Abstract
Poor sleep is often independently associated with greater pain sensitivity and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (e.g., greater basal cortisol and exaggerated stress-induced cortisol reactivity). However, the interactions among sleep, pain, and the HPA axis have not been adequately evaluated. In this study, 40 healthy adults provided self-report regarding perceived sleep quality over the past month prior to completion of an acute noxious physical stressor (i.e., cold pressor task; CPT). Following the CPT, they reported on the severity of pain experienced. Salivary cortisol was sampled before, immediately following, and during recovery from CPT. Using bootstrapped confidence intervals with a bias correction, results showed that poor sleep quality was significantly associated with greater reports of CPT-induced pain severity and greater cortisol reactivity (i.e., increase from baseline). Furthermore, greater cortisol reactivity to the CPT was found to significantly mediate the relationship between poor sleep and pain severity.
Keywords: sleep quality, HPA axis, pain, cortisol, reactivity
INTRODUCTION
Over recent years it has become increasingly evident that sleep quality is highly predictive of pain experiences as demonstrated in laboratory and clinical settings (Smith and Haythornthwaite, 2004). Specifically, research examining clinical pain reports and the responses of individuals exposed to controlled laboratory stimuli has documented reliable relations between poor sleep quality and increased pain severity (Raymond et al., 2001; Mystakidou et al., 2007; Edwards et al., 2009). At present, the mechanisms by which poor sleep quality exerts its nocent effects on the experience of pain have not been fully characterized, although the hypothalamic-pituitary-adrenal (HPA) axis has been proposed as a potential mediator of this relationship (Canivet et al., 2008). The HPA axis and its constituent neurohormones, particularly cortisol, are commonly examined in studies as an index of neuroendocrine stress reactivity. Previous research testing whether sleep quality predicts cortisol responses to stress has predominantly involved psychosocial stressors such as public speaking or mental stress test (Wright et al., 2007; Raikkonen et al., 2010). Acute pain represents a noxious physical stressor that also has been shown to elicit significant cortisol responses (Goodin et al., 2012), yet no studies to date have addressed whether sleep quality predicts cortisol reactivity to a noxious stressor and the resultant pain response. A direct examination of whether cortisol reactivity to a noxious stressor mediates the relationship between sleep quality and reports of pain severity may help to elucidate the physiological mechanisms linking poor sleep with pain sensitivity and is warranted at this time.
It has been revealed that poor sleep is directly associated with increased basal activity of the HPA axis, and it has further been suggested that poor sleep may potentiate the reactivity of this system to threat and challenge (Vgontzas and Chrousos, 2002; Buckley and Schatzberg, 2005; Meerlo et al., 2008). Support for this suggestion was provided in a recent review that reported robust relationships between poor sleep quality and subsequent dysregulation of the cortisol response to various stressors (Balbo, Leproult, & Van Cauter, 2010). In particular, poor sleep quality has been shown to predict exaggerated cortisol responses to psychological stressors (Raikkonen et al., 2010) and physiological stressors (Hori et al., 2011); however, it remains to be determined whether poor sleep also predicts cortisol response to a noxious physical stressor.
That poor sleep seems to promote exaggerated cortisol responses to stress is particularly relevant here because the HPA axis and cortisol have previously been found to be implicated in pain perception. In laboratory-based studies of healthy adults, it has been demonstrated that exposure to a cold pressor task (CPT) resulted in a significant increase in salivary cortisol from baseline, and this increase was significantly related with greater reports of pain intensity and pain unpleasantness (Zimmer et al., 2003; Goodin et al., 2012). Further, HPA axis activation (e.g., increased cortisol) has been associated with elevations in patient-related pain severity in samples with chronic widespread pain (Neeck and Riedel, 1999; Neeck, 2000). However, it is noteworthy that some previous experimental and clinical studies found inverse relationships between cortisol and pain, such that greater basal cortisol was associated with less severe pain (al’Absi et al., 2002) and diminished cortisol reactivity was associated with greater pain perception (Geiss et al., 1997). Thus, it appears that additional research is needed to further elucidate that nature of the relationship between cortisol and pain.
On balance, there is preliminary and indirect support for the view that poor quality sleep, by acting on stress systems like the HPA axis, may sensitize individuals to the experience of pain. However, it appears that no previous studies have evaluated whether sleep quality predicts aberrant HPA axis-related responses (i.e., cortisol) to a noxious physical stressor, and, in turn, whether cortisol response is related to reports of pain. Using a cold pressor task (CPT) and questionnaires, we tested three hypotheses. First, poor sleep quality will be significantly related with greater reported pain following the CPT. Second, poor sleep quality will also be significantly related with an increased cortisol response to the CPT. Third, the relationship between poor sleep quality and greater reported pain will be significantly mediated by the increase of cortisol in response to the CPT. Figure 1 displays our putative study model.
