ha−1) | GHGIb | ||
|---|---|---|---|---|---|---|---|---|---|
| Seasonal flux (kg CH4 ha−1) | GWPa (Mg CO2 ha−1) | Seasonal flux (kg N2O ha−1) | GWPa (Mg CO2 ha−1) | ||||||
| Continuous | Urea alone | 329 ± 13 | 4290 ± 95 | 124.0 ± 19 | 3.10 ± 0.04 | 0.55 ± 0.09 | 0.163 ± 0.007 | 3.26 ± 0.37 | 0.759 ± 0.05 |
| Urea + calcium carbide | 361 ± 17 | 4450 ± 107 | 118.0 ± 17 | 2.93 ± 0.03 | 0.29 ± 0.05 | 0.086 ± 0.002 | 3.01 ± 0.31 | 0.676 ± 0.03 | |
| Urea + calcium silicate | 445 ± 23 | 5150 ± 123 | 93.0 ± 13 | 2.30 ± 0.02 | 0.35 ± 0.05 | 0.104 ± 0.005 | 2.40 ± 0.25 | 0.466 ± 0.02 | |
| Urea + phosphogypsum | 425 ± 15 | 5070 ± 117 | 91.0 ± 11 | 2.26 ± 0.02 | 0.37 ± 0.06 | 0.110 ± 0.007 | 2.37 ± 0.27 | 0.467 ± 0.02 | |
| Urea + biochar | 419 ± 11 | 4980 ± 103 | 130.0 ± 21 | 2.23 ± 0.02 | 0.33 ± 0.05 | 0.098 ± 0.003 | 3.32 ± 0.39 | 0.667 ± 0.03 | |
| Intermittent | Urea alone | 357 ± 17 | 4350 ± 127 | 90.0 ± 11 | 2.19 ± 0.02 | 0.98 ± 0.19 | 0.292 ± 0.009 | 2.48 ± 0.33 | 0.570 ± 0.03 |
| Urea + calcium carbide | 399 ± 19 | 4580 ± 139 | 86.0 ± 9 | 2.13 ± 0.02 | 0.69 ± 0.15 | 0.199 ± 0.007 | 2.32 ± 0.27 | 0.506 ± 0.03 | |
| Urea + calcium silicate | 462 ± 21 | 5420 ± 128 | 61.0 ± 7 | 1.51 ± 0.01 | 0.77 ± 0.13 | 0.226 ± 0.007 | 1.73 ± 0.21 | 0.319 ± 0.02 | |
| Urea + phosphogypsum | 420 ± 13 | 5275 ± 116 | 56.0 ± 6 | 1.39 ± 0.01 | 0.79 ± 0.15 | 0.241 ± 0.008 | 1.63 ± 0.19 | 0.309 ± 0.02 | |
| Urea + biochar | 441 ± 16 | 5150 ± 77 | 96.0 ± 13 | 2.32 ± 0.04 | 0.73 ± 0.12 | 0.217 ± 0.007 | 2.59 ± 0.37 | 0.503 ± 0.03 | |
| ANOVA | A | *** | *** | *** | *** | *** | *** | *** | *** |
| B | *** | *** | *** | *** | *** | *** | *** | *** | |
| A × B | * | * | ** | ** | ** | ** | ** | * | |
aGWP of CH4 and N2O were calculated by multiplying 25 and 298 times on seasonal CH4 and N2O fluxes, respectively; The IPCC GWP factors (mass basis, kg CO2-equivalent ha−1) for CH4 and N2O are 25 and 298 in the time horizon of 100 years, respectively (Forster et al. 2007)
bGHGI (kg CO2-equivalent kg−1 grain yield) was calculated by dividing GWPs of CH4 and N2O emissions by rice yield
*, **, and *** means significant at 5, 1, and 0.1 % probability levels, respectively
The total seasonal N2O flux from the control plot was 0.550 kg N2O ha−1, which was decreased by 47, 36, 33, and 40 % with calcium carbide, calcium silicate, phosphogypsum, and biochar amendments, respectively, under continuous irrigated conditions. On the other hand, the seasonal N2O fluxes under intermittent irrigated conditions were increased by two folds in all treatments as compared to that of continuously irrigated rice fields. The seasonal N2O flux from the control plot was 0.98 kg N2O ha−1, which was decreased by 30, 21, 19, and 26 % with calcium carbide, calcium silicate, phosphogypsum, and biochor amendments, respectively, under intermittent irrigated conditions (Table 1). The interaction of soil amendments and irrigation water regimes significantly (p < 0.001) decreased total CH4 flux and N2O flux.
