Abstract
Psychiatric and neurological disorders have historically provided key insights into the structure-function relationships that subserve human social cognition and behavior, informing the concept of the ‘social brain’. In this review, we take stock of the current status of this concept, retaining a focus on disorders that impact social behavior. We discuss how the social brain, social cognition, and social behavior are interdependent, and emphasize the important role of development and compensation. We suggest that the social brain, and its dysfunction and recovery, must be understood not in terms of specific structures, but rather in terms of their interaction in large-scale networks.
Keywords: social brain, social cognition, brain networks, psychiatry, neurology, amygdala
Introduction
Several – perhaps most – psychiatric and neurological illnesses are characterized by prominent impairments in social functioning. These range from impaired processing of faces in autism [1–4] and prosopagnosia [5] to unusual tendencies to approach strangers in Williams Syndrome [6–8] and strange beliefs that one's spouse has been replaced by an impostor in Capgras syndrome [9,10]. Indeed, difficulty in social functioning is a key diagnostic criterion for several psychiatric disorders. Even those not necessarily associated specifically with social impairments, such as depression or schizophrenia, can nonetheless impact social relationships in profound ways (Table 1).
Table 1.
Examples of disorders in social behavior and functioning. The asterisk (*) indicates disorders that feature an uneven cognitive profile with disproportionate abnormality in the social domain, thought to arise from an abnormality in social processing. It should be emphasized that not all of these disorders arise from a primary impairment in social cognition (cf. main text).
| Examples of disorders that include alterations in social functioning |
|---|
| *Autism spectrum disorders |
| *Behavioral-variant frontotemporal dementia |
| *Developmental prosopagnosia |
| *Several monothematic delusions (e.g., Capgras syndrome, Fregoli delusion) |
| *Williams syndrome |
| Alzheimer's syndrome |
| Angelman's syndrome |
| Anxiety disorders |
| Childhood disintegrative disorder/Heller's syndrome |
| Down's syndrome |
| Fragile-X syndrome |
| Klinefelter's syndrome |
| Mood disorders |
| Prader-Willi syndrome |
| Rett's syndrome |
| Tuberous sclerosis |
| Turner syndrome |
| Schizophrenia |
Are human beings so social that essentially all aspects of cognition and behavior are in some way social, erasing any useful boundary between ‘social’ and ‘nonsocial’ [11,12]? Although much of human behavior occurs in a social context, we believe that it is important to delineate this domain of behavior, together with its underlying processing substrate. The advantages of attempting such delineation are twofold: first, it would provide a circumscribed domain of study for social neuroscience, and, second, it would reorient diagnosis and treatment of social disorders. However, it is important to note that we do not subscribe to older notions of strongly ‘modular’ processing [13] (Box 1); instead, we adopt a more nuanced view, which admits gradations and does not require all the processing features historically associated with modularity [14,15].
Box 1. Cognitive neuropsychology, past and future.
The study of dissociations in neurological and psychiatric disorders as a window into cognition has a long and fruitful history [142,143]. This approach was already apparent in the early models of language processing motivated by Broca's and Wernicke's classical findings: although the lesions that they studied were in specific regions of the brain, that information in and of itself was relatively meaningless, since next to nothing was known about the rest of the brain. They did, however, demonstrate what dissociations are possible and these dissociations began to inform processing models of language.
Classical cognitive neuropsychology and cognitive neuropsychiatry have taken precisely this approach. Single and double dissociations have informed processing architectures, without the requirement that they shed any light on the neurobiological specifics – or, indeed, sometimes without any interest in doing so [144]. The field derives much of its position from Jerry Fodor's seminal treatise [13] on how to think about cognitive architecture as, on the one hand, a collection of processing modules that dissociations could reveal (with particular properties, about which there is continuing debate [15]), and on the other hand, a general processing ability to think about the material that these modules provided as their outputs. More recently, modularity accounts have incorporated evolutionary data and this emphasis, in turn, has generated a plethora of putative processing modules concerned with social behavior [145,146]. There is evidence for modules that evolved to detect cheating and potential mates, to prevent incest and unfairness, and to help orchestrate much of our social interactions with other people. None of this work depends on elucidating the neurobiological substrates, although all of it derives from neurological processes.