Figure 1.
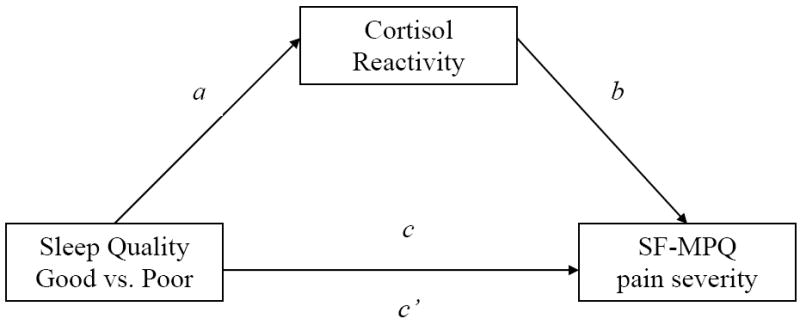
Putative study model
METHODS
Participants
Participants were 40 healthy adults, recruited from a college campus using posted advertisements, and individuals of both sexes were eligible for study enrollment. The sample was predominantly young adults (mean age = 20.2 ± 2.8 years old; range 18-24), with an equal number of men and women (50% women). Mean body mass index was 22.93 ± 3.28, which falls within the “ideal weight” range as determined by the National Institutes of Health (NIH, 1998). The majority indicated their race as either non-Hispanic white (35%) or Asian, Pacific Islander (35%), with the remainder being African American (25%) or of Hispanic decent (5%). Individuals were unable to participate if they met any of the following criteria: (a) age less than 18 or over 45 years; (b) ongoing chronic pain problems; (c) diagnosed sleep disorder or taking medication for sleep; (d) circulatory disorders; (e) history of cardiac events; (f) history of metabolic disease or neuropathy; (g) pregnant; (h) currently using prescription analgesics, tranquilizers, antidepressants, or other centrally acting agents; (i) use of nicotine, (j) use of prescription medication (e.g., corticosteriods, oral contraceptives), (k) psychiatric disorders (e.g., depression), or (l) chronic or acute health problems that affect the neuroendocrine or immune system. This study was carried out in accordance with the University’s appropriate guidelines for ethical conduct of research. Informed consent was obtained in accordance with approved protocol guidelines of an Institutional Review Board. All participants were compensated for their participation.
Procedures
Prior to the laboratory session, participants were asked to not use nonprescription medications or alcohol within 24 hours of their appointment. Participants were asked to refrain from exercise and consumption of caffeine for at least 2 hours prior to the testing session. To minimize potential error associated with the collection of oral fluid samples, participants were asked to not eat foods that may cause bleeding of the gums (e.g, potato chips) or brush their teeth for at least 2 hours prior to the testing session. This is because blood leakage from microinjuries of the oral mucosa may confound the measurement of salivary cortisol (Kivlighan et al., 2004). All study procedures were carried out between the hours of 4 P.M. and 7 P.M. to control for diurnal variations in neuroendocrine parameters and because afternoon sessions have been associated with greater cortisol responses (Dickerson and Kemeny, 2004).
Upon arrival to the study site, participants rested comfortably in a chair for 15 minutes to adapt to the experimental setting. During this time participants completed behavioral and psychological questionnaires that assessed perceived sleep quality and negative affect. Participants then provided a saliva sample for cortisol assessment (initial sample). The initial sample was intended to familiarize participants with the saliva collection procedures and is not included in data analysis. Participants then rested an additional 15 minutes and subsequently provided a second saliva sample (baseline sample) that was collected prior to the initiation of the CPT. Additional salivary cortisol samples were collected immediately following termination of the CPT and at various intervals during recovery (15, 20, 25, 30 and 40 minutes following initiation of the CPT). These sampling time-points were chosen based on a meta-analysis of prior research showing that peak changes in cortisol occur at approximately 30 minutes following stressor onset (Dickerson and Kemeny, 2004). Also following completion of the CPT and cortisol sampling, participants completed a short questionnaire describing their pain experiences.
Acute Pain Stressor
Cold Pressor Task
The CPT procedure is a psychophysiological pain test that involved a NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) filled with circulating cold water maintained at approximately 4°C (± 0.2°C). Participants were instructed to place their dominant hand into the cold water up to their wrist. In an effort to maximize participants’ exposure to the CPT and promote a corresponding cortisol response, standardized instructions asked participants “please try to keep your hand immersed in the water for at least two minutes or we may not be able to use your data”. However, participants were then immediately informed that they could remove their hand from the water at any time should it become intolerable. Unbeknownst to participants, the maximum allowable duration of the CPT was 300 seconds. While prior research has used different cutoff times, our 300 second cutoff is consistent with many previous studies (Walsh, Schoenfeld, Ramamurth, & Hoffman, 1989). Whether participants completed the entire CPT, or terminated the task prior to the allotted maximum time of exposure, the duration of exposure was recorded and classified as cold pressor pain tolerance (CPTo).
