Abstract
Objective
Selective serotonin reuptake inhibitors are often recommended in combination with established cognitive behavioral therapies for posttraumatic stress disorder (PTSD), but combined initial treatment of PTSD has not been studied under controlled conditions. There are also few studies of either treatment in PTSD related to terrorism. This study compared combined prolonged exposure (a cognitive behavioral therapy) plus paroxetine (a selective serotonin reuptake inhibitor) to prolonged exposure plus placebo in the treatment of terrorism-related PTSD.
Method
Adult survivors of the World Trade Center attacks of September 11, 2001 with PTSD were randomized to 10 weeks of treatment with combined prolonged exposure (10 sessions) plus paroxetine (N=19) versus prolonged exposure plus placebo (N=18). After week 10, patients discontinued prolonged exposure and were offered 12 additional weeks of continued randomized treatment.
Results
Patients treated with prolonged exposure plus paroxetine experienced significantly greater improvement in PTSD symptoms (incidence rate ratio=0.50; 95% CI=0.30–0.85; p=.013) and remission status (odds ratio=12.6; 95% CI=1.23–129; p=.034) during 10 weeks of combined treatment than patients treated with prolonged exposure plus placebo. Response rate and quality of life also improved significantly more with combined treatment. The subset of patients who continued randomized treatment for 12 more weeks showed no group differences.
Conclusions
Initial treatment with combined paroxetine plus prolonged exposure was more efficacious than prolonged exposure plus placebo for PTSD related to the World Trade Center attacks. Combined medication and prolonged exposure treatment deserves further study in larger samples with diverse forms of PTSD, and over longer periods of follow-up.
Introduction
Posttraumatic stress disorder (PTSD) has a lifetime prevalence of 8 to 12% and is associated with significant comorbidity and impaired quality of life (1, 2). Traumas commonly associated with PTSD include combat, rape, and natural disasters, but treatment of terrorism-related PTSD has been relatively little studied. Six months after September 11, 2001, 91,000 New York City residents were estimated to have PTSD related to the World Trade Center (WTC) attacks (3). The single randomized clinical trial to date for PTSD related to the WTC attacks studied cognitive-behavioral therapy (CBT) for rescue workers (4).
Although the selective serotonin reuptake inhibitors (SSRIs) paroxetine and sertraline have a U.S. Food and Drug Administration indication for PTSD based upon efficacy in several randomized clinical trials (5–7), the role of medication in the treatment of PTSD remains unclear (8–10). Some PTSD guidelines recommend SSRIs among first-line treatments for PTSD (8, 10), but others question the magnitude of response, and recommend that SSRIs be a second-line treatment or adjunct to CBT (9, 10). Trauma-focused CBT approaches such as prolonged exposure (PE) have strong empirical support, based on over two dozen randomized clinical trials (10, 11), but remission rates among completers have been under 50% in some studies (12). Because medication and CBT monotherapies each have limitations yet are very different approaches, combining these treatments might maximize efficacy (13).
No randomized clinical trials have studied combined medication and trauma-focused CBT for PTSD from the outset of treatment in a sample that was not pre-selected for treatment-resistance. One very small trial (N=10) and a subgroup analysis of a second trial reported an advantage for combined SSRI plus CBT treatment over SSRI monotherapy for adult PTSD nonresponders to pharmacotherapy (14, 15). Another small study of patients who had remained unremitted after 8 weeks of CBT (N=23) reported that augmentation with an SSRI was not superior to augmentation with placebo (16). A recent meta-analysis of 11 studies across all anxiety disorders, however, found combined CBT plus medication to be significantly more effective than CBT plus placebo at post-treatment, but not at 6 months follow-up (17), supporting need for further study of this issue in PTSD.
The goal of this study was to compare combined medication and CBT to the widely recommended first-line treatment of CBT alone in the initial treatment of PTSD. Enrollment was limited to persons with PTSD related to the World Trade Center attacks in order to obtain a sample that would be relatively homogeneous with respect to the stressor and to learn more about the treatment of PTSD related to terrorism. The primary hypothesis was that 10 weeks of treatment with combined prolonged exposure plus paroxetine, compared to prolonged exposure plus placebo, would be more efficacious in reducing symptoms and increasing remission rates in persons with PTSD related to the attacks.
Method
Design
This study was approved by an institutional review board and conducted at the Anxiety Disorders Clinic of New York State Psychiatric Institute/Columbia University from December, 2004 to February, 2009. After complete description of the study to the subjects, written informed consent was obtained. Patients with chronic PTSD related to the World Trade Center attacks were randomly assigned to 10 weeks of double-blind treatment with prolonged exposure plus paroxetine or plus placebo. To examine maintenance of gains, completers of 10 weeks of treatment were offered 12 additional weeks of continued double-blind treatment with paroxetine or placebo alone.
Participants
The sample included 37 adults referred by clinicians, responding to advertisements, or responding to direct mail to individuals who had either: 1) sought help for World Trade Center attack-related difficulties from the Mental Health Association of New York City, or 2) participated in the World Trade Center Health Registry and had screened positive for possible PTSD (scored ≥50 on the PTSD Checklist) (18).