The total GWP of CH4 and N2O significantly decreased with soil amendments as compared to those of control plots in both continuous and intermittent irrigations. The total GWP from the continuously irrigated control plot was 3.26 Mg CO2 ha−1 which was decreased by 7, 25, and 27 % with calcium carbide, calcium silicate, and phosphogypsum amendments, respectively, biochar increased total GWP by 1.8 %. Conversely, under intermittent irrigation the total GWP from the control plot was 2.48 Mg CO2 ha−1 which was decreased by 6, 30, and 34 % with calcium carbide, calcium silicate, and phosphogypsum amendments, respectively. Biochar increased total GWP by 4.0 % under continuous and intermittent irrigations. In general, intermittent irrigation system in combination with soil amendments significantly reduced the total GWPs (Table 1), even though N2O showed twofold higher GWP in all treatments under intermittent irrigation system compared to those of continuous irrigation system. The GHGI also decreased remarkably with calcium silicate, biochar, and phosphogypsum amendments under both irrigation methods (Table 1).
Soil amendments such as calcium silicate, biochar, and phosphogypsum amendments with urea fertilization significantly increased the panicle number per unit land area which contributed to increased rice yield (Table 1). Rice grain yield was significantly (p < 0.01) increased by soil amendments in both irrigation systems. Using quadratic equation model, the maximum increases in grain yields were estimated 4, 20, 18, and 16 % over the control (4290 kg ha−1) with calcium carbide, calcium silicate, phosphogypsum, and biochar amendments, respectively, under continuous irrigated conditions; whereas grain yields were increased by 6, 25, 21, and 18 % over the control (4350 kg ha−1) with calcium carbide, calcium silicate, phosphogypsum, and biochor amendments, respectively, under intermittent irrigated conditions (Table 1). The concentrations of active iron, NH4+-N, NO3−, SO42−, and ferrous iron oxides in soil significantly increased with the application of soil amendments in both continuous and intermittent irrigated field plots (Table 2), which acted as electron acceptors in soil and eventually decreased CH4 fluxes. Higher concentrations of NH4+-N were observed in the CaC2 and biochar amended soils as compared to other treatments (Table 2), which acted as nitrification inhibitors in soil and eventually decreased N2O fluxes. In addition, intermittent irrigation system significantly increased the soil porosity, iron oxides, and the content of soil organic matter (Table 2).
Table 2.
Physico-chemical properties of soil prior to rice harvesting stage
| Soil properties | Continuous irrigation | Intermittent irrigation | LSD0.05 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea alone | Urea + calcium carbide | Urea + calcium silicate | Urea + PG | Urea + biochar | Urea alone | Urea + calcium carbide | Urea + calcium silicate | Urea + PG | Urea + biochar | ||
| Soil porosity | 0.52 | 0.54 | 0.56 | 0.55 | 0.56 | 0.53 | 0.55 | 0.57 | 0.56 | 0.59 | 0.01 |
| Soil pH (1:5 with H2O) | 6.6 | 6.8 | 7.1 | 6.8 | 6.9 | 6.5 | 6.6 | 6.9 | 6.7 | 6.8 | 0.03 |
| Soil Eh | −95.0 | −73.0 | −61.0 | −65.0 | −68.9 | −71.0 | −60.0 | −43.0 | −37.0 | −40.0 | 5.9 |
| Organic matter (g kg−1) | 24.5 | 25.9 | 28.5 | 26.9 | 31.6 | 24.1 | 25.1 | 27.9 | 26.6 | 29.5 | 4.5 |