Questionnaires
Pittsburgh Sleep Quality Index (PSQI)
Sleep quality was measured before completion of the CPT using the PSQI. The PSQI is a self-rated questionnaire that retrospectively assesses sleep quality and disturbances over a one month time interval (Buysse et al., 1989). Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. Each of the seven component scores is weighted equally on a scale from 0 to 3, 0 indicating no difficulty and 3 indicating severe difficulty. The sum of scores for these seven component scores yields one global score, ranging from 0 to 21. Higher scores indicate worse sleep quality, and a global PSQI score > 5 is consistent with poor sleep quality. The seven component scores of the PSQI have previously been shown to possess good internal consistency (α = .83), and the overall global score has demonstrated good test-retest reliability (r = .87) (Buysse et al., 1989.) In the current study, internal consistency for the PSQI components was acceptable (α = .75)
Positive and Negative Affect Schedule (PANAS)
Negative affect was also measured prior to the CPT using the negative affect subscale of the Positive and Negative Affect Schedule (Watson et al., 1988). Given the positive relationship between negative affect and pain reports (Staud et al., 2006), this subscale was included to examine the influence of general negative affect on key study variables and determine the need for statistical control. The negative affect subscale (PANAS-neg) includes 10 negative affects (e.g., distressed, upset), and participants were asked to indicate on a five-point Likert scale (1 = not at all, 5 = very much so) the strength of the emotion for them. The total negative affect score for each participant was the sum of the 10 items, with a possible range of 10 to 50. The PANAS has good psychometric properties (Watson et al. 1988) and the internal consistency of the scale was adequate in the current study (α = 0.77).
Short Form-McGill Pain Questionnaire (SF-MPQ
The Short-Form McGill Pain Questionnaire (SF-MPQ) allows quantitative, multidimensional pain ratings to be obtained in a brief period of time (Melzack, 1987). In the current study, respondents rated 15 pain descriptors on a scale from 0 (none) to 3 (severe) following the CPT and cortisol sampling, and a sum of all rankings was used to compute a total pain rating score. The SF-MPQ is a reliable and valid instrument commonly used in clinical and research applications (Melzack, 1987). The instructions used in the current study asked participants about “the painful procedure you just experienced.” The internal consistency of the SF-MPQ in the current study was excellent (α = 0.93).
Cortisol Reactivity
Consistent with the procedures incorporated by Dickerson and colleagues (2004), the cortisol response in this study was obtained from oral fluids, which provide an established method for assessing cortisol (Kirschbaum and Hellhammer, 1994). Furthermore, oral fluid collection seems to be a less reactive and invasive means for reliably measuring adrenocortical activity relative to a needle stick with blood draws. Salivettes (Sarsted, Leicester, UK) were placed into the mouth, on top of the tongue for 2.5 minutes per sampling time-point, for salivary cortisol collection. Cortisol in saliva is in its unbound, biologically active form, and its concentration is independent of saliva flow rate. After obtaining the saliva samples, they were immediately refrigerated before being transferred and stored at -80°C until batch assayed. Cortisol was measured using high sensitivity salivary cortisol immunoassay kits (Salimetrics, State College, PA). Intra- and interassay variability was less than 8%.
Data Reduction and Analysis
For the purpose of sleep quality, the 40 study participants were classified as either good sleepers (PSQI global score </= 5) or poor sleepers (PSQI global score > 5) according to a clinically validated cut value previously shown to be effective for the detection of insomnia (Buysse et al., 2008). This resulted in 12 (30%) individuals being categorized as poor sleepers, while the remaining 28 (70%) individuals were categorized as good sleepers. A small portion of missing data existed for the salivary cortisol samples (< 5% of the total salivary cortisol samples across the sampling time range), which appeared to be missing at random. Rather than exclude the individuals, or their respective sampling time points, for which missing data existed from analysis, a simple data imputation method was completed using the macro for Hot Deck imputation (Myers, 2011). This data imputation method is well validated and accepted in the statistical community, and resulted in complete study data for each of the 40 study participants. Salivary cortisol data were then subjected to logarithmic transformation using a log10 function, which was effective for reducing skew according to Shapiro-Wilk’s tests (p’s > 0.05). A measure of area under the curve (AUCI) was calculated using the trapezoid formula to summarize cortisol reactivity to the CPT (Pruessner et al., 2003). AUCI is a parameter that emphasizes the changes of a physiological marker over time and is most related to the sensitivity of the system being studied, in this case the HPA axis. This study specifically focused on AUCI as a summary indicator of cortisol reactivity to the CPT. Bivariate relationships among all study variables were examined using Pearson correlations. Differences in sleep quality, pain severity, and the summary indicator AUCI of cortisol reactivity between good and poor sleepers were examined using analysis of covariance (ANCOVA). Finally, the bootstrapping technique and macro put forth by Preacher and Hayes (2008) for obtaining a 95% bias corrected (BC) confidence interval (CI) was utilized to test whether cortisol reactivity significantly mediated the association between sleep quality and reports of pain severity. Bootstrapping has been shown to be superior to other statistical techniques for testing the effects of the intervening mediator between the independent and dependent variables (Williams and MacKinnon, 2008). The probability value for acceptable Type I error was set at < .05. All analyses were two-tailed and completed using SPSS, version 19.