Eligibility was determined by clinical interview, Clinician Administered PTSD Scale (CAPS) (19), and Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (20, 21). Participants were age 18–70 with a principal DSM-IV diagnosis of PTSD that was related to the World Trade Center attacks, at least three months in duration, and at least moderately severe (CAPS score ≥45). Exclusion criteria were: Prominent suicidal ideation; current psychotic disorder; unstable medical illness; pregnancy or nursing; alcohol or substance use disorder in the past 3 months; history of seizure disorder; for women of childbearing potential, unwillingness to use contraception; conditions that contraindicate study treatments, such as failure or intolerance of paroxetine treatment or three SSRI trials, or of prolonged exposure therapy; psychotropic medication during 2 weeks (4 weeks for fluoxetine or monoamine oxidase inhibitors) before randomization, except zolpidem for insomnia.
Randomization and Blinding
Patients were randomized in blocks of 10 to prolonged exposure plus paroxetine versus prolonged exposure plus matching pill placebo by the data manager with no patient contact. Controlled-release paroxetine and matching placebo tablets were provided by GlaxoSmithKline (Brentford, United Kingdom). They were packed in bottles consecutively numbered for each patient according to the randomization schedule by a pharmacist with no patient contact. Patient allocation was concealed from all research personnel for the full duration of a patient's participation in the study. To minimize risk of unblinding of independent evaluators in particular, secondary outcome measures of depressive symptoms and adverse effects (described below) were administered by pharmacotherapists.
Treatments
Paroxetine and placebo were administered by psychiatrists experienced in pharmacotherapy of PTSD. Visits were 30 minutes weekly for 6 weeks, every 2 weeks for 4 weeks, then every 4 weeks. Pharmacotherapists offered support, monitored compliance using pill counts, reviewed symptoms, and prescribed paroxetine controlled-release 12.5 mg/day or matching placebo for 1 week, 25 mg/day for 3 weeks, then increased as tolerated to a maximum of 50 mg/day.
Prolonged exposure therapy uses guided exposure to traumatic memories and situations to enhance emotional processing. It was conducted in 10 weekly 90-minute sessions, following methods of Foa (23). It was conducted by psychiatrists and PhD-level psychologists experienced in CBT, who completed a 2–4 day training and supervised training case. Therapist adherence was monitored in individual and group supervision. All sessions were videotaped, and 10% were randomly selected and reviewed by two independent raters using a treatment fidelity manual. Therapists completed 89% of essential components. Three sessions were rated independently by both raters, and inter-rater reliability (percentage agreement) was .88.
Assessments
Independent evaluators were masters- or doctoral-level clinicians. They conducted major assessments at weeks 0, 5, 10, and for patients in the maintenance phase, at weeks 14, 18, and 22. PTSD severity was assessed by the CAPS and the Clinical Global Impression Change Scale (CGI-C) (24), a 7-point scale (very much worse to very much improved).
Pharmacotherapists administered the Hamilton Rating Scale for Depression (HRSD, 17-item) (25) at major assessments and used a checklist (available on request) to rate 29 potential adverse effects at every visit on a 0–3 scale (none, mild, moderate, or severe). An adverse event was considered treatment-emergent if its severity at any point in the study was at least one point greater than at baseline.
Patients completed the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) (26), a reliable and valid measure of enjoyment and satisfaction in eight domains.
Statistical analyses
Primary outcome variables were CAPS score and remission status at weeks 5 and 10. Remission is considered an important goal for treatment of PTSD (27), and it is particularly relevant for a study combining two efficacious treatments to maximize improvement. Remission was defined by a CAPS score of ≤20 and a CGI-C score of 1 (very much improved). Response was a secondary outcome measure, defined by a CGI-C score of 1 or 2 (much or very much improved).
Continuous variables were modeled using longitudinal mixed effect analyses (MEM) with appropriate link functions (28, 29). For instance, because CAPS scores were overdispersed (mean<variance) and had a right-skewed distribution, they were modeled using negative binomial distribution with log link function (30). Binary outcome variables were modeled using longitudinal logistic mixed effect analyses (LogMEM)(28, 29, 31).
All models included predictors of time, treatment, and time-by-treatment interactions, and adjustment for baseline values transformed according to used link function. Subjects were modeled as random factors, with temporal autoregressive (AR(1)) correlation structure within each subject. If time-by-treatment interaction was not significant, the outcome variable was modeled using main effects of time and treatment, adjusted for baseline CAPS score. Results are reported using incidence rate ratios (models with negative binomial link function) or odds ratios (models with logistic link function). Three-way interaction of baseline CAPS score by treatment by time in the model was used to assess the moderator effect of dichotomized (above and below median) baseline CAPS score on treatment over time.
Tests of main effects were considered significant at α=0.05, and tests of interaction terms were considered significant at α=0.15 (32). All tests were two-tailed and used intent-to-treat samples. Missing observations were investigated using logistic regression. Because no significant predictors of missingness were found, missing observations were assumed missing at random (i.e. no imputation methods were used). Analyses were performed using PROC GLIMMIX in SAS (SAS Institute, Cary, N.C.).
