Abstract
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved protein kinase that exquisitely regulates protein metabolism in skeletal muscle. mTOR integrates input from amino acids, growth factors and intracellular cues to make or break muscle protein. mTOR accomplishes this task by stimulating the phosphorylation of substrates that control protein translation while simultaneously inhibiting proteasomal and autophagic protein degradation. In a metabolic twist of fate, sepsis induces muscle atrophy in part by the aberrant regulation of mTOR. In this review we track the steps of normal mTOR signaling in muscle and examine where they go astray in sepsis and inflammation.
Introduction: mTOR and its binding partners
The mammalian target of rapamycin (mTOR) is a serine (S)/threonine (T) kinase lying at the intersection of nutrient, growth factor and stress signaling, and regulates diverse cellular functions. The majority of our knowledge related to the regulation of this protein has been derived from in vitro studies using sophisticated cellular and molecular approaches and a number of excellent reviews have summarized these reports (20, 43, 118). However, until recently, most of these studies have used model systems (e.g., Drosophila, C. elegans, transformed cell lines) which, although easily controlled and manipulated, may not fully recapitulate in vivo mammalian physiology. Furthermore, few reviews have focused on mTOR regulation in skeletal muscle per se. Therefore, the primary emphasis of the current review is to summarize recent studies on the central role of this kinase in regulating both basal and stress-related changes in skeletal muscle protein balance.
mTOR exists in two functionally distinct complexes referred to as mTOR complex 1 (mTORC1) and mTORC2, which were originally defined as being rapamycin-sensitive and – insensitive, respectively (Figure 1) (110). Although both complexes contain mTOR, mTORC1 primarily mediates the cellular growth response to anabolic stimuli. Rapamycin and the FK506 binding protein FKBP12 allosterically inhibit mTOR by binding to one surface of an mTOR dimer (135). Rapamycin initially weakens the interaction of mTORC1 with its binding partners which is eventually followed by disassociation of the complex. The regulatory associated protein of mTOR (raptor) is a core component of mTORC1 mediating the initial sensitivity of the complex to rapamycin. In contrast, the presence of the rapamycin insensitive companion of mTOR (rictor) differentiates mTORC2 from mTORC1 and in part explains their different physiological functions in vivo. Novel roles for additional mTORC1 and -2 components have emerged from studies using genetic screens and mass spectrometry to identify binding partners for mTOR (79, 124, 125). Moreover, the essential role of mTORC1 and -2 in growth control and development is underscored by the embryonic lethality resulting from the deletion of mTORC-related proteins in mice (Table 1). As will be described in more detail later in the review, mTORC1 is also a predominant loci for the inhibitory effects of sepsis and inflammation on protein synthesis in skeletal muscle.
Figure 1.
Protein components of the mTOR complexes. mTORC1 is comprised of the core proteins mTOR, raptor, and mLST8 (aka GβL). In addition, mTORC1 binds a number of non-conserved or associated proteins in a species- and condition-specific manner, such as PRAS40, DEPTOR, the Rag proteins A–D, and Rheb-GTP. TSC1/2-Rheb is the major upstream regulator of this rapamycin-sensitive protein complex and integrates input from cellular energy levels, growth factors, and nutrients to regulate protein translation by phosphorylating S6K1 and 4E-BP1. In contrast, mTORC2 includes core proteins mTOR, rictor, and, mLST8, as well as various associated proteins such has mSin1, DEPTOR, and PROTOR/PPR5. The mTORC2 controls cell structure and survival by regulating SGK and Akt.
Table 1.
Effect of genetic modification of mTOR-related proteins on muscle phenotype
| Protein | Modification | Muscle Response | Citation |
|---|---|---|---|
| mTOR | −/− (whole-body) | Embryonic lethal | (34, 83) |
| mTOR | −/+ (whole-body) | Decreased LBM, GC weight & protein synthesis | (62) |
| mTOR | −/− (muscle-specific) | Decreased mass of TA, GC, and PLA; decreased CSA; decreased S6 phosphorylation | (101) |
| Raptor | −/− (whole-body) | Embryonic lethal | (37) |
| Raptor | −/− (muscle-specific) | Decreased mass of TA, EDL, soleus; decreased fiber size; decreased S6 & 4E-BP1 phosphorylation | (6) |
| Rictor | −/− (whole-body) | Embryonic lethal | (37) |
| Rictor | −/− (muscle-specific) | No change in fiber size | (6) |
| Sin1 | −/− (whole-body) | Embryonic lethal | (101) |
| mLST8 | −/− (whole-body) | Embryonic lethal | (37) |
| S6K1 | −/− (whole-body) | Decreased CSA | (91) |
| S6K2 | −/− (whole-body) | No change in CSA | (91) |
| S6K1/S6K2 | −/− (whole-body DK) | Decreased mass of TA & GC, decreased CSA; decrease muscle wt gain to refeeding | (91) |
| 4E-BP1 | −/− (whole-body) | No reported muscle phenotype | (9) |
| 4E-BP1/2 | −/− (whole-body DK) | No reported muscle phenotype | (67) |
| TSC1 or 2 | −/− (whole-body) | Embryonic lethal | (55, 92) |
| TSC1 | Overexpression (muscle-specific) | Decreased weight and CSA in TA & EDL, decreased muscle wt gain to refeeding | (127) |
| Rheb | Overexpression (muscle-specific) | Increased CSA, S6K1 & S6 phosphorylation in TA | (35) |
| Akt1/2 | −/− (whole-body DK) | Decreased CSA, decreased S6K1 & 4E-BP1 phosphorylation | (94) |
| Akt | caAkt (muscle-specific) | Increased CSA | (10) |
| AMPKα1 | −/− (whole-body) | Increased CSA and enhanced hypertrophic response | (82) |
| AMPKα1/α2 | −/− (muscle-specific) | Increased CSA, S6K1 phosphorylation | (66) |
Observations made in theabove table summarize representative data specifically pertaining to skeletal muscle. A more in depth analysis may be found inthe cited references. Abbreviations: TA, tibialis anterior; GC, gastrocenmius; PLA, plantaris;EDL, extensor digitorum longus;CSA, cross-sectional area; DK, double knockout; ca, constitutively active; LBM, lean body mass
mTOR activity is positively regulated by growth factors (e.g., insulin) and amino acids (e.g., leucine) and both signals are thought to converge just upstream of mTOR on Rheb (Ras homolog enriched in brain). Rheb is active when bound to GTP and when colocalized with mTOR stimulates mTORC1 by enhancing the recruitment of its substrates (111). Thus, Rheb does not alter the intrinsic kinase activity of the enzyme but facilitates the binding of substrates that contain a TOR signaling (TOS) motif. Rheb activated substrate mobilization requires raptor and therefore explains why this Ras homolog activates only mTORC1 while simultaneously inhibiting mTORC2 (69, 111). In addition, only mTORC1 phosphorylates the ribosomal protein S6 kinase (S6K)-1 on T389 and only mTORC2 phosphorylates Akt on S473 in in vitro kinase assays (Figure 1) (134). Moreover, the specificity of Rheb towards the phosphorylation of mTORC1 substrates is replicated in skeletal muscle where over expression of Rheb is sufficient to stimulate mTORC1 activity, but not mTORC2, and produce hypertrophy (35).
mTOR: A highly regulated regulator
Rheb, like all small G-proteins, is a GTP sensitive switch. The switch is “on” and mTOR activated when Rheb contains bound GTP. Conversely, the switch is in the “off” position in the presence of GDP which prevents phosphorylation of mTOR substrates. The in vitro activation of mTORC1 by Rheb demonstrates a direct interaction of these two proteins. The nucleotide binding state and the interaction of Rheb with mTOR is coordinately controlled by the tuberous sclerosis proteins TSC1 (aka hamartin) and TSC2 (aka tuberin). TSC2 functions as a GTPase activating protein (GAP) that turns Rheb off by accelerating the GTPase activity of Rheb thereby converting Rheb to a GDP bound state and inhibiting mTOR activity (Figure 2) (78). Although both TSC1 and -2 have GAP domains, it appears that only the GAP activity of TSC2 is functional. Yet, the presence of TSC1 is necessary for optimal TSC2 activity because formation of a TSC1/TSC2 heterodimeric complex stabilizes TSC2. TSC1 physically prevents the ubiquitin-mediated proteolytic degradation TSC2 and likely explains the greater Rheb GAP activity of the TSC1/TSC2 complex when TSC1 is over expressed (7). The inhibitory effect of TSC1 is underscored by the severe muscle atrophy that occurs when TSC1 is over expressed in skeletal muscle (Table 1) (127).