RESULTS
Zero-order correlations and test of covariates
Pearson correlations are presented in Table 1. Greater PANAS-neg (i.e., negative affect) was significantly correlated with more severe pain reports on the SF-MPQ (r = .49, p < .001). CPTo demonstrated a positive correlation with SF-MPQ that was non-significant but trended toward significance (r = .30, p = .06). Lastly, there was a large and positive correlation between cortisol reactivity AUCI and SF-MPQ (r = .58, p < .001), such that a greater increase in cortisol from baseline was associated with more severe pain. Participants’ sex was not significantly correlated with any of the primary study variables; however, several of these relationships were moderate in size. Given their moderate to large relationships with SF-MPQ and cortisol reactivity AUCI, we decided to statistically adjust for participants’ sex, PANAS-neg, CPTo, and baseline cortisol in all analyses where cortisol reactivity AUCI and SF-MPQ were included as dependent variables.
Table 1.
Zero-order correlations
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
|
|
|||||
| 1) Sex | — | ||||
| 2) PANAS-neg | -.12 | — | |||
| 3) CPTo | .18 | .14 | — | ||
| 4) Baseline Cortisol (μg/dL) | .20 | .05 | .31 | — | |
| 5) Cortisol Reactivity AUCI | -.09 | .18 | .22 | .27 | — |
| 6) SF-MPQ | -.06 | .49** | .30 | .26 | .58** |
Note: Sex is coded 0 = women, 1 = men; PANAS-neg = negative affect subscale from Positive and Negative Affect Scale; CPTo = cold pressor tolerance; μg/dL = micrograms per deciLiter; AUCI = area under the curve for cortisol reactivity; SF-MPQ = Short Form-McGill Pain Questionnaire.
p < .01
Sleep quality, cortisol reactivity AUCI, and pain severity
Differences between good and poor quality sleepers on key study variables can be seen in Table 2. The distribution of participants’ sex was not significantly different across good and poor quality sleepers (χ2 = .48, p = ns). Poor quality sleepers had significantly greater baseline cortisol than their good sleep quality counterparts (F1,38 = 4.53, p < .05). Compared to good quality sleepers, poor quality sleepers demonstrated significantly greater cortisol reactivity AUCI (F1,34 = 7.61, p < .01; Figure 2). More specifically, 11/12 (92%) poor quality sleepers responded to the CPT with an increase in cortisol from baseline, whereas 20/28 (71%) good quality sleepers responsed with an increase in cortisol from baseline. Those that did not respond either remained flat or decreased from baseline. This corresponds to a mean percent increase in cortisol from baseline to maximum of 63% for poor quality sleepers and 48% for good quality sleepers (Figure 3). Poor quality sleepers reported more severe pain on the SF-MPQ in response to the CPT than did good quality sleepers (F1,34 = 4.95, p < .05; Figure 4). There were no significant differences in negative affect or CPTo between good and poor quality sleepers (see Table 2). Although the sample size of good and poor quality sleepers was uneven, results do not appear to have been meaningfully influenced by heterogeneity of variance according to Levene’s tests (p’s > 0.05).
Table 2.
Overall means and mean differences among key study variables between good and poor quality sleepers
| Overall | Good Quality Sleepers PSQI </= 5 | Poor Quality Sleepers PSQI > 5 | Sig. | |
|---|---|---|---|---|
| PSQI global | 4.47 | 3.43 | 6.92 | <.001 |
| PANAS-neg | 18.03 | 17.96 | 18.17 | .93 |
| CPTo (seconds) | 232.68 | 211.39 | 282.33 | .06 |
| Baseline Cortisol (μg/dL) | 0.13 | 0.09 | 0.15 | .04 |
| Cortisol Reactivity AUCI | 4.30 | 1.30 | 11.30 | .009 |
| SF-MPQ | 12.75 | 11.23 | 16.17 | .03 |
Figure 2.