Results
Sample
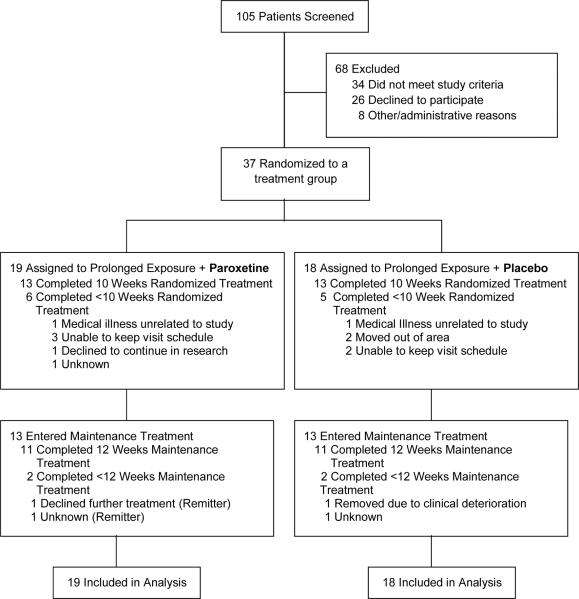
Figure 1 summarizes the flow of participants through the study. Paroxetine and placebo groups did not differ in rates of discontinuation prior to week 10 (6/19 [31.6%] vs. 5/18 [27.8%], χ2 =.06, p=0.80) or prior to week 22 (8/19 [42.1%] vs. 7/18, [33.3%], χ2 =.04, p=0.84). Patients discontinuing prematurely did not differ on baseline measures from those who completed each phase. After week 10, 13 patients continued on paroxetine and 13 patients continued on placebo, and 11 patients in each group completed the 12 week maintenance phase.
FIGURE 1.
Flow of Participants Through the Trial
Randomized groups did not differ significantly in demographic or clinical characteristics except for years of education (p=.02), as shown in Table 1. All patients reported having been in the vicinity of the World Trade Center at the time of the attacks or building collapse (in the World Trade Center (N=8), in nearby lower Manhattan (N=24), arrived in immediate aftermath to help (N=5). Thirty-one (83.8%) were emergently evacuated. Twelve (32.4%) reported loss of an immediate family member or close friend. Twenty-five (67.6%) reported at least some prior treatment of the index episode of PTSD. Adequacy of prior PTSD treatment was not systematically documented, but of the 15 previously-medicated patients only 9 (3 in placebo group, 6 in paroxetine group) reported any prior SSRI treatment, and of the 20 patients reporting therapy, none reported an adequate course of ≥10 sessions of trauma-focused CBT. The most common current psychiatric comorbidities were mood disorders (N=25, 65.8%), and treatment groups did not differ significantly in rate of comorbidity, or in severity of PTSD or depressive symptoms at baseline.
TABLE 1.
Baseline Demographic and Clinical Characteristics of PTSD Patients
| Treatment Group | ||||
|---|---|---|---|---|
|
| ||||
| Baseline Characteristic | Paroxetine (N=19) | Placebo (N=18) | ||
| N | % | N | % | |
|
| ||||
| Female | 8 | 42.1 | 12 | 66.7 |
|
| ||||
| Marital Status | ||||
| Married | 6 | 31.6 | 7 | 38.9 |
| Single, never married | 8 | 42.1 | 5 | 27.8 |
| Divorced | 5 | 26.3 | 6 | 33.3 |
|
| ||||
| Ethnicity/Race | ||||
| White | 13 | 68.4 | 12 | 66.7 |
| Black | 4 | 21.1 | 1 | 5.6 |
| Hispanic | 1 | 5.3 | 4 | 22.2 |
| Other | 1 | 5.3 | 1 | 5.6 |
|
| ||||
| Employment | ||||
| Full-time employment | 6 | 31.6 | 5 | 27.8 |
| Part-time/homemaker/retired | 6 | 31.6 | 3 | 16.7 |
| Unemployed/disabled | 7 | 36.8 | 10 | 55.6 |
|
| ||||
| Current Axis I Comorbid Diagnosis | 14 | 73.7 | 12 | 66.7 |
|
| ||||
| Current Axis II Diagnosis | 2 | 10.5 | 4 | 22.2 |
|
| ||||
| PTSD episode prior to 9/11/01 | 2 | 10.5 | 1 | 5.6 |
|
| ||||
| History of trauma prior to 9/11/01 | 5 | 26.3 | 9 | 50.0 |
|
| ||||
| Psychotherapy for PTSD post-9/11/01 | 10 | 52.6 | 10 | 55.6 |
|
| ||||
| Pharmacotherapy for PTSD post-9/11/01 | 8 | 42.1 | 7 | 38.9 |
|
| ||||
| Any treatment for PTSD post-9/11/01 | 13 | 68.4 | 12 | 75.0 |
|
| ||||
| Mean | SD | Mean | SD | |
|
| ||||
| Age, years | 49.1 | 8.0 | 51.5 | 8.0 |
|
| ||||
| Education, years | 15.5 | 1.6 | 14.2 | 1.7 |
|
| ||||
| Age of PTSD onset, years | 43.3 | 8.2 | 44.3 | 10.3 |
|
| ||||