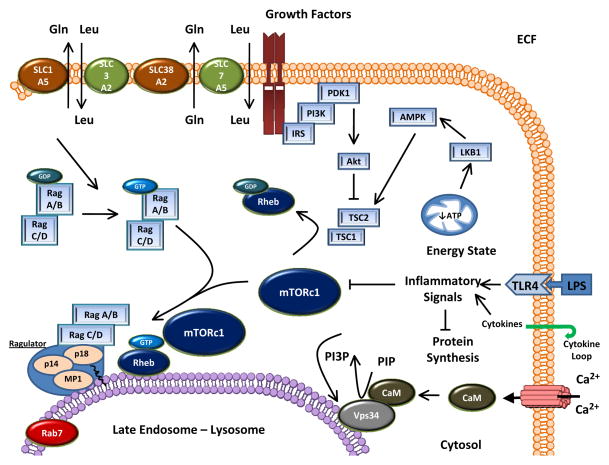
Figure 2.
Activation of mTORC1 by nutrients (e.g., leucine) and growth factors (e.g., insulin and IGF-I). mTORC1 functions as the central regulatory hub of these anabolic inputs, but is also the site where various catabolic insults (e.g., inflammatory cytokines, excess glucocorticoids, hypoxia, and cellular energy stress) negatively impact mTOR kinase activity and protein synthesis in mammalian skeletal muscle. A key component of the activation of mTOR occurs when leucine is taken up by amino acid transporters (SLC3A2 and SLC7A5) in a heteroexchange for glutamine (SLC1A5 and SLC38A2)(5, 86). An increase in intracellular leucine activates Rag heterodimers to translocate mTOR to the lysosomal membrane where mTOR can be activated by Rheb in a “Ragulator” dependent process (19, 28, 107, 108). Amino acids may also signal to mTOR via the Ca+2/CaM dependent activation of the lysosomal membrane protein Vps34 (38). Negative regulation of mTOR is imposed by LPS and inflammatory cytokines that may impinge on upstream amino acid signaling and mTOR itself to prevent the phosphorylation of mTOR substrates and translation initiation as summarized in Table 2 and a recent review (63). Abbreviations used include ECF; extracellular fluid, LPS; lipopolysaccharide, TLR; Toll-like receptor, AMPK; AMP-dependent kinase, SLC; solute carrier proteins (amino acid transporters), Ragulator; complex of p14, p18, and MP1, Rheb; Ras homolog enriched in brain, PIP; phosphatidylinositol phosphate, PI3P; phosphatidylinositol 3-phosphate, Vps34; vacuolar protein sorting 34, CaM, calmodulin. Other abbreviations used in the figure are defined in the text.
Although less well studied, Rheb is theoretically reactivated by guanine nucleotide exchange factors (GEFs) that specifically exchange GDP for GTP on Rheb. To date, two putative GEFs have been identified for Rheb. The first GEF, referred to as the translationally controlled tumor protein (TCTP), binds more avidly to nucleotide-free Rheb and stimulates the exchange of GDP for GTP in vitro. RNA interference of TCTP expression in Drosophila reduces both cell and organ size similar to that seen in Rheb and mTOR deletion mutants (42, 137). While these characteristics suggest TCTP is a candidate GEF for Rheb, the validity of TCTP in this role has been challenged (19, 129).
Given the above, it appears that the TSC proteins integrate an array of cellular signals that impinge on Rheb and therefore mTOR. Not surprisingly, investigators have found that TSC2 phosphorylation acts as a major gate keeper of Rheb and mTOR activity downstream of growth factor and stress signals (43). In this regard, Akt activation by insulin-like growth factor (IGF)-I leads to an Akt-dependent phosphorylation of TSC2 on multiple serine residues including S939 and S981 (Figure 2). Although phosphorylation of these sites does not alter the intrinsic GAP activity of TSC2 toward Rheb, it does facilitate the binding of TSC2 to 14-3-3 proteins and the partitioning of TSC2 away from a membrane fraction and therefore away from Rheb (12). Hence, the Akt-stimulated spatial disassociation of TSC2 from Rheb drives the membrane bound Rheb towards a GTP bound state which activates mTOR consistent with Akt’s role in muscle hypertrophy (10). Similar findings have been made in IGF-I treated C2C12 myotubes where a single mutation of S939 to alanine prevents the IGF-I-induced release of TSC2 from membrane bound Rheb (81). Therefore, a dynamic balance exists between these two proteins (12, 127). Over expression of Rheb is sufficient to induce muscle hypertrophy independent of Akt suggesting that an excess of “free” Rheb in skeletal muscle facilitates the activation of mTOR (36).
As mTOR sets in motion a metabolic gene network that up regulates energy consuming processes, such as protein biosynthesis, it is not unreasonable to assume that organisms have evolved mechanisms to limit mTOR activity during stress (23, 118). Once again, TSC2 appears to be a major gateway by which stress signals are relayed to mTOR. For example, upon energy stress induced by 2-deoxyglucose, the phosphorylation of multiple mTOR substrates is reduced as is cell size (46). The decreased mTOR activity is TSC2-dependent since TSC2 null cells maintain mTOR substrate phosphorylation (47). The phosphorylation of TSC2 by AMP-dependent kinase (AMPK) occupies a critical step in transducing energy stress to mTOR, as evidenced by the lack of a stress-induced inhibition of mTOR when the three AMPK-dependent phosphorylation sites on TSC2 are mutated (S1337, S1331, and S1345) to alanine (Figure 1). Limiting mTOR activity in the presence of energy stress is a TSC2-dependent survival mechanism as successful down regulation of mTOR enhances cell survival whereas cells that lack TSC2 and maintain mTOR activity are more prone to die an apoptotic cell death (47). In this regard, AMPK activation and inhibition of mTOR substrate phosphorylation in myocytes and skeletal muscle by the AMPK kinase activator aminoimidazole carboxamide ribonucleotide (AICAR) may also be a protective mechanism that limits anabolic processes and nutrient signaling in exchange for cell survival (87, 97, 131).
mTOR regulation by amino acids: Too much, too little, too late
Most signals impinging on mTOR are transduced through the TSC complex to regulate Rheb. Yet, amino acids may have found a signaling back door to the G-protein. Cellular amino acids levels are regulated by their plasma concentration, their ability to enter the cell through amino acid transporters, and their appearance from the breakdown of cellular proteins (8, 86, 96, 99). The circulating concentrations of all amino acids rise after a meal, but the essential amino acids (EAA) in general and the branched-chain amino acids (BCAAs) in particular hold a unique place in the stimulation of protein synthesis because they serve as both substrates and intracellular signaling molecules. Oral administration of a single BCAA, leucine, can replicate activation of mTOR in skeletal muscle seen after a meal (53). Likewise, addition of leucine to amino acid deprived myotubes stimulates mTOR activity to a greater extent than any other single amino acid (3). Therefore, this amino acid has been used to assess whether specific catabolic conditions display leucine resistance as a potential mechanism underlying diminished mTOR signaling and muscle protein synthesis (53, 58–61, 65).
