Abstract
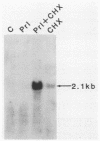
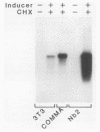
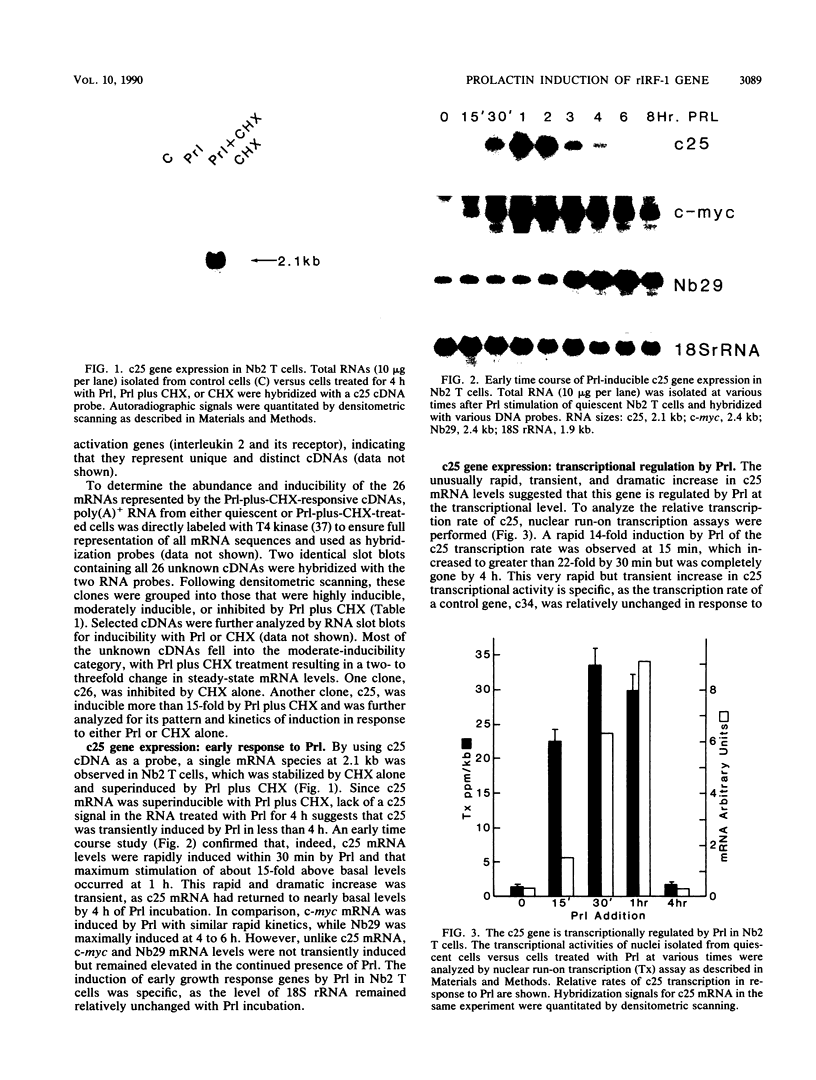
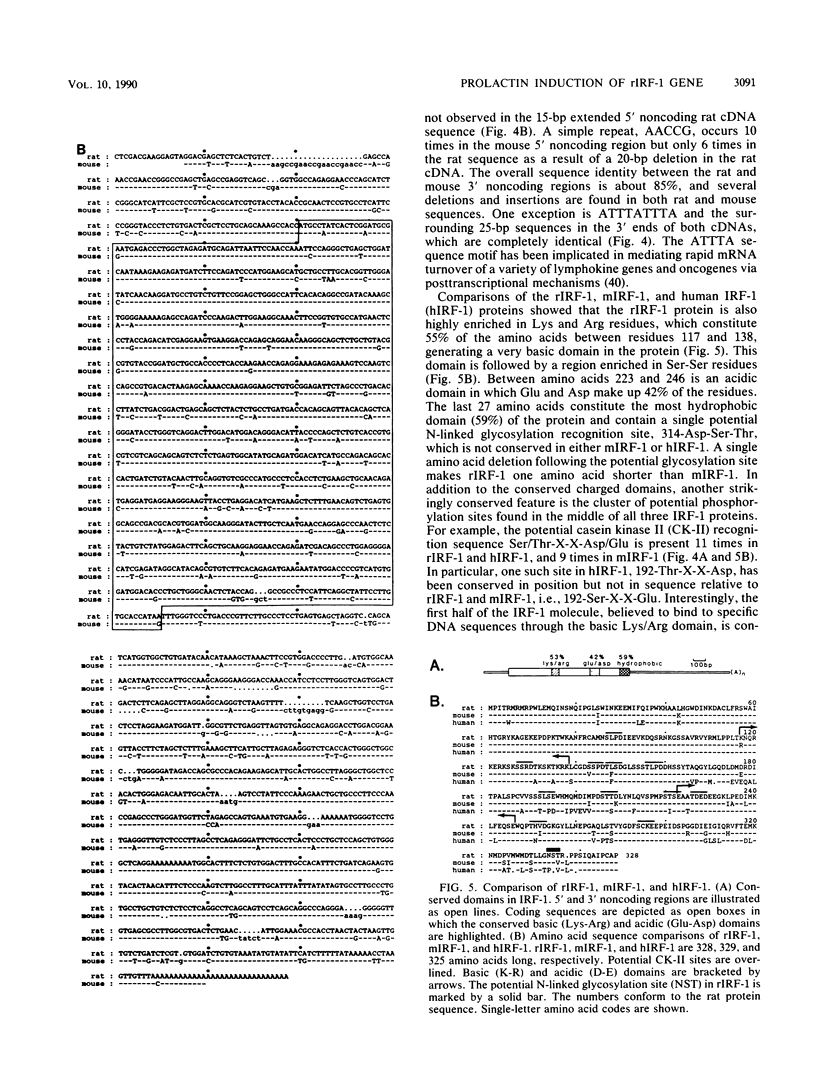
The pituitary peptide hormone prolactin (Prl) is a potent inducer of Nb2 T lymphoma cell proliferation. To analyze the early genetic response to the mitogenic signals of Prl, a cDNA library was constructed from Nb2 T cells stimulated for 4 h with Prl and the protein synthesis inhibitor cycloheximide. Of 26 distinct clones isolated by differential screening, one clone, designated c25, exhibited extremely rapid but transient kinetics of induction by Prl and superinduction by Prl plus cycloheximide. Run-on transcription analysis indicated that c25 gene transcription was induced greater than 20-fold within 30 to 60 min of Prl stimulation. Surprisingly, DNA sequence analysis of c25 cDNA revealed that this Prl-inducible early-response gene is the rat homolog of the mouse transcription factor interferon-regulatory factor 1 (IRF-1), sharing 91% coding sequence similarity with mouse IRF-1. At the protein level, rat IRF-1 shares 97% and 92% homology with mouse IRF-1 and human IRF-1, respectively, suggesting that this molecule has been functionally conserved throughout evolution. Our studies show that the gene for IRF-1 is an immediate-early gene in Prl-stimulated T cells, which suggests that IRF-1 is a multifunctional molecule. In addition to its role in regulating growth-inhibitory interferon genes, IRF-1 may, therefore, also play a stimulatory role in cell proliferation. The gene for IRF-1 is one of the earliest genes known to be transcriptionally regulated by Prl.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Rosen J. M. Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol Cell Biol. 1988 Aug;8(8):3183–3190. doi: 10.1128/mcb.8.8.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Two different virus-inducible elements are required for human beta-interferon gene regulation. EMBO J. 1989 Jan;8(1):101–110. doi: 10.1002/j.1460-2075.1989.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. H., Murphy P. R., Murphy L. J., Hatton T. W., Matusik R. J., Friesen H. G. Human growth hormone induces and maintains c-myc gene expression in Nb2 lymphoma cells. Endocrinology. 1985 Dec;117(6):2547–2549. doi: 10.1210/endo-117-6-2547. [DOI] [PubMed] [Google Scholar]