| Duration of PTSD, years | 5.8 | 2.7 | 7.2 | 8.3 |
|
| ||||
| Clinician Assessed PTSD Scale | 72.6 | 12.9 | 65.4 | 12.8 |
|
| ||||
| Hamilton Rating Scale for Depression | 16.9 | 4.9 | 16.6 | 4.9 |
Primary Outcome Measures
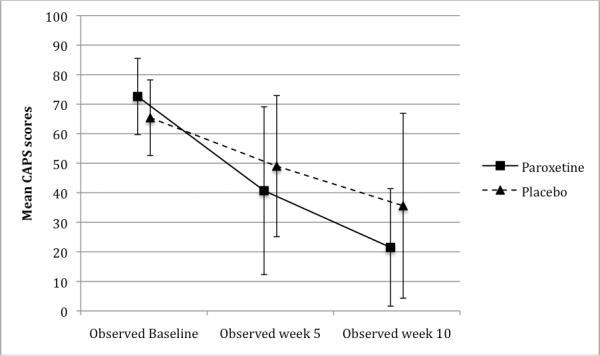
Interactions between time (from week 5 to week 10) and treatment were not significant, reflecting parallel improvement of both groups parallel from week 5 to week 10, so only change over time and treatment group effect were estimated. Each group's CAPS scores improved significantly from randomization to week 10 (p<.001), with significantly greater improvement in the combined treatment group than in the prolonged exposure plus placebo group (p=.01, Table 2, Figure 2). Patients in combined treatment had modeled CAPS scores at weeks 5 and 10 that were half (Incident Rate Ratio=.50) those of patients in the prolonged exposure plus placebo group. Analysis of dichotomized baseline CAPS scores did not provide evidence for baseline severity moderating group differences in treatment outcome.
TABLE 2.
Effects of Treatments and Time on Observed Values of Outcome Measures at Weeks 5 and 10
| Treatment group effect | Change over time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary outcomes | Paroxetine + PE | Placebo + PE | ||||||||||
| Nb | Mean | SD | Nb | Mean | SD | IRRa | 95% CI | p | IRRa | 95% CI | p | |
| Clinician-Administered PTSD Scale | ||||||||||||
| Baseline | 19 | 72.6 | 12.9 | 18 | 65.4 | 12.8 | ||||||
| Week 5 | 15 | 40.7 | 28.4 | 14 | 49.0 | 23.9 | .50 | .30 – .85 | .01 | .56 | .43 – .74 | <.001 |
| Week 10 | 13 | 21.5 | 19.9 | 13 | 35.6 | 31.3 | ||||||
| No./n | % | No./n | % | ORc | 95% CI | p | 95% CI | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Remission | ||||||||||
| Baseline | NA | NA | NA | NA | ||||||
| Week 5 | 2/14 | 14.3 | 0/14 | 0 | 12.6 | 1.23 – 129 | .03 | 20.8 | 2.44 – 176 | .007 |
| Week 10 | 8/13 | 61.5 | 3/13 | 23.1 | ||||||
| Treatment group effect at week 10 | Change over time (paroxetine group only) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary outcomes | Paroxetine + PE | Placebo + PE | ||||||||
| No./n | % | No./n | % | ORc | 95% CI | p | ORc | 95% CI | p | |
| Response d | ||||||||||
| Baseline | NA | NA | NA | NA | 23.8 | 1.21 – 469 | .04 | 27.0 | 1.88 – 333 | .02 |
| Week 5 | 6/14 | 42.9 | 6/14 | 42.9 | ||||||
| Week 10 | 12/13 | 92.3 | 7/13 | 53.8 | ||||||
| Nb | Mean | SD | Nb | Mean | SD | IRRa | 95% CI | p | IRRa | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamilton Rating Scale for Depression e | ||||||||||||
| Baseline | 19 | 16.9 | 4.9 | 17 | 16.6 | 4.9 | .14 | .79 | .50 – .93 | .02 | ||
| Week 5 | 15 | 11.7 | 5.9 | 16 | 11.8 | 5.6 | ||||||
| Week 10 | 13 | 7.7 | 3.7 | 14 | 11.4 | 6.7 | ||||||
| Quality of Life Enjoyment and Satisfaction Questionnaire f | ||||||||||||
| Baseline | 16 | 47.1 | 11.0 | 16 | 45.4 | 18.5 | ||||||
| Week 5 | 13 | 55.5 | 13.4 | 11 | 59.4 | 17.6 | 1.4 | 1.10 – 1.82 | .02 | .08 | ||
| Week 10 | 9 | 67.9 | 12.7 | 10 | 54.8 | 22.3 | ||||||
Abbreviations: PE, Prolonged Exposure Therapy, IRR, Incident Rate Ratio; CI, Confidence Interval; OR, Odds Ratios
Incident rate ratios are computed for modeled continuous outcome variables with negative binomial distribution.