Whole body inhibition of BCAA catabolism by deletion of the mitochondrial form of branched-chain amino acid transferase (BCATm) chronically raises plasma BCAAs 5- to 10-fold and enhances muscle protein synthesis compared to wild-type (WT) mice (64). BCATm null mice also exhibit enhanced phosphorylation of the mTOR substrate 4E binding protein (4E-BP1). Yet, BCATm null mice compensate for the increase in mTOR activity and muscle protein synthesis because lean body mass and individual muscle weight is not altered compared to WT mice. These data suggest that BCATm null mice balance their increased rate of muscle protein synthesis with enhanced proteasomal or autophagic degradation of muscle protein. This creates a futile cycle between protein synthesis and degradation as evidenced by the elevated metabolic rate in BCATm null mice (113). Finally, collectively, these data favor a model of mTORC1 regulation by leucine which does not invoke leucine catabolism.
The transport of leucine into cells and its stimulation of mTOR requires sufficient intracellular glutamine levels which facilitates the bidirectional transport of the two amino acids (Figure 2) (86). Inhibition of either glutamine transport with L-γ-glutamyl-p-nitro-analide (GPNA) or EAA transport with D-phenylalanine blocks mTOR activation. siRNA-mediated knockdown of solute carriers SLC7A5/SLC3A2 (the EAA transporter) or SLC1A5 (the glutamine transporter) ablates glutamine and EAA co-transport and mTOR signaling to S6K1 and 4E-BP1 (86). Baird et al have also provided evidence for functional coupling between the leucine transporter (SLC7A5 or LAT1) and the glutamine transporter (SLC38A2 or SNAT2) (5). Acidosis, a common manifestation of catabolic inflammatory conditions, also decreases glutamine transport and mTOR substrate phosphorylation via inhibition of the pH sensitive sodium-dependent neutral amino acid transporter (SNAT)-2 in myocytes (25). Therefore, down regulation of glutamine or leucine transport, which essentially mimics an amino acid deprived state, represents a potential mechanism by which mTOR activity might be inhibited in various catabolic conditions. Work by Drummond et al suggest that EAA transiently increase the expression of SLC7A5 and SNAT2 mRNA in human skeletal muscle (22). Teleologically, this suggests that EAA may influence the future transport of these amino acids but the increase in SLC7A5 protein compared to SLC7A5 mRNA was modest (22). Moreover, in addition to their transport functions, amino acid transporters may also function as sensors for both intra- and extra-cellular amino acid availability (44). However, the exact mechanism of action and the cellular location for the elusive leucine sensor remains to be definitively established (24).
Because EAA and glutamine continue to stimulate the phosphorylation of mTOR substrates in cells treated with TSC2 siRNA, the activation of mTOR by amino acids appears to proceed via a TSC2-independent mechanism (Figure 2) (86). Likewise, mTOR substrate phosphorylation continues to be suppressed upon amino acid withdrawal in TSC2 null cells (115). When small GTPases mediating the amino acid signal are over expressed in their GTP bound state they also activate Rheb and mTOR in a parallel pathway that is TSC2-independent (52, 108). Additionally, incubation of the extensor digitorum longus (EDL) with leucine inhibits AMPK activity and stimulates protein synthesis in the absence of changes in the phosphorylation of TSC2 or raptor (105).
To understand the mechanism by which amino acids stimulate mTOR, the importance of its intracellular localization must be appreciated. mTOR has been reported to localize to the cytoplasm (4, 103), nucleus (70, 71), mitochondria (98), and a late endosomal/lysosomal compartment (39, 108). As might be expected, mTORC1 and mTORC2 also have different subcellular locations (21). All of these sites may be critical for specific mTOR functions. For example, perturbing the conversion of early endosomes to late endosomes, but not other steps in endocytic trafficking (by expression of constitutively active Rab5a), antagonized amino acid-dependent activation of mTORC1 (28). Interestingly, the defect in mTOR activation could be circumvented by over expression of Rheb but not by relieving the natural inhibition of Rheb by TSC2. Such data suggest that inhibition of the conversion of early endosomes to late endosomes abrogates the ability of Rheb, Rheb activators, and mTOR to co-localize. In contrast, over expression of Rheb artificially forces its interaction with mTOR by mass action thereby stimulating the phosphorylation of mTOR substrates (28).
It now appears that amino acids trigger the movement of mTOR to the surface of lysosomes where amino acid sensitive GTPases (Rag proteins A through D) and Rheb are colocalized (Figure 2) (52, 107, 108). Amino acids increase the GTP loading state of Rag heterodimers allowing the Rags to interact with mTOR (52, 108). A complex of 3 proteins — p14, p21, and MP1— known collectively as the “Ragulator” helps tether the complex to the lysosome. The localization of mTOR to the lysosome is sufficient to activate mTOR independent of the Rag and Ragulator proteins, but not Rheb (107).
The vacuolar sorting protein (Vps34) is a class III phosphatidyl-inositide-3OH-kinase (PI3K) that specifically phosphorylates the D3 position in PtdIns to produce PI(3)P. In addition to its role in endosomal trafficking and autophagy, Vps34 has been posited to mediate mTOR activation by amino acids and to reside in endosomes/lysosomes. Over expression of Vps34 specifically stimulates mTOR to phosphorylate S6K1 in the presence but not the absence of amino acids, and siRNA-mediated knockdown of Vps34 reduces amino acid stimulation of S6K1 (38, 88). Although an amino acid-induced increase in intracellular calcium (Ca+2) can facilitate the binding of Ca+2/calmodulin to Vps34 resulting in elevated PI(3)P and enhanced mTORC1 signaling (38), such a Ca+2 – dependence has not been reported by others (133). Thus, comparable to the Rag proteins, Vps34 is perfectly positioned to mediate the activation of mTOR by amino acids. Vps34 activity is elevated after high resistance muscle contraction during the same time frame in which mTOR substrates are phosphorylated (74). These authors have also shown leucine to activate Vps34 in C2C12 myotubes. Yet in Drosophila, Vps34 does not mediate the effects of nutrients on mTOR signaling and Vps34 abundance is unchanged in skeletal muscle in response to insulin and amino acids (49, 120). Given the above, determining the relative importance of Vps34 and the Rag/Ragulator complex in amino acid-induced mTOR signaling in mammalian skeletal muscle will be an area of active investigation.
With an appreciation of how mTOR integrates diverse signals from growth factor receptors, upstream kinases, amino acid transporters, GAPs, GEFs and Rheb we next examine where this system may become dysfunctional in a pathological state. We will focus on the skeletal muscle loss occurring during sepsis because this disease exhibits a clear inhibition of basal mTOR activity and resistance to the potentially beneficial effects of amino acids. In addition, the observed decrease in mTOR activity and muscle mass is consistent with the loss of muscle mass and function that occurs in mice in which the mTORC1 signaling pathway has been genetically altered (Table 1).
mTOR signaling in sepsis: Where does it all go wrong?