- Gaddy-Kurten D., Hickey G. J., Fey G. H., Gauldie J., Richards J. S. Hormonal regulation and tissue-specific localization of alpha 2-macroglobulin in rat ovarian follicles and corpora lutea. Endocrinology. 1989 Dec;125(6):2985–2995. doi: 10.1210/endo-125-6-2985. [DOI] [PubMed] [Google Scholar]
- Gout P. W., Beer C. T., Noble R. L. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980 Jul;40(7):2433–2436. [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Hartzell S., Ryder K., Lanahan A., Lau L. F., Nathan D. A growth factor-responsive gene of murine BALB/c 3T3 cells encodes a protein homologous to human tissue factor. Mol Cell Biol. 1989 Jun;9(6):2567–2573. doi: 10.1128/mcb.9.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman N. D. A prolactin-inducible gene product which is a member of the calpactin/lipocortin family. Mol Endocrinol. 1989 May;3(5):773–779. doi: 10.1210/mend-3-5-773. [DOI] [PubMed] [Google Scholar]
- Irving S. G., June C. H., Zipfel P. F., Siebenlist U., Kelly K. Mitogen-induced genes are subject to multiple pathways of regulation in the initial stages of T-cell activation. Mol Cell Biol. 1989 Mar;9(3):1034–1040. doi: 10.1128/mcb.9.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph L. J., Le Beau M. M., Jamieson G. A., Jr, Acharya S., Shows T. B., Rowley J. D., Sukhatme V. P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification of an inducible factor that binds to a positive regulatory element of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 May;85(10):3309–3313. doi: 10.1073/pnas.85.10.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Kim G. S., Prystowsky M. B., Lancki D. W., Sabath D. E., Pan J. L., Weissman S. M. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci U S A. 1987 May;84(9):2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipes M. A., Napolitano M., Jeang K. T., Chang N. T., Leonard W. J. Identification, cloning, and characterization of an immune activation gene. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9704–9708. doi: 10.1073/pnas.85.24.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretz C. A., Bern H. A. Prolactin and osmoregulation in vertebrates. An update. Neuroendocrinology. 