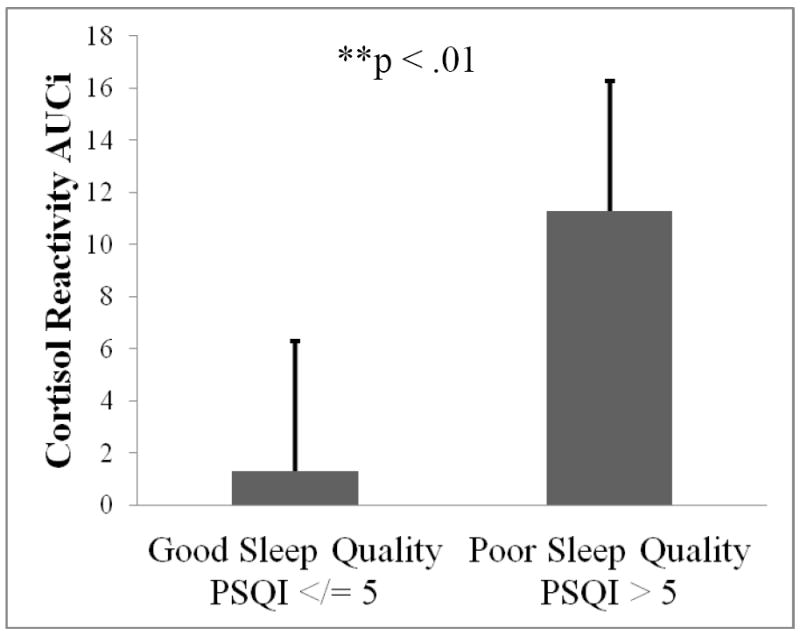
Difference in cortisol reactivity to the cold pressor between good and poor quality sleepers; higher scores represent greater cortisol increase from baseline.
Figure 3.
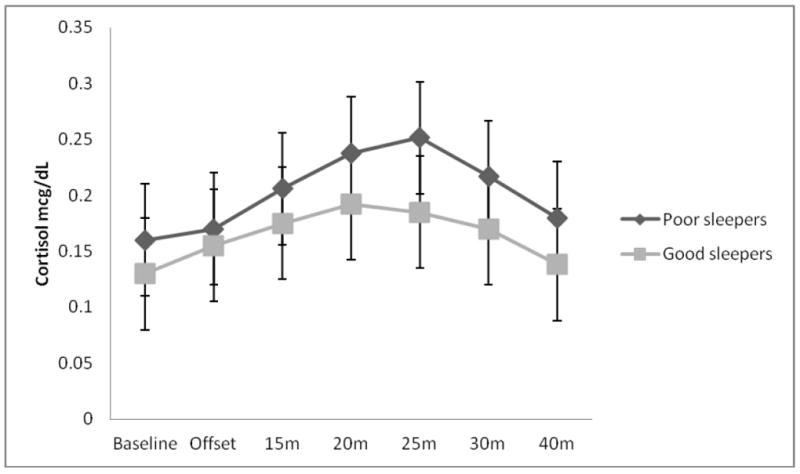
Difference in the cortisol profile over time between good and poor quality sleepers.
Figure 4.
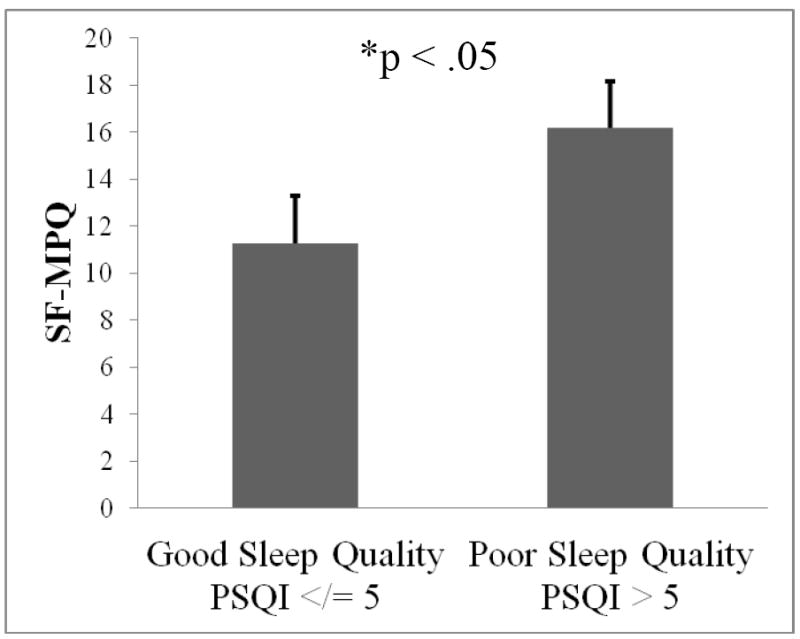
Difference in reported pain severity following the cold pressor between good and poor quality sleepers; higher scores represent greater pain severity.