Gram-negative infection and the resulting inflammatory sequelae yield a striking loss of lean body mass characterized by a pronounced deficit in skeletal muscle protein and mechanical function (80, 116). Although inflammation also enhances protein degradation, a significant portion of the sepsis-induced muscle atrophy is due to a failure of muscle to maintain protein synthesis (1, 13, 63, 73). Given the importance of maintaining skeletal muscle mass in disease prevention, survival, and recovery, elucidating how and why the inflammatory response inhibits protein synthesis in this tissue is of paramount importance (26, 106).
Early work by Vary and Kimball demonstrated that sepsis preferentially targets protein synthesis in fast-twitch but not slow-twitch muscles (126). Sepsis did not alter ribosomal number, but instead increased the number of free ribosomal subunits, implicating a defect in translation initiation and efficiency. Because translation initiation is largely controlled by the loading of the 43S ribosomal subunit onto RNA and the ability of the eukaryotic initiation factor (eIF)-4F to facilitate RNA binding, there are numerous reports on the sepsis-induced decrease in eIF-4F activity. In brief, eIF-4F contains a critical subunit, eIF-4E, that binds to the 7-methyl guanosine cap structure on RNA (100). The binding eIF-4E is facilitated by another initiation factor, eIF-4G, but is also competitively inhibited by a family of eIF-4E binding proteins (4E-BPs) that sequester eIF-4E away from eIF-4G and the 48S initiation complex. Growth factors, hormones and amino acids stimulate the phosphorylation of 4E-BP1, trigger the release eIF-4E, facilitate eIF-4E binding to eIF-4G, and ultimately the formation of a functional eIF-4F complex (100). In contrast, sepsis and the related inflammatory insult produced by endotoxin (LPS) restrain the above cascade and curtail muscle protein synthesis by inhibiting the kinase that phosphorylates 4E-BP1, eIF-4G, and other factors involved in cap-dependent translation, namely mTOR (reviewed in (63), Table 2). Although not specifically covered here, the inhibition of mTOR kinase activity is in part due to the over production of proinflammatory cytokines as evidenced by the ability of specific inhibitors of these cytokines to prevent the sepsis-induced decrease in 4E-BP1 and S6K1 phosphorylation and restore muscle protein synthesis (30, 31, 63) (Figure 2, Table 2).
Table 2.
Effect ofSepsis/LPS on signaling to and from mTOR substrates and regulatory proteins
| Protein | Modification | Direction/Magnitude | Citation |
|---|---|---|---|
| Rat pAkt | T308, S473 | Decreased 50% | (14, 32, 50) |
| Mouse pAkt | S473 | No Change | (62) |
| pPRAS40 | T246 | Decreased 50% | (32, 50) |
| pmTOR | S2448/S2481 | Decreased 20–50% | (50, 54, 59) |
| pTSC2 | S939, T1462 | No Change | (32, 50) |
| TSC1/2 | Association | No Change | (32, 50) |
| pRaptor | pS792 | Increased 80% | (32, 50) |
| pAMPKα | T172 | Increased 50% | (50) |
| Raptor/mTOR | Association | No Change | (50) |
| Raptor/eIF3b | Association | Decreased 50% | (50, 64) |
| pS6K1 | T389 | Decreased Basal 70% | (32, 54) |
| pS6K1 | T389 | Decreased Leu stimulation 70% | (59, 60) |
| Raptor/S6K1 | Association | Increased 60% | (50) |
| pS6 | S240/S244 S235/S236 |
Decreased basal, Leu, and IGF-I stimulation 75% | (32, 60) |
| p4E-BP1 | T37/T46 | Decreased basal and Leu stimulation 80% | (33, 54, 59, 60) |
| 4E-BP1/4E | Association | Increased 50% | (54, 59, 60) |
| 4E-BP1/4E | Leu induced Disassociation | Blocked Completely | (59, 60) |
| Mouse 4E-BP1/Raptor | Association | Decreased 60% | (64) |
| Rat 4E-BP1/Raptor | Association | Increased 50% | (50) |
| Deptor | Total Protein/Mouse C2C12 | Decreased 30% | (33) |
| Deptor | Total Protein/Rat Muscle | Increased 60% | (50) |
Observations made in the above table summarize representative data specifically pertaining to skeletal muscleand in some cases C2C12 myotubes. A more in depth analysis may be found in the cited references.
Given the importance of Akt activity in growth factor and insulin-induced muscle hypertrophy it is quite reasonable to assume that sepsis might inhibit Akt signaling which would then have a secondary effect on mTOR (10). Indeed numerous investigators have demonstrated that Akt phosphorylation is decreased by a 4 or 24 h treatment with LPS as well as after cecal ligation and puncture in rats (14, 15, 50, 60) (Table 2). Likewise, LPS/INFγ treated C2C12 myotubes exhibit a decrease in the phosphorylation of Akt on the S473 site and a decrease in the phosphorylation of multiple Akt substrates including the proline rich Akt substrate of 40 kDa (PRAS40) (29, 32). In myotubes, the changes in Akt and Akt substrate phosphorylation, as well as the phosphorylation of mTOR substrates and protein synthesis are all prevented by nitric oxide synthase (NOS) inhibitors suggesting a role of NO in regulating the Akt-mTOR signaling cassette (32). We speculate that nutrients, such as leucine, may not be able to overcome sepsis-induced inhibition of Akt-mTOR signaling but that strong activators of the pathway, such as IGF-I, may provide a robust enough force to restore muscle protein synthesis (33, 59, 60)
mTOR substrates in sepsis: Friend or foe?
The phosphorylation of multiple mTOR substrates including 4E-BP1 and S6K1are inhibited in the gastrocnemius of septic rats or animals treated with LPS. These changes are temporally associated with a marked reduction in muscle protein synthesis, whereas the total content of multiple mTOR substrates are unchanged from controls. In addition, the phosphorylation of potential downstream substrates including ribosomal protein S6, eIF-4G, and eIF-4B are also reduced (59, 60). Likewise, the phosphorylation of mTOR itself by S6K1 (S2448) is diminished (41). Although mTORC1 and -2 activity per se have not been evaluated in muscle, the reduction in mTOR autophosphorylation on S2481 (a marker of kinase activity) and the diminished phosphorylation of both mTORC1 and-2 substrates suggests the kinase activity of both complexes is impaired (50) (Table 2). A similar decrease in protein synthesis has been reported in cultured myotubes treated with a combination of LPS and interferon gamma (IFNγ) to replicate the septic condition (32, 54).
Currently it is not possible to identify specifically whether the decreased phosphorylation of 4E-BP1 or S6K1 is principally responsible for the sepsis-induced reduction in muscle protein synthesis, but it seems likely that both changes are causally related. Such speculation is supported by data from S6K1 null mice which are smaller than their WT littermates and exhibit disproportionate muscle atrophy (91). However, as S6K1 phosphorylates at least 10 substrates in addition to S6, the relatively importance of these individual proteins is yet to be assessed (40). Finally, while a muscle phenotype has not been yet reported in the 4E-BP1/BP-2 double knockout mouse (possibly because of a compensatory hyper-stimulation of S6K1), data from Drosophila clearly demonstrate the importance of 4E-BP1 in regulating cap-dependent translation during stress (123). Hence, the ability of sepsis to coordinately down regulate both S6K1 and 4E-BP1 appears causally related to the observed inhibition of mRNA translation and protein synthesis in skeletal muscle.