Sample sizes vary by time point and measure due to attrition and missing data.
Odds ratios are computed for modeled dichotomous outcome variable.
Significant 2-way interaction time*treatment, p value = .09
Non-significant 2-way interaction time*treatment was omitted from the model, p value = .20
Higher scores represent better quality of life. Significant 2-way interaction time*treatment, p value = .06
FIGURE 2.
Clinician-Administered PTSD Scale (CAPS) Scores During Acute Treatment, By Group with Standard Deviation Bars
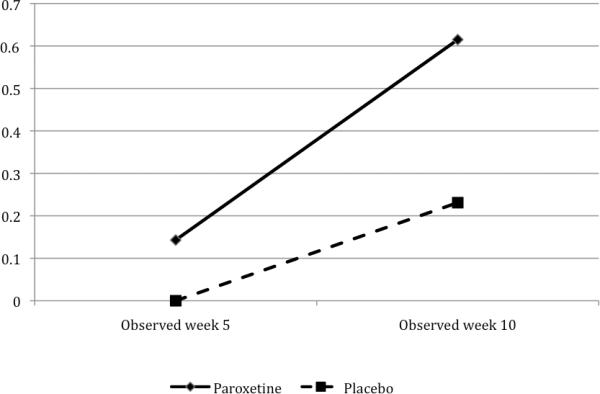
Remission in each group increased significantly over time (p<.007), and remission was significantly more frequent in the combined treatment group (p=.03, Table 2, Figure 3). Remission rates for the intent-to-treat sample at week 10 were 8/19 (42.1%) for combined treatment versus 3/18 (16.7%) for prolonged exposure plus placebo. Modeled data showed that combined treatment increased the odds of remission at weeks 5 and 10 to 12.6 times that of the prolonged exposure plus placebo treatment. The wide 95% CI (1.23, 129), however, reflects limited precision of this estimate due to the small number of subjects in the study.
FIGURE 3.
Remission Rates During Acute Treatment, by Group
Secondary Outcomes
Table 2 shows secondary outcomes. Response rates for the intent-to-treat sample at week 10 were 12/19 (63.2%) for combined treatment versus 7/18 (38.9%) for prolonged exposure plus placebo. Interactions between time (weeks 5 and 10) and treatment were significant for response status and quality of life outcomes, so the treatment group effect was different at each time point, and the effect of change over time was different for each treatment group. In the prolonged exposure plus placebo group, neither response status nor quality of life outcome had significant treatment effect at week 5 or significant change from week 5 to week 10. The combined treatment group improved significantly in quality of life (p=.02) and in response rate (p=.04) at week 10 compared to the prolonged exposure plus placebo group. From week 5 to week 10 only the combined treatment group response rate improved significantly (p=.02). Both groups' depression scores improved over time (p=.04), with no significant treatment group effect.
Among patients who continued treatment after week 10 with paroxetine (N=13) or placebo (N=13), no outcome measures showed any significant improvement or group differences during weeks 10–22. In the paroxetine group, of the eight remitters at week 10, two discontinued prematurely (both remitters at time of discontinuation), five remained remitters at week 22, and one was a non-remitter at week 22; of the five non-remitters at week 10, one became a remitter at week 22. In the placebo group, all three of the remitters at week 10 remained remitters at week 22; of the ten non-remitters at week 10 two discontinued prematurely and two became remitters at week 22. Among completers, week 22 remission rates were 5/11 (45.5%) in each group, and mean CAPS scores were 30.4 (SD=30.7) for paroxetine and 27.9 (SD=21.1) for placebo group (p=.83).
Mean maximum daily dose of paroxetine CR attained for at least one week was 32.2 mg/day (SD=13.4) in the combined treatment group and did not differ significantly from the paroxetine CR equivalent of 36.8 mg/day (SD=12.1) in the placebo group. Treatment-emergent adverse events were numerically greater in the paroxetine group but did not differ significantly from the placebo group.
Discussion
Combined prolonged exposure plus paroxetine was superior to prolonged exposure plus placebo in reducing the symptoms of PTSD related to the World Trade Center attacks over 10 weeks of treatment. This finding offers the strongest evidence to date that combining trauma-focused CBT with medication may be a more efficacious initial treatment strategy than CBT alone for PTSD. Both primary outcome measures (CAPS and remission rate) and secondary measures (response rate and quality of life) demonstrated significant advantages for combined treatment. Based on remission rates for each group at week 10, the number needed to treat is three (i.e. three patients would need to be treated with combined treatment to yield one additional remission during initial CBT treatment). Given the evidence supporting trauma-focused CBT as a treatment of choice for PTSD, the findings here advance the field by demonstrating that a combined treatment approach can further improve acute response. More study will be needed, however, to determine if these benefits persist.
The advantage of combined medication and CBT in the initial treatment of PTSD may reflect additive mechanisms. Prolonged exposure is believed to act through learning, including basic processes of extinction of conditioned responses and re-appraisal of cognitive schemas linked to the trauma (33). Paroxetine decreases presynaptic reuptake of serotonin, which may lead to stabilization of CNS circuits mediating hyperarousal and activation of memories by conditioned aversive stimuli.