1982 Oct;35(4):292–304. doi: 10.1159/000123397. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Kuenzel E. A., Krebs E. G., Eisenman R. N. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 1989 Apr;8(4):1111–1119. doi: 10.1002/j.1460-2075.1989.tb03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Tsuyuki D., Myal Y., Shiu R. P. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J Biol Chem. 1987 Nov 5;262(31):15236–15241. [PubMed] [Google Scholar]
- Rodgers J. R., Johnson M. L., Rosen J. M. Measurement of mRNA concentration and mRNA half-life as a function of hormonal treatment. Methods Enzymol. 1985;109:572–592. doi: 10.1016/0076-6879(85)09116-9. [DOI] [PubMed] [Google Scholar]
- Russell D. H. New aspects of prolactin and immunity: a lymphocyte-derived prolactin-like product and nuclear protein kinase C activation. Trends Pharmacol Sci. 1989 Jan;10(1):40–44. doi: 10.1016/0165-6147(89)90106-5. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Jongstra J., Dyer B. J., Jorgensen J., Clayberger C., Davis M. M., Krensky A. M. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988 Aug 1;141(3):1018–1025. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirone F., Shooter E. M. Early gene regulation by nerve growth factor in PC12 cells: induction of an interferon-related gene. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2088–2092. doi: 10.1073/pnas.86.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y. Prolactin stimulates transcription of growth-related genes in Nb2 T lymphoma cells. Mol Cell Endocrinol. 1990 Jan 2;68(1):21–28. doi: 10.1016/0303-7207(90)90165-5. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Rosen J. M. A transfected alpha-casein minigene bypasses posttranscriptional control by hormones, but retains cell-substratum regulation in mammary epithelial cells. Mol Endocrinol. 1988 May;2(5):431–443. doi: 10.1210/mend-2-5-431. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deToledo S. M., Murphy L. J., Hatton T. H., Friesen H. G. Regulation of 70-kilodalton heat-shock-like messenger ribonucleic acid in vitro and in vivo by prolactin. Mol Endocrinol. 1987 Jun;1(6):430–434. doi: 10.1210/mend-1-6-430. [DOI] [PubMed] [Google Scholar]