There are several reasons why we believe that future approaches in this vein will incorporate neuroanatomical information: There are now some solid examples that combine classical neuropsychology and purely cognitive psychological approaches with neurobiology. Face perception, for example, shows strong evidence for modularity in terms of anatomical localization and in terms of single-cell selectivity; the evidence is so compelling that it is natural to try to put them all together [20,44]. We now know so much more about the brain that finding a particular dissociation to depend on damage to a particular region is informative, whereas in Broca's and Wernicke's days it was not (see Conclusions).
Perhaps of the broadest significance: we do not actually know what the processes are that we should attempt to slot into the boxes of our processing architecture. They can certainly be inferred from behavioral results, or from evolutionary considerations, but there is no reason to think that the picture so produced in fact characterizes the mind we are trying to understand, rather than simply the mind of the experimenter trying to explain it. Given how much we now know about the brain, understanding the biological substrates of a particular cognitive dissociation is informative. This may be particularly the case for social cognition, for which models of processing architectures are not nearly as mature as those of language processing, visual perception, memory, or attention.
Thus, although there is no reason to abandon the goal of cognitive neuropsychology in using dissociations to build models of processing architectures, it is important that neurobiological data begin to inform those models.
If the ‘social’ is a proper domain of study (as we believe it is), is there a corresponding ‘social brain’? We review the current status of this question and highlight recent developments that have replaced the view that a collection of isolated neural structures are important to social cognition with a network view. This network view also emphasizes the issue of compensatory processing: which nodes of the network are indispensable and which can be compensated for by other parts of the network (or, indeed, other networks altogether)?
Of course, all of these issues are prominently informed by functional MRI (fMRI) studies in healthy individuals as well, not to mention a significant body of experimental research in animal models. We focus here on disorders of the mind and brain, both because they provide the largest historical corpus of data and a complementary emphasis and because the application of social neuroscience concepts gleaned from the study of disorders in turn provides specific avenues for a better understanding of those disorders.
The social brain, social cognition, and social functioning
Social cognition refers to processing that is elicited by, about, and directed towards other people (or, more species-general, towards conspecifics). Thus, the term ‘social’ must be anchored in the processing demands made by particular classes of stimuli. Looking at a face and thinking about what somebody will do next are both social; looking at an apple, thinking about the weather, and driving a car on an empty road are not. Such distinctions at the level of stimuli and behavior naturally lead to corresponding distinctions in social cognition and its neural substrates, distinctions that are borne out not only by dissociations observed in healthy brains, but also by the dissociations caused by psychiatric and neurological disorders, as we review below [16,17].
Levels of ‘social’
It is essential at the outset to clarify the various ‘social’ phenomena commonly referred to in the literature – the social brain, social cognition, social behavior, and social functioning – and how they relate to one another. Social behavior, the anchor for all these different levels of explanation, comprises the readily observable inter actions between an individual and other people (or, more generally, an animal and conspecifics or even individuals of another species). Social cognition, in turn, refers to the various psychological processes (both conscious and non-conscious) that underlie social behavior. We use the term ‘social cognition’ relatively broadly here, to include any cognitive processing (e.g., perception, reasoning, memory, attention, motivation, and decision-making) that underlies a social ability or social behavior, but that is to some degree distinct from broader, nonsocial abilities and behaviors. The processing of social stimuli and the generation of social behavior typically engage some processing that appears to be relatively specialized for the social domain (recognizing faces, thinking about what another person is thinking, hearing somebody call one's name) and other processes that also participate, but are more general in function.