A specific contribution of prolonged exposure to response in the combined therapy group is suggested by the prolonged exposure + placebo group's clinically meaningful within-group effect size on the CAPS (Cohen's d=1.12). This is within the range reported for exposure therapy for PTSD in prior trials, although larger effects have been reported at some expert sites (34). Without a group controlling for nonspecific effects of prolonged exposure, this study cannot determine the contribution of techniques specific to prolonged exposure to response in either group.
Outcomes for the prolonged exposure plus placebo group in this study could have been influenced by factors related to study design and implementation. This study limited prolonged exposure to the 10-week course established in prior trials, but a longer course continuing through weeks 10–22 might have produced greater improvement. Both treatments in this study appear to have been adequately implemented and tolerated, based on assessment of therapy tapes, paroxetine doses, and attrition rates of 36.8% for combined treatment and 27.8% for prolonged exposure plus placebo, which are comparable to the 30.3% and 20.5% rates reported across all PTSD randomized trials of SSRI and exposure therapy, respectively (35, 36). Features of study treatments that may have contributed to attrition include the confrontation of traumatic memories in prolonged exposure therapy and adverse effects of medication. This is the first report of efficacy for the controlled release form of paroxetine for PTSD, which yields slower release of paroxetine and thus slightly more stable plasma levels than the immediate release form that has established efficacy for PTSD (22).
Features of the sample may also have affected outcome. Characteristics of persons with PTSD, such as avoidance behavior and loss of trust may tend to increase attrition. Although most subjects reported inadequate response to some prior treatment, which might suggest treatment-resistance, few had received an adequate trial of an evidence-based treatment. Features specific to the WTC attacks also might have affected outcomes. The violence was both intentional and catastrophic, which tends to increase severity of PTSD (37–39). Ongoing stressors related to high rates of personal loss, additional terrorist threats following 9/11, and the downward spiral of consequences of chronic illness (job loss, family conflict, divorce) could have reduced treatment-responsiveness. Good prognostic factors, however, include relatively high educational status, and the fact that the index trauma was a single event in adulthood, which generally has better treatment outcome than severe childhood trauma or the multiple traumatic exposures typical of combat- or abuse-related PTSD. Thus, on balance, we do not believe this sample can be characterized as treatment-resistant or uniquely distinct from other traumatized populations in respect to treatment responsiveness. Generalizability of these findings, however, will need to be tested in other PTSD samples.
The sample was also distinguished by openness to trying both medication and CBT treatments, as was required by the study design. Persons with PTSD have been shown to have strong treatment preferences, especially favoring non-medication treatments (40). Participants may have been less compliant with their less-favored treatment than participants entering studies of a single treatment modality. Future studies of combined treatment should assess treatment preferences and their impact on outcome.
Over the 12 weeks after PE was discontinued and patients were maintained on double-blind paroxetine or placebo, no group differences were observed. Interpretation is subject to important limitations: The diminished sample size in this phase limited power to detect smaller effects, and patients who entered this phase were not a random selection, which may have further obscured treatment differences. Future studies with larger samples will need to address the important question of whether the initial advantage of combined treatment persists over time.
The primary limitation of this study is its relatively small sample. The full sample of 37 patients, however, represents the largest randomized clinical trial to date in persons with World Trade Center-related PTSD. The findings here of superiority for combined treatment diverge somewhat from those of the one PTSD study that failed to find an advantage for paroxetine over placebo augmentation for non-remitters to 8 weeks of prolonged exposure treatment (16). Design differences in that study included a smaller randomized sample (N=23), randomization of only those patients who remained symptomatic after a course of prolonged exposure, and continued provision of PE during placebo-controlled augmentation, which could have obscured any drug-specific effects. Nevertheless, the paroxetine group in that study had more than double the remission rate of the placebo group (33% vs. 14%), though the effect was not statistically significant in the small sample. A methodological advantage shared by these studies is the incorporation of pill placebo as a control. None of the PTSD studies that have reported superiority of CBT augmentation of SSRIs over SSRI treatment alone incorporated any form of “placebo” therapy to control for nonspecific effects of CBT, such as therapist attention.
The study findings support clinical consideration of combined paroxetine and prolonged exposure treatment at the outset for patients with PTSD, due to superior efficacy for the initial treatment of PTSD symptoms. These advantages must be weighed against potential disadvantages of the greater cost of combined treatments, the risk of adverse effects of medication, and the risk that eventual discontinuation of medication might be associated with risk of relapse, as has been shown after discontinuation of SSRI monotherapy (41). Future studies should assess moderators of response to combined treatments and monotherapy, with the goal of developing clinically useful predictors of treatment selection. The finding of medication effects also underscores the importance of assessing the impact of concurrent medication use in any studies assessing psychosocial treatments of PTSD.