Recent studies have identified multiple potential mTOR inhibitors. One of these proteins, the proline-rich Akt substrate of 40 kDa (PRAS40) contains a TOS motif and interacts with raptor as a competitive inhibitor of other mTOR substrates (109, 125). As an Akt substrate, PRAS40 becomes phosphorylated on T246 which facilitates its interaction with 14-3-3 proteins. As a result, the inhibitory effect of PRAS40 on mTOR is relieved and permits other mTOR substrates (such as 4E-BP1 and S6K1) to bind and be phosphorylated (111). mTORC1 may also assist this process by phosphorylating PRAS40 on S221 which reduces PRAS40 binding to raptor and relieves PRAS40-mediated substrate competition (128). PRAS40 phosphorylation on T246 is dramatically deceased in septic muscle and in myotubes treated with LPS/IFNγ suggesting that it should strongly inhibit mTOR activity (Table 2). However, such a mechanism does not appear operational as the amount of PRAS40 and other substrates bound to raptor was not altered by the decrease in PRAS40 phosphorylation (32, 50). In addition, an 80% knockdown of PRAS40 in C2C12 myotubes had no affect on mTOR substrate phosphorylation or global protein synthesis suggesting that overall levels of PRAS40 and its phosphorylation status (T246) may have little influence on mTOR activity in fully differentiated muscle (51).
The sepsis-related decrease in mTOR activity is also associated with an increased expression of a potential inhibitor of mTOR referred to as DEPTOR (the disheveled, egl-10, and pleckstrin homology domain containing regulator of mTOR) (Table 2) (50). DEPTOR is unique among the mTOR binding proteins in that it associates with both the mTORC1 and -2, and unlike PRAS40 interacts directly with the mTOR protein. siRNA-mediated knockdown of DEPTOR increases the phosphorylation of mTORC1 and -2 substrates, whereas its over expression selectively inhibits mTORC1 while activating mTORC2 (95). The activation of mTORC2 by DEPTOR may be a consequence of the relief of mTORC1-mediated inhibition of mTORC2 normally imposed by the phosphorylation of rictor on T1135 by S6K1 (18). We speculate that the observed increase in DEPTOR protein in muscle from septic rats may be due to increased DEPTOR mRNA expression or alternatively a stabilization of existing DEPTOR protein which occurs under nutrient and growth factor deprived conditions (50, 95).
eIF3 is the largest of the mammalian eukaryotic initiation factors and functions as a docking site for multiple proteins, including S6K1(40). Over expression of eIF3f in mouse myoblasts or skeletal muscle induces hypertrophy (56). Conversely, siRNA-mediated knockdown of eIF3f induces atrophy in myotubes (56). During muscle atrophy, the ubiquitin ligase MAFbx ubiquitinates eIF3f leading to its proteasomal degradation (16). The protein level of this initiation factor is also reduced in myocytes during starvation and oxidative stress (56). However, although sepsis does not decrease the total eIF3f protein in muscle, the amount of eIF3f bound to mTORC1 (e.g., raptor) was reduced (50). Such data are consistent with the observed sepsis-induced decrease in S6K1 phosphorylation.
Why can’t BCAA do that?
Sepsis and LPS not only inhibit basal mTOR activity but also lead to the development of muscle leucine resistance, in the context of both mTORC1 and -2 (59, 60). Whereas an oral gavage of leucine, which raised plasma leucine 5- to 7-fold, increased mTOR activity and muscle protein synthesis in control rats, this anabolic response to leucine is virtually absent in septic animals. One possibility is that although control and septic rats exhibit comparable plasma leucine levels, that sepsis impairs either the transport of leucine into muscle or the ability of muscle to “sense” leucine. A similar defect in essential amino acid (EAA) signaling occurs with aging. Cuthbertson demonstrated that the elderly exhibit decreased activation of muscle protein synthesis and mTOR signaling in response to EAA suggesting that a similar mechanism may underlie amino acid resistance in a variety of muscle wasting states (17). Because leucine transport is highly dependent upon the prevailing intracellular concentration of glutamine and the two amino acids are bi-directionally co-transported across cell membranes, a deficit in glutamine could impair leucine transport or sensing in skeletal muscle (86). Glutamine is released from skeletal muscle during sepsis and the intramuscular levels of this amino acid are rapidly depleted (48, 104, 126).
Secreted sphingomyelinase activity is elevated in serum after acute inflammation and may act in an endocrine fashion on various tissues, including cardiac and skeletal muscle, where it converts sphingomyelin in muscle membranes to ceramide (27, 132). In addition, pro-inflammatory cytokines increase the generation of ceramide in skeletal myocytes and inhibit protein synthesis (119). Recent work suggests that ceramide decreases the expression of the SNAT2 amino acid transporter in myotubes and may be responsible for the decreased intracellular glutamine levels and mTOR activity in these cells (45). Sepsis-induced hyper-lacticacidemia may also have a similar effect on SNAT2 expression and explain how metabolic acidosis inhibits glutamine transport and mTOR activity in L6 myoblasts (25). Given the importance of glutamine for co-transport of leucine, future work will need to focus on whether inhibition of muscle sphingomyelinase in sepsis can replenish intracellular pools of muscle glutamine and therefore restore leucine-stimulated mTOR activity.
Another alternative is that sepsis and inflammatory mediators prevent leucine from being detected by a yet to be defined leucine receptor/sensor. As noted above, leucine stimulates the GTP loading of Rag GTPases and facilitates the coalescing of Rheb and mTOR on the surface of lysosomes. It is possible that sepsis and LPS disturb one or more of the Rag or Ragulator proteins leading to the formation of a non-functional complex. For example, a sepsis-induced loss of the p18 protein or a decrease in its lipidation would be expected to inhibit the tethering of the Rag/Ragulator complex to the lysosome making mTOR less sensitive to leucine (84). In some cell types, LPS can disrupt and/or colocalize with lysosomes after trafficking from the cell surface with Toll-like receptor (TLR)-4 (72, 93, 122). The small G-protein Rab5a, which perturbs the conversion of early to late endosomes and ablates amino acid- and insulin-stimulated mTOR activity, can also be activated by infection and IFNγ (2, 75). Thus, there may be one or more Rag, Ragulator, and/or endosomal mechanisms by which sepsis disturbs the interaction of mTOR and Rheb culminating in leucine resistance.
Although sepsis and LPS produce leucine resistance in muscle at the level of mTOR activity, the stimulation of mTOR by IGF-I remains largely intact. Indeed, increasing the local bioavailability of IGF-I in either the general circulation or specifically in skeletal muscle over a sustained period of time also prevents sepsis-induce atrophy (89, 121). Yet, if sepsis-induced mTOR inhibition was strictly through defective Rag GTPases, one would predict that IGF-I could not rescue this phenotype as amino acids are necessary for insulin-stimulated phosphorylation of S6K1. For example, Rag proteins that mimic the GDP bound state inhibit both the amino acid and insulin-stimulated phosphorylation of mTOR substrates. Likewise, siRNA knockdown of the Rag proteins also antagonizes insulin-stimulated mTOR activity (108). These results suggest that sepsis may only partially inhibit Rag/Ragulator function or that IGF-I can restore intracellular amino acids more efficiently than achieved by leucine supplementation alone.