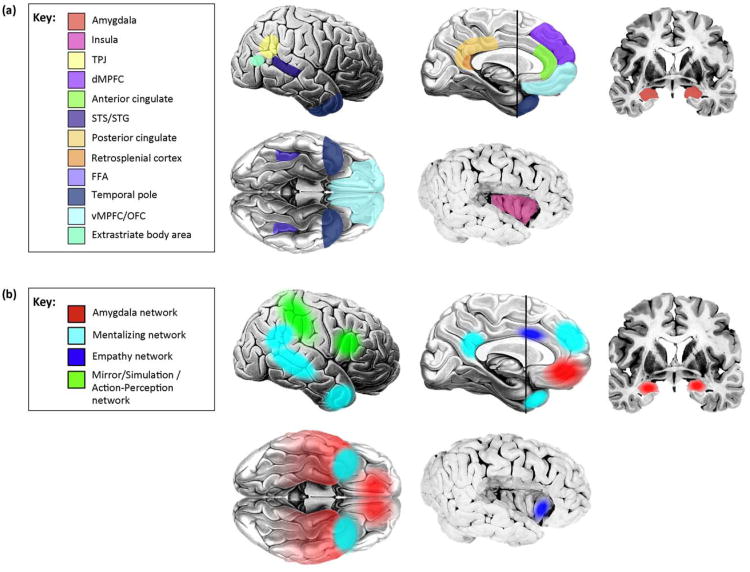
Mapping the social/nonsocial distinction at the behavioral and cognitive levels onto the brain poses a challenge, however. The ‘social brain’ historically refers to those brain structures that subserve social processes [18], often in a relatively domain-specific way: regions in the temporal lobe for processing faces [19–21], the temporoparietal junction and medial prefrontal cortex for representing other people's beliefs [22–24], and so forth. All of these regions have been retained in subsequent writings on the topic, with more added [16,25] (Figure 1a), in particular from the substantial number of fMRI studies that are now being pooled together into meta-analyses of social cognition [26]. These newly added regions encompass structures related to social perception, social attribution, and other aspects of social cognition. Nevertheless, even though damage within these regions can result in relatively specific impairments (Table 2), no social process can be attributed to a single structure alone; instead a network view of brain function is required (Figure 1b). Some initial steps in this direction consist of simply linking together the known individual structures and assigning the outputs from one ‘module’ as the input to the next (e.g., [27,28]). Another approach is to remain agnostic about information flow within the system, but nonetheless assign social cognitive processes to networks rather than to single structures (e.g., [29,30]), an approach facilitated by recent developments in network analysis, which yields insights even from resting-state fMRI data [31] (Figure 2).
Figure 1.
The social brain: from structures to networks. (a) Structures. There are a number of brain regions, only a subset of which are depicted here, that are now known to be involved in social cognition. Some of these are implicated because damage to them impairs aspects of social cognition and behavior; others are implicated because they are differentially activated in healthy brains when people perform social tasks in an MRI scanner. TPJ, temporoparietal junction; dMPFC, dorsomedial prefrontal cortex; STS/STG, superior temporal sulcus/gyrus, FFA: fusiform face area; vMPFC/OFC, ventromedial prefrontal cortex/orbitofrontal cortex. (b) Networks. Several core social cognition networks have been described. Not surprisingly, most of these encompass structures from the original ‘social brain’ [see Panel (a)], although a few new ones have been added, as well. We outline four here. One is a network centered on the amygdala [62,64,135]; the functions of this network (which will likely fractionate into several that are linked to specific amygdala nuclei eventually) range from triggering emotional responses to detecting socially salient stimuli to social affiliative behaviors. A second is the so-called mentalizing network, a collection of structures correlated at rest and activated by thinking about the internal states of others [29,136,137]. A third is recruited when individuals empathize with others [138,139]. A fourth network is activated during observation of the actions of others, including their emotional expressions [28,140,141]. Please note that, for simplicity and clarity, not all regions implicated in the networks are shown; several networks also involve other subcortical and brainstem structures not illustrated here.
Table 2.