Patient Perspectives
On September 11, 2001 Mr. J, a 35-year-old, married man with two daughters, was working for a financial company at the World Trade Center. After the planes hit, he was evacuated from his building and eventually made his way to safety. He had no prior psychiatric history, and Mr. J remembers thinking immediately after the event that he would be fine if he just resumed working and went back to his usual optimistic coping style. As time went by, however, he noticed that he had intrusive memories of 9/11, was emotionally disconnected from his family, had trouble getting to work in downtown Manhattan, and was increasingly avoidant of trains and airports. By the time he presented for treatment, he had lost his job and his marriage, had become distant from his kids and reported feeling “panicky, anxious, and for the first time in my life, hopeless.”
Mr. J enrolled in the study, feeling it was his last chance, and he threw himself into the prolonged exposure treatment with a deliberate seriousness. During imaginal exposure exercises he described his 9/11 experience with great affect: “I watched over and over as people jumped off the tower. I can still clearly see them; hear their bodies hitting the ground.” After evacuating from his building, he remained by the towers as others ran away, waiting for his close friend Peter to emerge. Suddenly the tower collapsed and Mr. J was overtaken by a choking cloud of white debris: “I put my newspaper around my face and dove under a car for cover. I was thinking, `You're going to die,' and then I thought, `Oh no, I can't die like this, I have a 3-month-old who needs me,' and then I lost consciousness.” The next thing he remembered was a cop, pulling him out by his feet, and yelling, “This one's still alive.” Others around him were dead, and he recalled seeing hundreds of women's shoes that must have been abandoned in the streets as they fled. When he finally got home that night, he cleaned up and went to Peter's house. “It was full of people crying and praying. Peter's wife was crying and asked me if Peter made it out. I lied and told her he was probably in a hospital, but after what I saw, I knew he was dead.” During the first imaginal exposure session Mr. J was intensely distressed (Subjective Units of Distress Score=100), but his distress decreased with each retelling. Between sessions he conscientiously completed his behavioral exposure exercises to confront his multiple avoidances. Afterwards, he explained, “I'm a straightforward sort of person and I liked this therapy because it was straightforward. You explained everything, and told me exactly what to do. I did it and I got a lot better.” Mr. J also took study medication daily (he had been randomized to active paroxetine), which he tolerated without significant side effects. After ten weeks of combined treatment Mr. J was significantly improved, with minimal anxiety or avoidance symptoms: “I'm back to my old self. I can connect to my kids, planes don't scare me anymore, and I was even able to visit the memorial stone for Peter for the first time. I'm feeling optimistic again, and I think I can work in Manhattan without freaking out.”
Supplementary Material
Acknowledgments
Dr. Schneier has received research funding from Forest Labs. Dr. Marshall is currently employed by Sunovion Pharmaceuticals.
This study was supported by National Institute of Mental Health Grant MH068173 to Drs. Marshall and Schneier. GlaxoSmithKline provided paroxetine and matching placebo used in this study.
ClinicalTrials.gov identifier number: NCT01130103
Footnotes
All other authors report no financial relationships with commercial interests.
Presented in part at the International Society for Traumatic Stress Studies 26th Annual Meeting, Montreal, Canada, November 4–6, 2010, and the Anxiety Disorders Association of America 31st Annual Conference, New Orleans, LA, March 24–27, 2011
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galea S, Vlahov D, Resnick H, Ahern J, Susser E, Gold J, Bucuvalas M, Kilpatrick D. Trends of probable post-traumatic stress disorder in New York City after the September 11 terrorist attacks. Am J Epidemiol. 2003;158:514–524. doi: 10.1093/aje/kwg187. [DOI] [PubMed] [Google Scholar]
- 4.Difede J, Malta LS, Best S, Henn-Haase C, Metzler T, Bryant R, Marmar A randomized controlled clinical treatment trial for World Trade Center attack-related PTSD in disaster workers. J Nerv Ment Dis. 2007;195:861–865. doi: 10.1097/NMD.0b013e3181568612. [DOI] [PubMed] [Google Scholar]
- 5.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–8. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 6.Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 7.Marshall RD, Lewis-Fernandez R, Blanco C, Simpson HB, Lin SH, Vermes D, Garcia W, Schneier F, Neria Y, Sanchez-Lacay A, Liebowitz MR. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety. 2007;24:77–84. doi: 10.1002/da.20176. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association Practice Guideline for the Treatment of Patients with ASD and PTSD American Psychiatric Association. 2004 http://www.psychiatryonline.com/pracGuide/pracGuideTopic_11.aspx.