Testing cortisol reactivity AUCI to CPT as a simple mediator
Examination of the path coefficients for Figure 1 (shown in Table 3) shows that the path representing differences between good and poor quality sleepers for cortisol reactivity AUCI (path a) is statistically significant (t = 2.53, p = 0.02). Additionally, the path representing the relationship between cortisol reactivity AUCI and pain severity is statitically significant and positive (t = 2.22, p = 0.03), suggesting that greater cortisol reactivity AUCI is related to greater pain severity. Overall, the study model accounted for 52% of the variance in SF-MPQ pain severity (R2 = .42, p < .01) and the statistical power for detecting effects was adequate (1-β >.80).
Table 3.
Simple mediation model of sleep quality on SF-MPQ pain severity through cortisol reactivity AUCI adjusted for covariates (Figure 1).
| Effect | Coefficient | SE | t | Sig. | 95% CI Bias Corrected |
|---|---|---|---|---|---|
| c | 7.05 | 2.53 | 2.79 | 0.01 | |
| a | 9.06 | 3.58 | 2.53 | 0.02 | |
| b | 0.25 | 0.11 | 2.22 | 0.03 | |
| c’ | 4.83 | 2.60 | 1.86 | 0.07 | |
| a × b | 2.17 | 1.32 | d | (LL = 0.41, UL = 6.16) |
Table shows unstandardized coefficients for the mediated effect of group differences in pain severity through group differences in sleep quality.
direct effect of IV to mediator;
direct effect of mediator on DV;
total effect of IV on DV;
direct effect of IV on DV;
indirect effect of IV on DV through proposed mediator; LL, lower limit; UL, upper limit.
A p-value for the indirect effect is not provided because such a p-value is contingent upon a normal distribution of the indirect effect. Given that the product of the a and b path coefficients is always positively skewed, interpretation of this p-value can be misleading.
A 95% BC and bootstrapped CI was carried out to test whether group differences (i.e., good versus poor quality sleepers) in cortisol reactivity AUCI significantly mediated group differences in pain severity after adjusting for covariates (Figure 1). The test of mediation was found to be significant (95% BC CI: 0.41 to 6.16 with 5000 resamples) in that the bootstrapped CI excluded zero (Table 3). This finding indicates that the indirect effect (i.e., mediation) represented by Figure 1 significantly differs from zero and, more specifically, that the greater pain severity reported by poor quality sleepers may be explained, at least in part, by their exaggerated cortisol response to the painful stressor (CPT).
DISCUSSION
Consistent with our hypothesis, individuals who reported having poor sleep quality over the past month also reported more severe pain in response to the CPT when compared to a group of individuals who reported good sleep quality. This finding is congruent with previous experimental studies that also demonstrated significant associations between poor sleep and greater sensitivity to evoked pain (Edwards et al., 2009; Smith et al., 2009). Also consistent with our hypothesis, the poor quality sleepers demonstrated greater mean basal cortisol and cortisol reactivity AUCI in response to the CPT than did the good quality sleepers. The finding that poor quality sleepers demonstrated greater baseline cortisol compared to good quality sleepers has been reported previously and is consistent with the literature (Vgontzas et al., 2001). Poor sleep quality has also previously been shown to predict exaggerated cortisol responses (i.e., increases) to psychological stress using the Trier Social Stress Test (Raikkonen et al., 2010) and to physiological stress using the combined dexamethasone/corticotrophin-releasing hormone test (Hori et al., 2011). Our findings suggest that poor sleep quality is also associated with a greater magnitude increase in cortisol following exposure to a noxious physical stressor. Of interest, the increase in cortisol was found to significantly and fully mediate the association between poor sleep and pain severity reports. The statistical mediation remained significant even after controlling for the confounding effects of basal cortisol, negative affect, participants’ sex, and the duration of exposure to the CPT (CPTo). To our knowledge, these are some of the first data to suggest that the cortisol response to a noxious physical stressor mediates the relationship between sleep quality and the severity of the pain produced by the noxious physical stressor.