| Available P2O5 (mg kg−1) | 63.5 | 71.5 | 105.0 | 98.0 | 97.0 | 59.5 | 67.5 | 73.5 | 91.5 | 89.0 | 12.0 |
| Available SiO2 (mg kg−1) | 59.0 | 79.5 | 117.0 | 108.0 | 93.0 | 57.5 | 67.0 | 97.9 | 93.0 | 85.0 | 15.0 |
| Ex. cations (cmol+ kg−1) | |||||||||||
| Ca | 3.9 | 4.1 | 5.6 | 6.9 | 6.3 | 3.6 | 3.6 | 5.7 | 6.3 | 5.5 | 0.35 |
| Mg | 1.1 | 1.3 | 1.6 | 1.5 | 1.5 | 1.1 | 1.2 | 1.4 | 1.30 | 1.4 | 0.11 |
| K | 0.21 | 0.29 | 0.35 | 0.38 | 0.39 | 0.22 | 0.34 | 0.41 | 0.43 | 0.47 | 0.03 |
| Active iron (g Fe kg−1)a | 6.50 | 7.80 | 11.50 | 10.20 | 9.50 | 6.70 | 9.20 | 12.50 | 11.50 | 10.30 | 7.16 |
| Ferrous iron (mg Fe2+ kg−1)b | 81.5 | 123.0 | 149.0 | 173.0 | 143.0 | 73.5 | 101.0 | 117.5 | 159.0 | 103.0 | 29.6 |
| NH4+-N (mg kg−1) | 37.5 | 49.5 | 39.5 | 37.9 | 35.5 | 30.7 | 37.9 | 28.5 | 31.5 | 29.8 | 4.6 |
| NO3−-N (mg kg−1) | 0.69 | 0.83 | 0.95 | 0.97 | 0.93 | 1.13 | 0.95 | 1.07 | 1.29 | 1.21 | 0.31 |
| SO42− (mg kg−1) | 33.5 | 57.5 | 95.0 | 139.5 | 121.5 | 39.5 | 47.0 | 129.5 | 148.5 | 119.0 | 23.5 |
CH4 flux showed a positive correlation with the availability of soil organic carbon, while there were negative correlations with soil porosity, soil pH, soil Eh, active iron, active Mn, NO3−, SO42−, and ferrous iron oxides in soil (Table 3). On the other hand, N2O fluxes showed strong positive correlations with soil porosity, soil nitrate (NO3), and soil Eh, while there were negative correlations with soil ammonium (NH4) content.
Table 3.
Correlation of CH4 and N2O emissions with selected plant parameters and soil properties
| Parameters (n = 15) | Correlation coefficient (r) | |||
|---|---|---|---|---|
| Continuous irrigation | Intermittent irrigation | |||
| CH4 emission | N2O emission | CH4 emission | N2O emission | |
| Panicle number | −0.584* | −0.456 | −0.493* | 0.427 |
| Grain yield | −0.547* | −0.643** | −0.641** | −0.601** |
| Organic carbon | 0.189 | −0.423 | 0.175 | −0.411 |
| Soil porosity | −0.649** | 0.645** | −0.689** | 0.868*** |
| Soil Ph | 0.656** | 0.265 | 0.743** | 0.715** |
| Soil Eh | −0.753*** | 0.667** | −0.693** | 0.647** |
| Active Fe | −0.688** | – | −0.665** | – |
| Ferrous Fe | −0.665** | – | −0.643** | – |
| NH4+ | 0.687*** | −0.564* | 0.567* | −0.669*** |
| NO3− | −0.647*** | 0.746*** | −0.623** | 0.864** |
| SO42− | −0.798*** | 0.645** | −.689*** | 0.686** |
*, ** and *** means significant at 5, 1 and 0.1 % probability levels, respectively
Discussion
In our study, the applied of soil amendments significantly decreased the seasonal CH4 and N2O emissions during rice cultivation under both continuous and intermittent irrigation practices. The lower CH4 emission from the amended paddy soil was due to increased aeration and stabilization of soil C, improved soil redox potential status, higher content of active iron oxides, increased sulfate and nitrate ionic compounds, which acted as electron acceptors, and eventually suppressed CH4 production. On the other hand, lower N2O evolution may be an effect of slower N cycling due to inhibition of nitrification and higher C/N ratio with application of selected soil amendments in rice paddy soils. Among the amendments calcium silicate and phosphogypsum significantly decreased CH4 emission rate due to their high content of active iron oxides and SO42− ion which acted as electron acceptors (Inubushi et al. 1984; Yagi and Minami 1990).
