Abstract
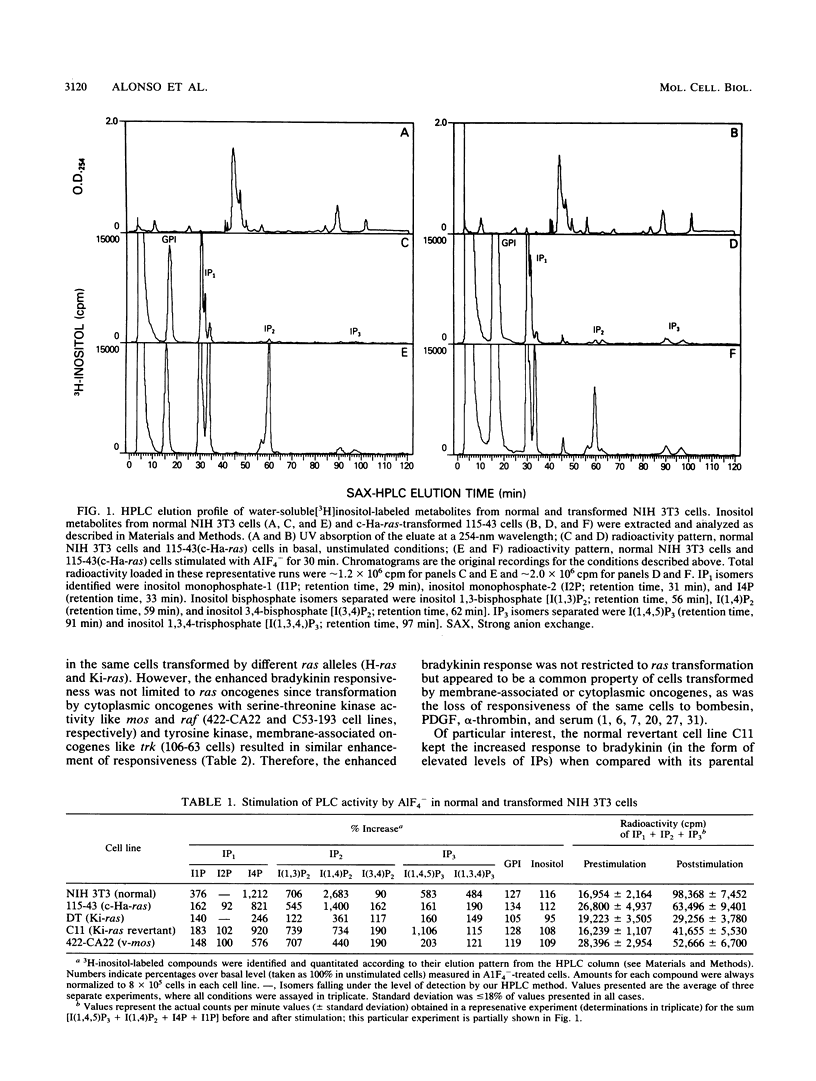
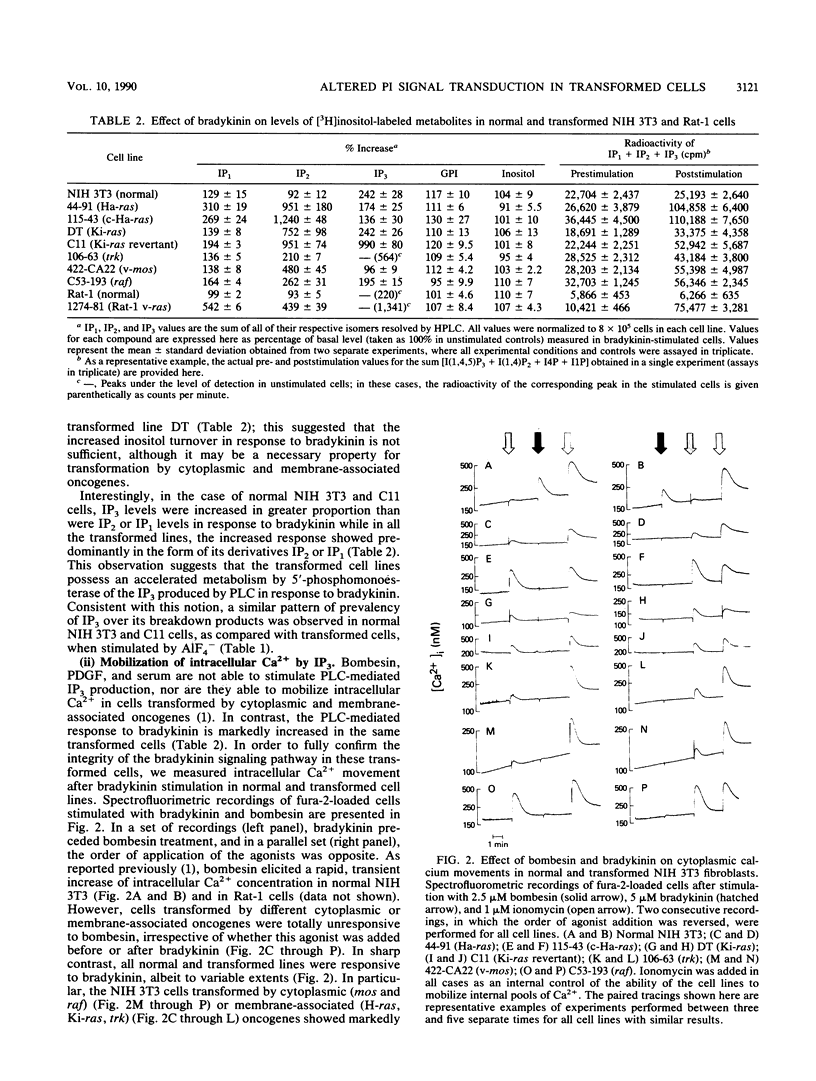
We showed previously that transformation by cytoplasmic and membrane-associated oncogenes including ras results in uncoupling between surface stimulation by platelet-derived growth factor, bombesin, and serum and activation of intracellular phospholipase C (PLC); this uncoupling does not involve alterations at the receptor or effector enzyme levels (T. Alonso, R. O. Morgan, J. C. Marvizon, H. Zarbl, and E. Santos, Proc. Natl. Acad. Sci. USA 85:4271-4275, 1988). In this study, we stimulated normal and oncogene-transformed NIH 3T3 cells with fluoroaluminate (AIF4-), thus directly activating PLC-associated G protein(s) and bypassing the receptor step. A1F4(-)-elicited PLC responses were significantly impaired in transformed cells when compared with those in their normal counterparts, suggesting that the uncoupling of PLC is the result, at least in part, of functional impairment at the G-protein level. Transformation by ras oncogenes has also been reported to result in enhanced PLC response to bradykinin resulting from increased receptor numbers (G. Parries, R. Hoebel, and E. Racker, Proc. Natl. Acad. Sci. USA 84:2648-2652, 1987; J. Downward, J. de Gunzburg, R. Riehl, and R. Weinberg, Proc. Natl. Acad. Sci. USA 85:5774-5778, 1988). We demonstrate here that transformation by other membrane-associated and cytoplasmic oncogenes also results in increased responsiveness to bradykinin ("supercoupling") and enhanced receptor numbers. However, there is no direct correlation between the number of receptors and the enhancement in responsiveness, suggesting that other factors besides receptor number are also involved in the enhanced responses. We propose that a common effect of transformation by cytoplasmic and membrane-associated oncogenes is functional alteration of coupling G proteins and that a similar modification of different kinds of G proteins may account for the pleiotropic alterations of signal transduction (uncoupling and supercoupling) observed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso T., Morgan R. O., Marvizon J. C., Zarbl H., Santos E. Malignant transformation by ras and other oncogenes produces common alterations in inositol phospholipid signaling pathways. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4271–4275. doi: 10.1073/pnas.85.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Benjamin C. W., Connor J. A., Tarpley W. G., Gorman R. R. NIH-3T3 cells transformed by the EJ-ras oncogene exhibit reduced platelet-derived growth factor-mediated Ca2+ mobilization. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4345–4349. doi: 10.1073/pnas.85.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. Loss of platelet-derived growth factor-stimulated phospholipase activity in NIH-3T3 cells expressing the EJ-ras oncogene. Proc Natl Acad Sci U S A. 1987 Jan;84(2):546–550. doi: 10.1073/pnas.84.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., de Gunzburg J., Riehl R., Weinberg R. A. p21ras-induced responsiveness of phosphatidylinositol turnover to bradykinin is a receptor number effect. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5774–5778. doi: 10.1073/pnas.85.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. B., Schonbrunn A. The bombesin receptor is coupled to a guanine nucleotide-binding protein which is insensitive to pertussis and cholera toxins. J Biol Chem. 1988 Feb 25;263(6):2808–2816. [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Kamata T., Kung H. F. Effects of ras-encoded proteins and platelet-derived growth factor on inositol phospholipid turnover in NRK cells. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5799–5803. doi: 10.1073/pnas.85.16.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R., Santone K., Melder D., Oakes S. G., Abraham R., Powis G. An increase in intracellular free Ca2+ associated with serum-free growth stimulation of Swiss 3T3 fibroblasts by epidermal growth factor in the presence of bradykinin. J Biol Chem. 1988 Dec 5;263(34):18030–18035. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero M., Srivastava S. K., Fleming T. P., Ron D., Eva A. NIH3T3 fibroblasts transformed by the dbl oncogene show altered expression of bradykinin receptors: effect on inositol lipid turnover. Oncogene. 1989 Jun;4(6):767–771. [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Lagarde A., Pouysségur J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 1988 Jan;7(1):161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Serum, bradykinin and vasopressin stimulate release of inositol phosphates from human fibroblasts. Biochem Biophys Res Commun. 1984 Sep 17;123(2):663–670. doi: 10.1016/0006-291x(84)90280-8. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Woolkalis M. J., Nakada M. T., Manning D. R. Alterations in components of adenylate cyclase associated with transformation of chicken embryo fibroblasts by Rous sarcoma virus. J Biol Chem. 1986 Mar 5;261(7):3408–3413. [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]