- 9.National Institute of Clinical Excellence. Clinical guideline 26: Posttraumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. National Collaborating Centre for Mental Health; London, UK: 2005. http://guidance.nice.org/CG26. [Google Scholar]
- 10.Forbes D, Creamer M, Bisson JI, Cohen JA, Crow BE, Foa EB, Friedman MJ, Keane TM, Kudler HS, Ursano RJ. A guide to guidelines for the treatment of PTSD and related conditions. J Trauma Stress. 2010;23:537–552. doi: 10.1002/jts.20565. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine (IOM) Treatment of posttraumatic stress disorder: An assessment of the evidence. The National Academies Press; Washington, DC: 2008. [Google Scholar]
- 12.Ponniah K, Hollon SD. Empirically supported psychological treatments for adult acute stress disorder and posttraumatic stress disorder: a review. Depress Anxiety. 2009;26(12):1086–109. doi: 10.1002/da.20635. [DOI] [PubMed] [Google Scholar]
- 13.Marshall RD, Cloitre M. Maximizing treatment outcome in post-traumatic stress disorder by combining psychotherapy with pharmacotherapy. Curr Psychiatry Rep. 2000;2:335–340. doi: 10.1007/s11920-000-0078-3. [DOI] [PubMed] [Google Scholar]
- 14.Otto MW, Hinton D, Korbly NB, Chea A, Ba P, Gershuny BS, Pollack MH. Treatment of pharmacotherapy-refractory posttraumatic stress disorder among Cambodian refugees: a pilot study of combination treatment with cognitive-behavior therapy vs sertraline alone. Behav Res Ther. 2003;41:1271–1276. doi: 10.1016/s0005-7967(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 15.Rothbaum BO, Cahill SP, Foa EB, Davidson JR, Compton J, Connor KM, Astin MC, Hahn CG. Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. J Trauma Stress. 2006;19:625–638. doi: 10.1002/jts.20170. [DOI] [PubMed] [Google Scholar]
- 16.Simon NM, Connor KM, Lang AJ, Rauch S, Krulewicz S, LeBeau RT, Davidson JR, Stein MB, Otto MW, Foa EB, Pollack MH. Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. J Clin Psychiatry. 2008;69:400–405. doi: 10.4088/jcp.v69n0309. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-Analytic review. Int J Cogn Ther. 2009;2:160–175. doi: 10.1521/ijct.2009.2.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 19.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-IV—Patient Version (SCID-P, Version 2.0) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 21.First M, Spitzer R, Gibbon M, Williams J, Benjamin L. Structured Clinical Interview for DSM-IV, Axis II Disorders (SCID-II) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 22.Pae CU, Patkar AA. Paroxetine: current status in psychiatry. Expert Rev Neurother. 2007;7:107–20. doi: 10.1586/14737175.7.2.107. [DOI] [PubMed] [Google Scholar]
- 23.Foa EB, Rothbaum BO. Treating the trauma of rape: A Cognitive-Behavioral Therapy for PTSD. Guilford; New York: 1998. [Google Scholar]
- 24.Guy W. ECDEU Assessment Manual for Psychopharmacology— Revised (DHEW Publ No ADM 76-338) U.S. Department of Health, Education, and Welfare; Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; NIMH Psychopharmacology Research Branch; Division of Extramural Research Programs; Rockville, MD: 1976. pp. 218–222. [Google Scholar]
- 25.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 26.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 27.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Brown H, Prescott R. Applied Mixed Models in Medicine. Wiley; West Sussex, UK: 1999. [Google Scholar]
- 29.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd edition Oxford University Press; Oxford: 2002. [Google Scholar]
- 30.Hu MC, Pavlicova M, Nunes EV. Zero-inflated and hurdle models of count data with extra zeros: Example from an HIV Risk Reduction Intervention Trial. Am J Drug Alc Abuse. 2011 doi: 10.3109/00952990.2011.597280. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedeker D. Generalized linear mixed models. In: Everitt B, Howell D, editors. Encyclopedia of Statistics in Behavioral Science. Wiley; New York: 2005. [Google Scholar]
- 32.Selvin S. Statistical Analysis of Epidemiological Data. Oxford University Press; New York: 1996. pp. 213–214. [Google Scholar]
- 33.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 34.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–27. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 35.Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress. 2003;16:555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- 36.Lurie I, Levine SZ. Meta-analysis of dropout rates in SSRIs versus placebo in randomized clinical trials of PTSD. J Nerv Ment Dis. 2010;198:116–124. doi: 10.1097/NMD.0b013e3181cc41b6. [DOI] [PubMed] [Google Scholar]
- 37.Norris FH, Friedman MJ, Watson PJ. 60,000 disaster victims speak: Part II. Summary and implications of the disaster mental health research. Psychiatry. 2002;65:240–260. doi: 10.1521/psyc.65.3.240.20169. [DOI] [PubMed] [Google Scholar]
- 38.Neria Y, Nandi A, Galea S. Posttraumatic stress disorder following disasters: A systematic review. Psychol Medicine. 2008;38:467–480. doi: 10.1017/S0033291707001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall RD, Bryant R, Amsel L, Cook J, Suh EJ, Neria Y. The psychology of ongoing threat: Relative risk appraisal, the September 11, 2001 attacks, and terrorism-related fears. American Psychologist. 2007;62:304–16. doi: 10.1037/0003-066X.62.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feeny NC, Zoellner LA, Mavissakalian MR, Roy-Byrne PP. What would you choose? Sertraline or prolonged exposure in community and PTSD treatment seeking women. Depress Anxiety. 2009;26:724–731. doi: 10.1002/da.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson J, Pearlstein T, Londborg P, Brady KT, Rothbaum B, Bell J, Maddock R, Hegel MT, Farfel G. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1974–1981. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.