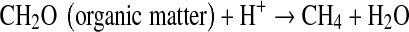 |
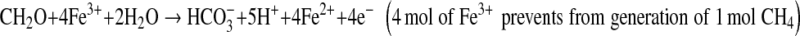 |
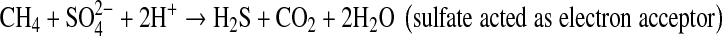 |
On the other hand, N2O fluxes were decreased remarkably with biochar and calcium carbide amendments due to their effects as nitrification inhibitors. Slow decay of biochar in soils together with tillage and transport activities may release a small amount of CH4 and CO2 to the atmosphere. The carbon in biochar prevented soil degradation and hold C in soil for long period of time (Bruno et al. 2002). It has also been shown that biochar-amended soil retains ammonium (NH4+) and nitrate (NO3−) ionic compounds (Liang et al. 2006; Cheng et al. 2008). Johannes (2007) reported that biochar amendments in soil substantially reduced nitrous oxide emission, probably due to the increased synchrony in the mineral N availability and plant uptake. The concentrations of active iron oxides (Fe2O3), NH4+, NO3−, SO42−, and ferrous iron oxides significantly (p < 0.001) increased in the amended paddy soil (Table 2). Seasonal CH4 emissions showed strong negative correlations with the active iron, SO42−, and ferrous iron concentrations in soil at harvesting stage (Table 3). This implies that the released iron oxides and sulfate from the applied soil amendments acted as electron acceptors and eventually suppressed CH4 emissions as supported by Jackel and Schnell (2000). On the other hand, seasonal N2O emissions showed strong positive correlations with the soil porosity, NO3−, soil pH, and soil Eh (Table 3). Bronson et al. (1991) reported that the slow release of acetylene from urea-encapsulated calcium carbide significantly reduced CH4 and N2O emissions during rice cultivation and increased rice yield. Rice grain yield was negatively correlated with seasonal CH4 flux (Table 3), which was supported by Denier van Der Gon et al. (2002). The increased carbon sources in paddy soil enhanced CH4 emissions but decreased N2O emissions were observed under anaerobic conditions (Hou et al. 2000). Zhang et al. (2010) reported that biochar amendments with 10–40 t ha−1 increased rice yields by 12–14 % in unfertilized soils and by 9–12 % in soils with N fertilization, respectively. They also found that total soil CH4–C emissions were increased by 34 and 41 % in soils amended with biochar at 40 t ha−1 compared to the treatments without biochar and with or without N fertilization, respectively. However, total N2O emissions were sharply decreased by 40–51 % and by 21–28 %, respectively, in biochar amended soils with or without N fertilization. Wang et al. (2011) reported that biochar incorporation (50 t ha−1) into paddy soils significantly decreased N2O emissions during the 60-day period by 73 %, while the inhibition ranged from 51 to 94 % (p < 0.05–0.01) in terms of cumulative emissions. Wang et al. (2012) reported that biochar application decreased N2O emissions up to 54 and 53 % during rice and wheat seasons. However, Clough et al. (2010) and Kristiina et al. (2011) found that biochar application had no effect on N2O emissions. Zhang et al. (2012) reported that biochar amendment (20 t ha−1) in a Chinese paddy soil increased GWP by 39 % in the first growing cycle; however, the overall GWP decreased (7–18 %) in the second consecutive growing season.
Soil amendments improved soil redox potential status and soil porosity under intermittent irrigation, provided a soil condition favorable for N2O production primarily through nitrification followed by denitrification (Hou et al. 2000). Soil pH was shifted toward neutral point (7.0–7.1) in the paddy rice field, which was favorable to the growth of methanogenic bacteria and other microbial organisms. The seasonal N2O emissions were low in all treatments under continuous irrigation as compared to those of intermittent irrigation, which may be due to less nitrification activities under anaerobic condition. Seasonal N2O emissions were increased by 16–43 % with intermittent irrigations compared with continuous flooding.
The N2O emission peaks were observed at 35 and 63 DAT, probably due to the split applications of nitrogenous fertilizers and maximum availability of mineral N until the middle season. Afterward a small flux of N2O was recorded in the late season. Draining the rice fields around 60–65 DAT increased O2 availability in the soil for N2O production as the intermediate product of either nitrification or denitrification (Xiong et al. 2007; Zheng et al. 2008). Moreover, midseason drainage improved root activities and accelerate soil organic C decomposition, which might produce more available C and N for soil microbes, and thereby favor N2O emissions (Zou et al. 2007). Rondon et al. (2005) observed a 50 % reduction in N2O emissions from soybean plots and almost complete suppression of CH4 emissions from biochar-amended (20 Mg ha−1) acidic soils in the Eastern Colombian Plains, possibly due to better aeration and greater stabilization of C. The lower N2O evolution may also be an effect of slower N cycling possibly due to a higher C/N ratio. Yanai et al. (2007) observed 85 % reduction in N2O production of rewetted soils containing 10 % biochar compared to soils without biochar. Roberts et al. (2010) reported that 30 % GHG emissions (−864 kg CO2 e Mg−1 dry corn stover) could be reduced with biochar application to soil rather than combusted for energy generation.