Sepsis strongly inhibits both basal and leucine-stimulated muscle protein synthesis in vivo. In contrast to this model where rodents are challenged with a bolus of leucine, little is known about whether a sustained elevation of BCAA is beneficial in sepsis and whether it could alleviate the negative effects of LPS on mTOR activity. As noted earlier, mice deficient in the key BCAA metabolizing enzyme BCATm exhibit a persistent elevation of plasma BCAA levels (64, 113). The elevation of BCAA concentrations appear to protect BCATm null mice from some of the negative effects of LPS because they maintain muscle protein synthesis rates similar to control mice when challenged with the inflammatory stimuli (64). BCAA, when elevated in this sustained fashion, also protect BCATm null mice by inhibiting an overzealous inflammatory response, as evidenced by a reduced expression of inflammatory markers and increased survival (64). It would be of interest to determine whether exogenous administration of leucine or BCAAs would also inhibit the inflammatory response and maintain muscle protein synthesis to distinguish the effect of elevated plasma amino acids from other metabolic changes that occur in BCATm null mice.
Finally, although the exact molecular mechanism for the sepsis-induced muscle leucine resistance remains to be defined, it appears to differ at least partially from that which impairs muscle protein synthesis and mTOR activity under basal post-absorptive conditions. For example, the sepsis-induced decrease in basal mTOR activity in muscle can be completely ameliorated by pretreatment of rats with a neutralizing TNF binding protein (TNFBP). However, in contrast, TNFBP alone failed to prevent the sepsis-induced leucine resistance, and it was necessary that rats be administered both TNFBP and RU486 (a glucocorticoid receptor antagonist) in order to prevent the development of leucine resistance (61). These results suggest that elevated concentrations of both TNFα and glucocorticoids interact in a cooperative manner to modulate the response to amino acids under this catabolic condition.
mTOR heterozygosity: What happens when the glass is half full?
mTOR and mTOR kinase activity are essential for embryonic development as mice that are either null for mTOR or mice carrying a knock-in mutation in the mTOR kinase domain are embryonic lethal (34, 83, 114) (Table 1). In contrast, mice with a muscle-specific deletion of mTOR or raptor both survive birth, but exhibit severe myopathy/dystrophy and premature death (6, 101). Interestingly, the weight of fast-twitch glycolytic muscles (e.g., gastrocnemius) are selectively targeted by the loss of mTOR, whereas the weight of slow-twitch oxidative muscles (e.g. soleus) were not affected (101). It is not known whether muscle protein synthesis or degradation are altered in muscle-specific mTOR null mice, but the expression of two atrogenes (MAFbx and MuRF-1) was markedly depressed making it unlikely that an increased proteasomal degradation accounts for the loss of muscle mass in mTOR knockout mice.
Because of the confounding effect of muscle-specific mTOR deletion causing muscle dystrophy and early death, whole-body mTOR heterozygous mice were used to determine whether a 50% decrease in mTOR would reduce muscle protein synthesis (62). Muscle mass and protein synthesis were both reduced, and the mTOR heterozygotes were less responsive than WT mice to leucine stimulation. Surprisingly, the basal level of mTOR substrate phosphorylation (S6K1 and 4E-BP1) and the ability of an oral leucine challenge to stimulate substrate phosphorylation were not significantly different in mTOR heterozygotes and WT mice. Such a response suggests that the relative amount of mTOR may alter protein translation and leucine sensitivity independent of mTOR substrate phosphorylation. One possibility is that the 50% reduction in muscle mTOR inhibits ribosome biogenesis and that this results in a long-term reduction in the number of active ribosomes that accounts for the fall in muscle protein synthesis in mTOR heterozygotes. A reduction in the number of ribosomes would also be consistent with the lack of change in either the basal or leucine-stimulated phosphorylation of 4E-BP1, S6K1, and S6 since translation of ribosomal RNAs is independent of the phosphorylation of these substrates (130).
The decreased mTOR protein in mTOR heterozygotes places a significant burden on the mice when stressed. mTOR heterozygotes, which already have a reduced rate of muscle protein synthesis, exhibit a disproportionally greater decrease when challenged with LPS (62). Additionally, skeletal muscle from mTOR heterozygotes that received this “second hit”, were unresponsive to leucine (62). Thus, although superficially mTOR heterozygotes appeared relatively normal compared to their homozygous brethren, they were less able to mount an anabolic response during an infectious stress. These data suggest that a partial reduction in muscle mTOR reserves can have unforeseen negative consequences when combined with stresses that inhibit mTOR activity.
Feedback inhibitors may co-opt mTOR activity
Because chronic mTOR activation stimulates anabolic processes that are energy demanding, over time the end products of mitochondrial oxidation (reactive oxygen species, ROS) accumulate (112). Indeed, rapamycin can rapidly inhibit mitochondrial function, shift energy production towards aerobic glycolysis, and in a protective coup inhibit ROS accumulation (98). Drosophila have evolved an mTOR inhibitory protein, sestrin, which naturally responds to oxidative stress, and prevents age-related pathologies (68). Over expression of sestrin inhibits the phosphorylation of S6K1 and 4E-BP1, whereas sestrin null flies exhibit excessive ROS accumulation and mitochondrial damage (68).
Sestrins are also induced by other stressors which activate AMPK by a direct binding mechanism independent of the cellular AMP/ATP ratio (11). Sestrin-activated AMPK becomes autophosphorylated and transphosphorylates TSC2. In sestrin over expressing cells, siRNA knockdown of TSC2 restores the activation of mTOR by upstream signals, suggesting the TSC complex is necessary for sestrin’s inhibitory activity.
It is not known whether sestrin mediates the sepsis-induced decrease in mTOR activity and leucine resistance in muscle. LPS does however stimulate muscle AMPK phosphorylation as well as the expression of FOXO1 and -3, potential activators of sestrin gene expression (90). In addition, artificial activation of AMPK in skeletal muscle with AICAR inhibits mTOR substrate phosphorylation and induces leucine resistance (97). Lastly, LPS and sepsis induce the accumulation of ROS in skeletal muscle essentially mimicking the conditions necessary for sestrin expression (85, 136). Thus, we speculate that the expression of sestrin or sestrin-like proteins remain a potential mechanism by which feedback inhibitors attempt to prevent additional ROS mediated damage by inhibiting mTOR activity.
ROS: the metabolic cost of doing business
Another feedback control mechanism by which cells regulate ROS is the elimination of mitochondria that have “gone bad”. In skeletal muscle, dysfunctional mitochondria are chiefly removed by an autophagic process (69, 117). Under normal conditions, autophagy performs “house-keeping” functions, but when ROS and AMPK activation drive the transport of FOXO3 into the nucleus, FOXO3 stimulates the expression of genes, such as BNIP3, that regulate mitochondrial fission and autophagy (57). Persistent and/or defective mitochondrial fission drives the AMPK-dependent elimination of mitochondria and the proteolytic side of muscle wasting (76, 77, 102). Although less well studied, the FOXO3- driven expression of BNIP3 also inhibits mTOR. BNIP3 binds to Rheb and inhibits the phosphorylation of 4E-BP1 and S6K1 (but not Akt on S473), consistent with BNIP3 exclusively inhibiting mTORC1. BNIP3 decreases GTP binding to Rheb and therefore may trigger the movement of Rheb away from the lysosomal surface where GTP loading is thought to occur (69). Teleologically, the inhibition of mTOR activity and protein synthesis in muscle during a catabolic state characterized by ongoing autophagic and proteasomal protein degradation makes perfect sense. In this context, mTOR inhibition contributes to a feedback loop that operates on the rationale “if they’re going to break it… don’t make it”.