In this sample of healthy adults without chronic pain, findings suggest that the more severe CPT-induced pain reported by individuals with poor sleep quality may be related to exaggerated activations of the HPA axis response to stress, particularly cortisol. Although the exact reasons for why cortisol activity might mediate the relationship between poor sleep and pain sensitivity remain speculative, several possibilities merit mentioning. If sleep is important for well-being and homeostasis, then it is plausible that poor sleep quality represents a stress for an organism and should be associated with activation of the stress system. Consistent with our study’s findings, previous research has demonstrated that poor quality and disturbed sleep is associated with greater basal cortisol levels in individuals’ with insomnia compared to matched controls without sleep disturbances (Vgontzas et al., 2001). The stress of poor sleep not only affects basal cortisol activity but, also, reactivity to new stressful stimuli. Poorly sleeping individuals exposed to stressful situations tend to respond with exaggerated cortisol reactivity (Raikkonen et al., 2010), a finding replicated by the current study. In turn, it has been suggested that cortisol may exert a hyperalgesic effect in healthy (Zimmer et al., 2003; Goodin et al., 2011) and clinical samples (McLean et al., 2005). Elevated cortisol levels have been associated with increased negative mood in at least one study examining the immediate effects of cortisol in healthy controls (Ellenbogen et al., 2002). Although the current study controlled for general negative affect, we did not account for momentary changes in mood related to being exposed to a noxious physical stressor. Thus, cortisol may influence pain via short-term alterations of cortically-mediated pain processing induced by mood. In addition to its effects on the central nervous system, cortisol may also influence pain processing via its actions in the periphery. Cortisol regularly interacts with inflammatory markers in response to stress and injury (Sorrells and Sapolsky, 2007). In the periphery, cortisol variation may influence the balance of peripheral pro- and anti-inflammatory cytokines, which might contribute to pain sensitivity via peripheral or central mechanisms (Watkins et al., 1995).
Although our data are consistent with prospective findings from clinical studies in which poorer sleep at baseline predicted later increase in cortisol (Vgontzas et al., 2001) and pain symptoms (Smith et al., 2008), the cross-sectional design of the current study precluded the possibility of testing whether the associations among poor sleep, cortisol reactivity to the CPT, and pain responsiveness may be bi-directional or co-occurring. Mounting evidence from longitudinal research in clinical samples, as well as experimental studies in healthy controls, suggests that the experiences of pain and sleep are bi-directionally connected (Smith and Haythornthwaite, 2004). For instance, it is all too common for a poor night’s sleep to be accompanied by an increase in pain severity on the subsequent day, and pain during the day to then be followed by another poor night’s sleep (Raymond et al., 2001). Further, the respective associations between pain and sleep with cortisol activity have also been shown to be bidirectional in nature. Chronically poor sleep stimulates greater cortisol secretion; conversely, greater amounts of circulating cortisol have been shown to predict arousal and wakefulness (Buckley & Schatzberg, 2005). Similarly, cortisol may exert a hyperalgesic influence that potentiates pain, but pain is itself a stressor that predicts activation of the HPA axis and cortisol (Dixon et al., 2004). On balance, results from the current study, along with evidence from past research, suggests that cortisol activity may constitute an important bi-directional mechanism by which sleep and pain interact; however, additional research into this matter is needed.
Some important limitations of this study will need to be addressed in future research. To begin, sleep quality was not objectively measured (e.g., via polysomnography, actigraphy) the night prior to individuals’ exposure to the CPT. Thus, the current study cannot adequately address the proximal influence of prior night’s sleep on next day’s pain. Although the PSQI is a well validated measure and is routinely used in studies to capture individuals’ subjective reports of their sleep quality, a multi-method approach to sleep assessment that also included objectively derived sleep parameters may have been beneficial. Accordingly, it will be important for future research attempting to replicate the current study’s findings to explore the relative contributions of subjective and objective sleep quality measurements to stress-induced cortisol reactivity and their respective relationships with pain. A multi-method approach to sleep quality assessment would allow for enhanced understanding of how specific domains of sleep (e.g., waking after sleep onset, latency, and sleep duration) might relate with the cortisol response to a noxious physical stressor. Another limitation is the use of a non-clinical sample of healthy college students. College students have previously been shown to regularly maintain atypical sleep habits that coincide with periods of intense studying and examination (Pilcher et al., 1997). As a consequence, the generalizability of these findings is not perfectly clear and the current results may or may not apply to individuals with chronic pain. However, given that previous clinical studies have shown that a poor night’s sleep is often accompanied by an increase in pain severity on the subsequent day (Smith & Haythornthwaite, 2004), and because both poor sleep and chronic pain represent stressors that consistently activate the HPA axis (Buckley & Schatzberg, 2005; McLean et al., 2005), it may be that the link between poor sleep and pain severity through exaggerated cortisol responses is even stronger in chronic pain populations. Replication of the current study’s findings in other non-clinical and clinical samples with chronic pain will be quite pertinent. Given that sleep deficits are typically greater and predictive of pain complaints in conditions such as fibromyalgia (Bigatti et al., 2008), the associations among sleep quality, pain, and stress-induced cortisol reactivity may well be stronger in clinical populations than was found in our non-clinical sample.