In our study, maximum level of NH4+-N (43.7 mg kg−1 soil) was observed in the CaC2 amended soils before rice harvesting (Table 2), which could be due to inhibition effect of CaC2 for nitrification and eventually caused minimum N2O production. Kashif et al. (2007) reported maximum concentration of NH4+-N (41.2 mg kg−1 soil) in the CaC2-amended (30 mg kg−1 soil) soil receiving 60 mg N kg−1 as urea, while lowest concentration of NH4+-N 14.3 mg kg−1 was recorded in the absence of calcium carbide. The application of encapsulated CaC2 significantly suppressed the oxidation of NH4+-N to NO3−-N in urea-fertilized soil, being supported by other researchers (Yaseen et al. 2006). Among the amendments silicate fertilizer and phosphogypsum, being industrial byproducts are easily available, economically feasible, and effective for mitigating seasonal CH4 flux and increasing rice productivity, while calcium carbide and biochar amendments in selected paddy soils seem to be effective for reducing N2O emission.
Conclusion
Calcium carbide, calcium silicate, and phosphogypsum amendments in paddy soil decreased total GWPs of CH4 and N2O gases by 6–34 %, whereas biochar amendments increased total GWP by 4.0 %. Rice grain yield was increased by 16–20 % over the control with biochar, phosphogypsum, and silicate fertilizer amendments with conventional urea fertilizer application. Among the amendments, phosphogypsum and silicate fertilizers were found effective for suppression of CH4 emissions, while calcium carbide and biochar for reducing N2O emissions from the flood plain paddy soils in Bangladesh. As the emission of CH4 and N2O gases from paddy soils depends on the inherent characteristics of soils, exogenous N-fertilizer sources, microbial activity, and soil ecosystem, therefore, economically viable biochar production technology and regional trials to different agro-ecological zones require further research.
Acknowledgments
The authors are grateful to the Muhammad Hussain Central Lab., BAU, Bangladesh and Environmental Soil Chemistry Lab., Gyeongsang National University, Republic of Korea for their analytical support.
Biographies
Muhammad Aslam Ali
is a professor in the Department of Environmental Science, Bangladesh Agricultural University, Mymensingh, Bangladesh. His research interests include mitigation of greenhouse gas emissions from rice paddy ecosystem and forest ecosystem, terrestrial carbon sequestration and soil fertility management for sustaining agricultural productivity in the changing climate, and disasters management for sustainable agricultural productivity under the changing climate. In recent years, the author mainly focussed his research programmes towards investigation of greenhouse gases emissions and their controlling strategies from different agro-ecosystems.
M. Anamul Hoque
is a professor in the Department of Soil Science, Bangladesh Agricultural University, Mymensingh, Bangladesh. His research interests include improvement of salinity tolerance, mitigation of heavy metal toxicity, antioxidant defense mechanisms, modification of protein by environmental stress, soil fertility, and plant nutrition.
P. J. Kim
is a professor in the Division of Applied Life Science, Gyeongsang National University, Chinju, South Korea. His research is focused on establishment of soil management strategy for (1) increasing phosphorus availability in arable soil to reduce P release to water body, (2) decreasing phytoextractability of cadmium (Cd) and arsenic (As) in heavy metal-contaminated soil, and (3) for increasing carbon sequestration for suppressing green house gas emission from arable land.
References
- Akiyama H, Yan X, Yagi K. Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: Summary of available data. Soil Science and Plant Nutrition. 2006;52:774–787. doi: 10.1111/j.1747-0765.2006.00097.x. [DOI] [Google Scholar]
- Ali MA, Lee CH, Kim PJ. Effect of silicate fertilizer on reducing methane emission during rice cultivation. Biology and Fertility of Soils. 2008;44:597–604. doi: 10.1007/s00374-007-0243-5. [DOI] [Google Scholar]
- Ali MA, Farouque G, Haque M, Kabir A. Influence of soil amendments on mitigating methane emissions and sustaining rice productivity in paddy soil ecosystems of Bangladesh. Journal of Environmental Science and Natural Resources. 2012;5:179–185. doi: 10.3329/jesnr.v5i1.11574. [DOI] [Google Scholar]
- Allison LE. Organic carbon. In: Black CA, Evans DD, White JL, Ensminger LE, Clark FE, editors. Methods of soil analysis, part 2. Madison, WI: American Society of Agronomy; 1965. pp. 1367–1376. [Google Scholar]
- Amon, B., T.T. Amon, G. Moitzi, and J. Boxberger. 2002. Nitrous oxide emissions from agriculture and mitigation options N2O emission aus der Landwirtschaft und Minderungsmoglichkeiten. Paper presented at Nussdorfel Laende A-1190, Vienna, Austria, 29–31.