Concluding Remarks
For survival, mammals take in complex carbohydrates, fats and proteins not only for energy but also to recycle their constituent parts. The amino acids released from digested protein facilitate this recycling process by acting as signaling molecules that stimulate protein synthesis. The BCAAs are essential for translation initiation and their absence triggers proteolytic systems designed to obtain them from internal stores, especially skeletal muscle. Sepsis and inflammation are quasi starvation-like states in which digested protein and even parenterally supplied BCAAs fail to optimally stimulate protein synthesis and mTOR activity. Although mTOR is a highly regulated kinase, the sepsis-induced defect in mTOR signaling appears to be independent of TSC2 because signaling can be restored by growth factors, such as IGF-I, that use this pathway. Sepsis-induced leucine resistance can be mimicked by AMPK activation, similar to that seen after LPS, suggesting that one or more AMPK-dependent mTOR inhibitors may promote the resistance phenotype. Because protein synthesis and degradation are coordinately controlled, it is likely that factors such as sestrins and BNIP3, that regulate autophagy and proteasomal protein degradation, may reciprocally inhibit mTOR and protein synthesis to prevent futile cycling of amino acids. When the world is deemed to be pathogen-free, hormones and growth factors may signal the “all clear” to muscle that it is once again safe to increase energy expenditure using amino acids to accrue muscle protein.
Acknowledgments
This work was supported in part by NIH grant GM38032. We thank our many collaborators who have helped make our work possible, especially Drs. L. Jefferson, S. Kimball, T. Vary, and C. Lynch.
References
- 1.Almendro V, Carbo N, Busquets S, Figueras M, Tessitore L, Lopez-Soriano FJ, Argiles JM. Sepsis induces DNA fragmentation in rat skeletal muscle. Eur Cytokine Netw. 2003;14:256–259. [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez C, Stahl PD. Interferon-gamma selectively induces Rab5a synthesis and processing in mononuclear cells. J Biol Chem. 1998;273:33901–33904. doi: 10.1074/jbc.273.51.33901. [DOI] [PubMed] [Google Scholar]
- 3.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann RA, Kim JH, Wu AL, Park IH, Chen J. A nuclear transport signal in mammalian target of rapamycin is critical for its cytoplasmic signaling to S6 kinase 1. J Biol Chem. 2006;281:7357–7363. doi: 10.1074/jbc.M512218200. [DOI] [PubMed] [Google Scholar]
- 5.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–829. doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- 6.Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Benvenuto G, Li S, Brown SJ, Braverman R, Vass WC, Cheadle JP, Halley DJ, Sampson JR, Wienecke R, DeClue JE. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000;19:6306–6316. doi: 10.1038/sj.onc.1204009. [DOI] [PubMed] [Google Scholar]
- 8.Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003;372:555–566. doi: 10.1042/BJ20021266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackshear PJ, Silver J, Nairn AC, Sulik KK, Squier MV, Stumpo DJ, Tuttle JS. Widespread neuronal ectopia associated with secondary defects in cerebrocortical chondroitin sulfate proteoglycans and basal lamina in MARCKS-deficient mice. Exp Neurol. 1997;145:46–61. doi: 10.1006/exnr.1997.6475. [DOI] [PubMed] [Google Scholar]
- 10.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 11.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37:S354–367. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol. 2008;586:5589–5600. doi: 10.1113/jphysiol.2008.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossland H, Constantin-Teodosiu D, Greenhaff PL, Gardiner SM. Low-dose dexamethasone prevents endotoxaemia-induced muscle protein loss and impairment of carbohydrate oxidation in rat skeletal muscle. J Physiol. 2010;588:1333–1347. doi: 10.1113/jphysiol.2009.183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csibi A, Cornille K, Leibovitch MP, Poupon A, Tintignac LA, Sanchez AM, Leibovitch SA. The translation regulatory subunit eIF3f controls the kinase-dependent mTOR signaling required for muscle differentiation and hypertrophy in mouse. PLoS One. 2010;5:e8994. doi: 10.1371/journal.pone.0008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 18.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Yang B, Li Y, Zhong C, Ding J. Molecular basis of the acceleration of the GDP-GTP exchange of human ras homolog enriched in brain by human translationally controlled tumor protein. J Biol Chem. 2009;284:23754–23764. doi: 10.1074/jbc.M109.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Drenan RM, Liu X, Bertram PG, Zheng XF. FKBP12-rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J Biol Chem. 2004;279:772–778. doi: 10.1074/jbc.M305912200. [DOI] [PubMed] [Google Scholar]
- 22.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edinger AL, Thompson CB. Akt maintains cell sizeand survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426–1436. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- 26.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol. 2010;299:C552–560. doi: 10.1152/ajpcell.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flinn RJ, Yan Y, Goswami S, Parker PJ, Backer JM. The late endosome is essential for mTORC1 signaling. Mol Biol Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 30.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci. 2008;86:E84–93. doi: 10.2527/jas.2007-0483. [DOI] [PubMed] [Google Scholar]
- 31.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 32.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock. 2009;32:416–426. doi: 10.1097/SHK.0b013e3181a034d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost RA, Pereyra E, Lang CH. Ethyl Pyruvate Preserves Insulin-Like Growth Factor (IGF)-I Sensitivity toward mTOR Substrates and Protein Synthesis in C2C12 Myotubes. Endocrinology. 2010 Nov; doi: 10.1210/en.2010-0248. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A Phosphatidylinositol 3-Kinase/Protein Kinase B-independent Activation of Mammalian Target of Rapamycin Signaling Is Sufficient to Induce Skeletal Muscle Hypertrophy. Mol Biol Cell. 21:3258–3268. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell. 2010;21:3258–3268. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 42.Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 43.Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. Faseb J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 46.Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 48.James JH, Hasselgren PO, King JK, James LE, Fischer JE. Intracellular glutamine concentration does not decrease in all muscles during sepsis. J Surg Res. 1993;54:558–564. doi: 10.1006/jsre.1993.1085. [DOI] [PubMed] [Google Scholar]
- 49.Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-Induced Alterations In Protein-Protein Interactions Within Mtor Complex 1 And The Modulating Effect Of Leucine On Muscle Protein Synthesis. Shock. 2010 doi: 10.1097/SHK.0b013e3181ecb57c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kazi AAM, Lang CHP. PRAS40 regulates protein synthesis and cell cycle in C2C12 myoblasts. Mol Med. 2010 doi: 10.2119/molmed.2009.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 54.Kimball SR, Orellana RA, O’Connor PM, Suryawan A, Bush JA, Nguyen HV, Thivierge MC, Jefferson LS, Davis TA. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E637–644. doi: 10.1152/ajpendo.00340.2002. [DOI] [PubMed] [Google Scholar]
- 55.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 56.Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, Segura CT, Leibovitch SA. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. Embo J. 2008;27:1266–1276. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landes T, Emorine LJ, Courilleau D, Rojo M, Belenguer P, Arnaune-Pelloquin L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010;11:459–465. doi: 10.1038/embor.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang CH. Elevated plasma free fatty acids decrease basal protein synthesis, but not the anabolic effect of leucine, in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E666–674. doi: 10.1152/ajpendo.00065.2006. [DOI] [PubMed] [Google Scholar]
- 59.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- 60.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 61.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang CH, Frost RA, Bronson SK, Lynch CJ, Vary TC. Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am J Physiol Endocrinol Metab. 2010;298:E1283–1294. doi: 10.1152/ajpendo.00676.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 64.Lang CH, Lynch CJ, Vary TC. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R935–944. doi: 10.1152/ajpregu.00297.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang CH, Pruznak AM, Frost RA. TNFalpha mediates sepsis-induced impairment of basal and leucine-stimulated signaling via S6K1 and eIF4E in cardiac muscle. J Cell Biochem. 2005;94:419–431. doi: 10.1002/jcb.20311. [DOI] [PubMed] [Google Scholar]