Despite these limitations, the results of this laboratory-based investigation raise the possibility that the relationship between poor sleep quality and greater reports of pain severity may be significantly mediated by cortisol reactivity to stress. Results of the present investigation represent a useful first step in demonstrating that poor sleep quality is significantly associated with noxious stressor-induced activation of the cortisol response, yet replication of our findings using a multi-method approach to subjective and objective sleep assessment will be important. Additionally, whether psychological treatments for pain that target sleep have effects through biological mechanisms such as HPA axis responses is not yet known, but may further support the primary role of cortisol in sleep quality and pain outcomes. In conclusion, we feel that additional research investigating the interface between sleep and pain with basal cortisol activity and stress reactivity is warranted.
Acknowledgments
Funding and support for this study and manuscript preparation was provided by NIH/NCCAM Grant R21AT003250-01A1 (L. M.), by an NIH Training Grant T32NS045551-06 provided to the University of Florida (B. R. G.), and by NIH/NIAMS Grant R01 AR05487 (M. T. S).
Footnotes
All authors declare that they do not have any conflicts of interest or financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. International Journal of Endocrinology. 2010:16. doi: 10.1155/2010/759234. Article ID 759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep distrubances in fibromyalgia syndrome: Relationships to pain and depression. Arthritis Care and Research. 2008;59:961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. REVIEW: On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of Clinical Endocrinology and Metabolism. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- Canivet C, Ostergren P-O, Choi B, Nilsson P, af Sillen U, Moghadassi M, et al. Sleeping problems as a risk factor for subsequent musculoskeletal pain and the role of job strain: Results from a one-year follow-up of the Malmo shoulder neck study cohort. International Journal of Behavioral Medicine. 2008;15:254–262. doi: 10.1080/10705500802365466. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. European Jouranal of Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Stress and selective attention: The interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neuroscience Letters. 1997;237:65–68. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- Goodin BR, Quinn NB, King CD, Page GG, Haythornthwaite JA, Edwards RR, et al. Salivary cortisol and soluble tumor necrosis factor-α receptor II responses to multiple experimental modalities of acute pain. Psychophysiology. 2012;49:118–127. doi: 10.1111/j.1469-8986.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, Teraishi T, Sasayama D, Ozeki Y, Matsuo J, Kawamoto Y, et al. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. Journal of Psychiatric Research. 2011;45:1257–1263. doi: 10.1016/j.jpsychires.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DC. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Hormones and Behavior. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis & Rheumatism. 2005;52:3660–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1987;1:227–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Myers TA. Goodbye listwise deletion: Presenting hotdeck imputation as an easy and effective tool for handling missing data. Communication Methods and Measures. 2011;5:297–310. [Google Scholar]
- Mystakidou K, Parpa E, Tsilika E, Pathiaki M, Gennatas K, Smyrniotis V, et al. The relationship of subjective sleep quality, pain, and quality of life in advanced cancer patients. Sleep. 2009;30:737–742. doi: 10.1093/sleep/30.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obesity Research. 1998;6:S51–S210. [PubMed] [Google Scholar]
- Neeck G. Neuroendocrine and hormonal perturbations and relations to the serotonergic system in fibromyalgia. Scandanavian Journal of Rheumatology. 2000;113:8–12. [PubMed] [Google Scholar]
- Neeck G, Riedel W. Hormonal perturbations in fibromyalgia syndrome. Annals of the New York Academy of Science. 1999;876:325–339. doi: 10.1111/j.1749-6632.1999.tb07657.x. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being, and sleepiness in college students. Journal of Psychosomatic Research. 1997;42:583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Pesonen A-K, Pyhala R, Paavonen EJ, Feldt K, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. Journal of Clinical Endocrinology and Metabolism. 2010;95:2254–2261. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized burn patients. Pain. 2001;92:381–388. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Klick B, Kozachik S, Edwards RR, Holavanahalli R, Wiechman S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138:497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, Behavior, & Immunity. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, Price DD. Overall fibromyalgia pain is predicted by ratings of local pain and pain-related negative affect – possible role of peripheral tissues. Rheumatology. 2006;45:1409–1415. doi: 10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin H-M, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nytochemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. The Journal of Clinical Endocrinology & Metabolism. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: Multiple interactions and disturbances in sleep disorders. Endocrinology and Metabolism Clinics of North America. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- Walsh N, Schoenfeld L, Ramamurth S, Hoffman J. Normative model for cold pressor test. American Journal of Physical Medicine and Rehabilitation. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams J, MacKinnon DP. Resampling and distribution of the product methods for testing indirect effects in complex models. Structural Equation Modeling. 2008;15:23–51. doi: 10.1080/10705510701758166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Valdimarsdottir HB, Erblich J, Bovbjerg DH. Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biological Psychology. 2007;74:319–327. doi: 10.1016/j.biopsycho.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Basler H-D, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. Clinical Journal of Pain. 2003;19:233–239. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]