- Aulakh MS, Singh K, Doran J. Effects of 4-amino 1,2,4 triazole, dicyandiamide and encapsulated calcium carbide on nitrification inhibition in a subtropical soil under upland and flooded conditions. Biology and Fertility of Soils. 2001;33:258–263. doi: 10.1007/s003740000317. [DOI] [Google Scholar]
- Balesdent J, Chenu C, Balabane M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Research. 2000;53:215–230. doi: 10.1016/S0167-1987(99)00107-5. [DOI] [Google Scholar]
- Bhatia A, Pathak H, Jain N, Singh PK, Singh AK. Global warming potential of manure mended soils under rice–wheat system in the Indo-Gangetic plains. Atmospheric Environment. 2005;39:6976–6984. doi: 10.1016/j.atmosenv.2005.07.052. [DOI] [Google Scholar]
- Bronson KF, Mosier AR. Effect of encapsulated calcium carbide on dinitrogen, nitrous oxide, methane, and carbon dioxide emissions from flooded rice. Biology and Fertility of Soils. 1991;11:116–120. doi: 10.1007/BF00336375. [DOI] [Google Scholar]
- Bruno G, Johannes L, Wolfgang Z. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal. Biology and Fertility of Soils. 2002;35:219–230. doi: 10.1007/s00374-002-0466-4. [DOI] [Google Scholar]
- Bruun EW, Muller Stover D, Ambus P, Hauggaard-Nielsen H. Application of biochar to soil and N2O emissions: Potential effects of blending fast-pyrolysis biochar with anaerobically digested slurry. European Journal of Soil Science. 2011;62:581–589. doi: 10.1111/j.1365-2389.2011.01377.x. [DOI] [Google Scholar]
- Cheng CH, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochimica et Cosmochimica Acta. 2008;72:1598–1610. doi: 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- Clough TJ, Bertram JE, Ray JL, Condron M, O’Callaghan M, Sherlock PR, Wells NS. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Science Society of America Journal. 2010;74:852–860. doi: 10.2136/sssaj2009.0185. [DOI] [Google Scholar]
- Conrad R. Soil micro-organisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O and NO) Microbiological Reviews. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, E.A., W.T. Swank, and T.O. Perry. 1986. Distinguishing between nitrification and denitrification as source of gaseous nitrogen production in soil. Applied Environmental Microbiology 52: 1280–1286. [DOI] [PMC free article] [PubMed]
- Denier van Der Gon HAC, Kropff MJ, van Breemen N, Wassmann R, Lantin RS, Aduna E, Corton TM. Optimizing grain yields reduces CH4 emissions from rice paddy fields. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12021–12024. doi: 10.1073/pnas.192276599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominic W, James E, Amonette F, Alayne SP, Lehmann J, Stephen J. Sustainable biochar to mitigate global climate change. Nature Communications. 2010 doi: 10.1038/ncomms1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran JW, Smith MS. Organic matter management and utilisation of soil and fertilizer nutrients. In: Follett RF, Stewart JWB, Cole CV, editors. Soil fertility and organic matter as critical components of production systems. Madison, WI: American Society of Agronomy; 1987. pp. 53–72. [Google Scholar]
- Forster, P., V. Ramaswamy, P. Artaxo, T. Berntsen, R. Betts, D.W. Fahey, J. Haywood, J. Lean, D.C. Lowe, G. Myhre, J. Nganga, R. Prinn, G. Raga, M. Schulz, and R. Van Dorland. 2007. Changes in atmospheric constituents and in radiative forcing. In Climate change 2007: the physical science basis. Contribution of working group i to the fourth assessment report of the intergovernmental panel on climate change, ed. S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor, and H.L. Miller, 130–234. Cambridge: Cambridge University Press.