- 66.Lantier L, Mounier R, Leclerc J, Pende M, Foretz M, Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. Faseb J. 2010;24:3555–3561. doi: 10.1096/fj.10-155994. [DOI] [PubMed] [Google Scholar]
- 67.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 70.Lince-Faria M, Maffini S, Orr B, Ding Y, Claudia F, Sunkel CE, Tavares A, Johansen J, Johansen KM, Maiato H. Spatiotemporal control of mitosis by the conserved spindle matrix protein Megator. J Cell Biol. 2009;184:647–657. doi: 10.1083/jcb.200811012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu GH, Guan T, Datta K, Coppinger J, Yates J, 3rd, Gerace L. Regulation of myoblast differentiation by the nuclear envelope protein NET39. Mol Cell Biol. 2009;29:5800–5812. doi: 10.1128/MCB.00684-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Hong Z, Ding J, Liu J, Zhang J, Chen S. Predominant release of lysosomal enzymes by newborn rat microglia after LPS treatment revealed by proteomic studies. J Proteome Res. 2008;7:2033–2049. doi: 10.1021/pr7007779. [DOI] [PubMed] [Google Scholar]
- 73.Lunemann JD, Schmidt J, Dalakas MC, Munz C. Macroautophagy as a pathomechanism in sporadic inclusion body myositis. Autophagy. 2007;3:384–386. doi: 10.4161/auto.4245. [DOI] [PubMed] [Google Scholar]
- 74.MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol. 2009;587:253–260. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maganto-Garcia E, Punzon C, Terhorst C, Fresno M. Rab5 activation by Toll-like receptor 2 is required for Trypanosoma cruzi internalization and replication in macrophages. Traffic. 2008;9:1299–1315. doi: 10.1111/j.1600-0854.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 78.Marshall CB, Ho J, Buerger C, Plevin MJ, Li GY, Li Z, Ikura M, Stambolic V. Characterization of the intrinsic and TSC2-GAP-regulated GTPase activity of Rheb by real-time NMR. Sci Signal. 2009;2:ra3. doi: 10.1126/scisignal.2000029. [DOI] [PubMed] [Google Scholar]
- 79.Martin J, Masri J, Bernath A, Nishimura RN, Gera J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–583. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal Muscle wasting and contractile performance in septic rats. Muscle Nerve. 2005;31:339–348. doi: 10.1002/mus.20268. [DOI] [PubMed] [Google Scholar]
- 81.Miyazaki M, McCarthy JJ, Esser KA. Insulin like growth factor-1-induced phosphorylation and altered distribution of tuberous sclerosis complex (TSC)1/TSC2 in C2C12 myotubes. Febs J. 2010;277:2180–2191. doi: 10.1111/j.1742-4658.2010.07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. Faseb J. 2009;23:2264–2273. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- 83.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. Embo J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nethery D, DiMarco A, Stofan D, Supinski G. Sepsis increases contraction-related generation of reactive oxygen species in the diaphragm. J Appl Physiol. 1999;87:1279–1286. doi: 10.1152/jappl.1999.87.4.1279. [DOI] [PubMed] [Google Scholar]
- 86.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niesler CU, Myburgh KH, Moore F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp Physiol. 2007;92:207–217. doi: 10.1113/expphysiol.2006.034736. [DOI] [PubMed] [Google Scholar]
- 88.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nystrom G, Pruznak A, Huber D, Frost RA, Lang CH. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism. 2009;58:787–797. doi: 10.1016/j.metabol.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nystrom GJ, Lang CH. Sepsis and AMPK Activation by AICAR Differentially Regulate FoxO-1, -3 and -4 mRNA in Striated Muscle. Int J Clin Exp Med. 2008;1:50–63. [PMC free article] [PubMed] [Google Scholar]
- 91.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- 92.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panaro MA, Cianciulli A, Gagliardi N, Mitolo CI, Acquafredda A, Cavallo P, Mitolo V. CD14 major role during lipopolysaccharide-induced inflammation in chick embryo cardiomyocytes. FEMS Immunol Med Microbiol. 2008;53:35–45. doi: 10.1111/j.1574-695X.2008.00397.x. [DOI] [PubMed] [Google Scholar]
- 94.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350(Pt 2):361–368. [PMC free article] [PubMed] [Google Scholar]
- 97.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–1894. doi: 10.1093/jn/138.10.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106:22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136:264S–268S. doi: 10.1093/jn/136.1.264S. [DOI] [PubMed] [Google Scholar]
- 100.Rhoads RE. eIF4E: new family members, new binding partners, new roles. J Biol Chem. 2009;284:16711–16715. doi: 10.1074/jbc.R900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, Richard-Bulteau H, Vignaud A, Baas D, Defour A, Freyssenet D, Tanti JF, Le-Marchand-Brustel Y, Ferrier B, Conjard-Duplany A, Romanino K, Bauche S, Hantai D, Mueller M, Kozma SC, Thomas G, Ruegg MA, Ferry A, Pende M, Bigard X, Koulmann N, Schaeffer L, Gangloff YG. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. Embo J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosner M, Hengstschlager M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008;17:2934–2948. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- 104.Roth E, Funovics J, Muhlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr. 1982;1:25–41. doi: 10.1016/0261-5614(82)90004-8. [DOI] [PubMed] [Google Scholar]
- 105.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine-and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saini A, Faulkner S, Al-Shanti N, Stewart C. Powerful signals for weak muscles. Ageing Res Rev. 2009;8:251–267. doi: 10.1016/j.arr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 107.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 111.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284:12783–12791. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 113.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shor B, Cavender D, Harris C. A kinase-dead knock-in mutation in mTOR leads to early embryonic lethality and is dispensable for the immune system in heterozygous mice. BMC Immunol. 2009;10:28. doi: 10.1186/1471-2172-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 116.Smith IJ, Alamdari N, O’Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol. 2010;42:701–711. doi: 10.1016/j.biocel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. 2009;1793:154–170. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 118.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- 120.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol. 2010;109:1448–1454. doi: 10.1152/japplphysiol.00428.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Svanberg E, Frost RA, Lang CH, Isgaard J, Jefferson LS, Kimball SR, Vary TC. IGF-I/IGFBP-3 binary complex modulates sepsis-induced inhibition of protein synthesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1145–1158. doi: 10.1152/ajpendo.2000.279.5.E1145. [DOI] [PubMed] [Google Scholar]
- 122.Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 123.Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19:1840–1843. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, Arrieumerlou C, Hall MN. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 126.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast-and slow-twitch muscles. Am J Physiol. 1992;262:C1513–1519. doi: 10.1152/ajpcell.1992.262.6.C1513. [DOI] [PubMed] [Google Scholar]
- 127.Wan M, Wu X, Guan KL, Han M, Zhuang Y, Xu T. Muscle atrophy in transgenic mice expressing a human TSC1 transgene. FEBS Lett. 2006;580:5621–5627. doi: 10.1016/j.febslet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 128.Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG. Reevaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 131.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006;291:E80–89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]
- 132.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan Y, Flinn RJ, Wu H, Schnur RS, Backer JM. hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem J. 2009;417:747–755. doi: 10.1042/BJ20081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS One. 2008;3:e2086. doi: 10.1371/journal.pone.0002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li Y, Qi W, Zhang BX, Van Remmen H. Loss of manganese superoxide dismutase leads to abnormal growth and signal transduction in mouse embryonic fibroblasts. Free Radic Biol Med. 2010;49:1255–1262. doi: 10.1016/j.freeradbiomed.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]