- Frolking S, Li C, Braswell R, Fauglestvedt J. Short- and long-term greenhouse gas and radiative forcing impacts of changing water management in Asia rice paddies. Global Change Biology. 2004;10:1180–1196. doi: 10.1111/j.1529-8817.2003.00798.x. [DOI] [Google Scholar]
- Graedel TE, Paul J, Crutzen WH. Atmospheric change, an earth system perspective. New York: Freeman and Company; 1992. [Google Scholar]
- Hou AX, Chen GX, Wang ZP, Van Cleemput O, Patrick WH., Jr Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Science Society of American Journal. 2000;64:2180–2186. doi: 10.2136/sssaj2000.6462180x. [DOI] [Google Scholar]
- Houghton IT, Meira F, Callander LG, Harris BA, Kattenberg A, Maskell K, editors. Scientific technical analysis. Cambridge: Cambridge University Press; 1995. The science of climate change: Climate change, impacts, adaptations and mitigation of climate change. [Google Scholar]
- Houghton IT, Meira F, Callander LG, Harris BA, Kattenberg A, Maskell K, editors. Climate change 2001. The scientific basis of climate. Cambridge: Cambridge University Press; 2001. The Third Assessment Report. [Google Scholar]
- Inubushi K, Wada H, Takai Y. Easily decomposable organic matter in paddy soil. Soil Science and Plant Nutrition. 1984;30:198–1894. [Google Scholar]
- Jackel U, Schnell S. Suppression of methane emission from rice paddies by ferric iron fertilization. Soil Biology & Biochemistry. 2000;32:1811–1814. doi: 10.1016/S0038-0717(00)00094-8. [DOI] [Google Scholar]
- Johannes L. Bio-energy in the Black. Frontiers in Ecology and the Environment. 2007;5:381–387. doi: 10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2. [DOI] [Google Scholar]
- Kashif SR, Yaseen M, Arshad M, Abbas M. Evaluation of calcium carbide as a soil amendment to improve nitrogen economy of soil and yield of okra. Soil and Environment. 2007;26:69–74. [Google Scholar]
- Keeney DR, Nelson DW. Nitrogen inorganic forms. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analysis, part 2: Chemical and microbiological properties. Madison, WI: American Society of Agronomy; 1982. pp. 643–698. [Google Scholar]
- Kristiina K, Matilla T, Irina B, Kristiina R. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity-results from a short-term pilot field study. Agriculture, Ecosystems & Environment. 2011;140:309–313. doi: 10.1016/j.agee.2010.12.005. [DOI] [Google Scholar]
- Kumada K, Asami T. A new method for determining ferrous iron in paddy soils. Soil Plant Food. 1958;3:187–193. doi: 10.1080/00380768.1957.10431920. [DOI] [Google Scholar]
- Lehmann, C.J., and M. Rondon. 2006. Bio-char soil management on highly-weathered soils in the tropics. In Biological approaches to sustainable soil systems, ed. N.T. Uphoff, 517–530. Boca Raton, CRC Press.
- Liang B, Lehmann J, Solomon D, Kinyan J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG. Black carbon increases cation exchange capacity in soils. Soil Science Society of America Journal. 2006;70:1719–1730. doi: 10.2136/sssaj2005.0383. [DOI] [Google Scholar]
- Loeppert RH, Inskeep WP. Iron. In: Sparks DL, Page AL, Loeppert RH, Johnston CT, Sumner ME, Bigham JM, editors. Methods of soil analysis, part 3, chemical methods. Madison, WI: Soil Science Society of America and American Society of Agronomy; 1996. pp. 639–664. [Google Scholar]
- Mosier AR, Halvorson AD, Reule CA, Liu XJ. Net global warming potential and greenhouse gas intensity in irrigated cropping systems in Northeastern Colorado. Journal of Environmental Quality. 2006;35:1584–1598. doi: 10.2134/jeq2005.0232. [DOI] [PubMed] [Google Scholar]
- RDA, Rural Development Administration. 1988. Method of soil chemical analysis. National Institute 7 of Agricultural Science and Technology, Suwon, Korea.
- Reddy KR, Overcash MR, Khaleel R, Westerman PW. Phosphorus adsorption–desorption characteristics of two soils utilized for disposal of animal wastes. Journal of Environmental Quality. 1980;9:86–92. doi: 10.2134/jeq1980.00472425000900010020x. [DOI] [Google Scholar]
- Roberts KG, Brent AG, Stephen J, Norman RS, Lehmann J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environmental Science and Technology. 2010;44:827–833. doi: 10.1021/es902266r. [DOI] [PubMed] [Google Scholar]
- Robertson GP, Grace PR. Greenhouse gas fluxes in tropical and temperate agriculture: The need for a full-cost accounting of global warming potentials. Environment, Development and Sustainability. 2004;6:51–63. doi: 10.1023/B:ENVI.0000003629.32997.9e. [DOI] [Google Scholar]
- Rolston, D.E. 1986. Gas flux. In Methods of soil analysis, part 1, 2nd edn, Agronomy monograph 9, ed. A. Klute, 1103–1119. Madison, WI: ASA and SSSA.
- Rondon, M., J. A. Ramirez, and J. Lehmann. 2005. Charcoal additions reduce net emissions of greenhouse gases to the atmosphere. Paper read at proceedings of the 3rd USDA symposium on greenhouse gases and carbon sequestration, March 21–24, 2005, at Baltimore, USA.
- SAS/STAT User’s guide. 1990. ACECLUS-FREQ, version 6, 4th edn, vol. 1. Cary, NC: SAS Institute, Inc.
- Synder CS, Bruulsema TW, Jensen TL, Fixen PE. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agriculture, Ecosystems & Environment. 2009;133:247–266. doi: 10.1016/j.agee.2009.04.021. [DOI] [Google Scholar]
- Wang Y, Wang Y. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Advance Atmospheric Science. 2003;20:842–844. doi: 10.1007/BF02915410. [DOI] [Google Scholar]
- Wang J, Zhang M, Xiong Z, Liu P, Pan G. Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biology and Fertility of Soils. 2011 [Google Scholar]
- Wang J, Pan X, Liu Y, Xiong XZ. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant and Soil. 2012 [Google Scholar]
- Xiong ZQ, Xing GX, Zhu ZL. Nitrous oxide and methane emissions as affected by water, soil and nitrogen. Pedosphere. 2007;17:146–155. doi: 10.1016/S1002-0160(07)60020-4. [DOI] [Google Scholar]
- Yagi K, Minami K. Effect of organic matter application on methane emission from some Japanese paddy fields. Soil Science & Plant Nutrition. 1990;36:599–610. doi: 10.1080/00380768.1990.10416797. [DOI] [Google Scholar]
- Yanai Y, Toyota K, Okazaki M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Science & Plant Nutrition. 2007;53:181–188. doi: 10.1111/j.1747-0765.2007.00123.x. [DOI] [Google Scholar]
- Yaseen M, Arshad M, Khalid A. Effect of acetylene and ethylene gases released from encapsulated CaC2 on growth and yield of wheat and cotton. Pedobiologia. 2006;50:405–411. doi: 10.1016/j.pedobi.2006.08.002. [DOI] [Google Scholar]
- Zhang A, Cui L, Pan G, Li L, Hussain Q, Zhang X, Zheng J, David C. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agriculture, Ecosystems & Environment. 2010;139:469–475. doi: 10.1016/j.agee.2010.09.003. [DOI] [Google Scholar]
- Zhang A, Bian R, Pan G, Cui L, Hussain Q, Lee L, Zheng J, Zhang X, Han X, Yu X. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of two consecutive rice growing cycles. Field Crops Research. 2012;153:160. [Google Scholar]
- Zheng X, Mei B, Wang Y, Xie B, Wang Y, Dong H, Xu H, Chen G, Cai Z, Yue J, Gu J, Su F, Zou J, Zhu J. Quantification of N2O fluxes from soil–plant systems may be biased by the applied gas chromatograph methodology. Plant and Soil. 2008;11:211–234. doi: 10.1007/s11104-008-9673-6. [DOI] [Google Scholar]
- Zou J, Huang Y, Jiang J, Zheng X, Sass RL. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Global Biogeochemical Cycles. 2005;19:GB2021. doi: 10.1029/2004GB002401. [DOI] [Google Scholar]
- Zou J, Huang Y, Lu Y, Zheng X, Wang Y. Direct emission factor for N2O from rice–winter wheat rotation systems in southeast China. Atmospheric Environment. 2005;39:4755–4765. doi: 10.1016/j.atmosenv.2005.04.028. [DOI] [Google Scholar]
- Zou J, Huang Y, Zheng X, Wang Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmospheric Environment. 2007;41:8032–8042. doi: 10.1016/j.atmosenv.2007.06.049. [DOI] [Google Scholar]