Example structure-function relationships based on evidence from lesions. The table lists some of the disproportionate impairments in social processing that arise from damage to one of the structures from Figure 1a. There is often substantial hemispheric asymmetry and, in all cases where data is available, bilateral lesions are the most severe. The color scheme is the same as for the ‘social brain’ regions shown in Figure 1a.
| Lesion site | Social deficit |
|---|---|
| Occipitotemporal cortex/ FFA (R>L) | Face agnosia |
| Temporal Pole (L > R) | Naming people |
| Amygdala (bilateral) | Fear recognition |
| Ventromedial prefrontal cortex (R > L) | Social emotions, social decisions |
| Insula | Empathy, social context |
| Somatosensory cortex (not shown) (R > L) | Emotion recognition |
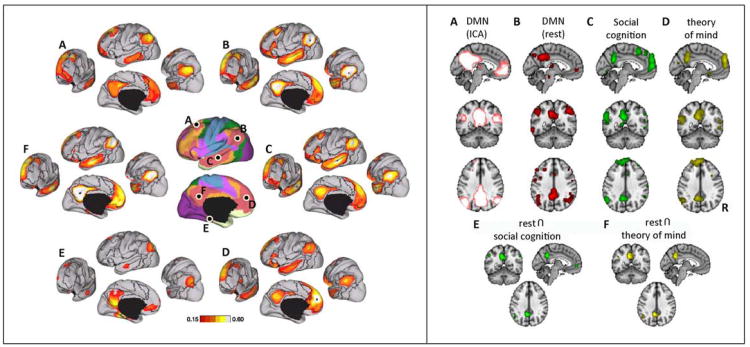
Figure 2.
Default-mode and social networks from resting-state data. (a) Connectivity of default-mode network regions, many of which are also implicated in social cognition. The central images show lateral and medial views of the brain, with different resting-state networks indicated in different colors (pink is the default-mode). The letters indicate seeds within key nodes of the network, and the surrounding plots show the functional connectivity that seed has with other regions of the brain. Reproduced, with permission, from [113]. (b) Overlap between default-mode network and regions activated by social cognition tasks. Reproduced, with permission, from [115].
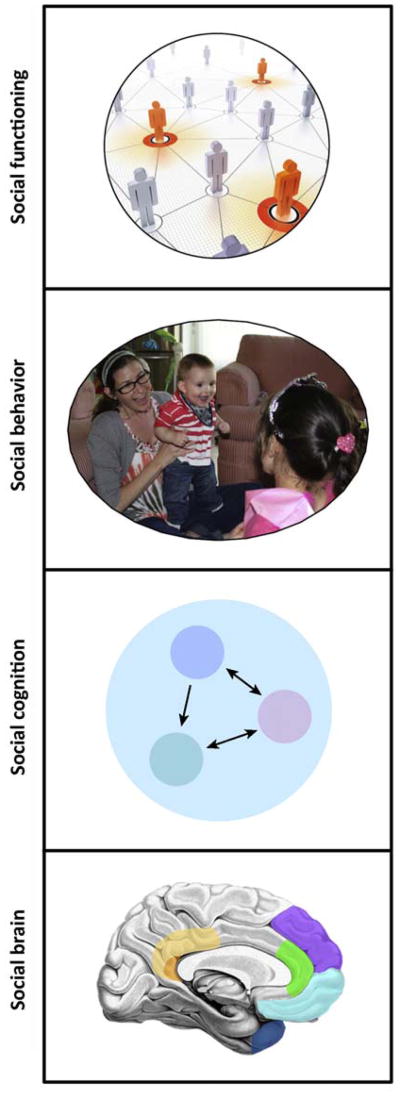
A final level requiring clarification is that of ‘social functioning’, a level of description as important clinically as it is difficult to relate to the other levels we discussed above. Social functioning is broader than social behavior in that it refers to the long-term, contextualized ability of an individual to interact with others (e.g., a person's behavior within a community over the past months). In principle, the relationship between the different levels of ‘social’ is straightforward: the social brain implements social cognition, which in turn causes social behavior, which in turn constitutes social functioning when integrated over time and context (Figure 3).
Figure 3.
Four ‘social’ levels of description and analysis. The levels we discuss in the text suggest particular relationships: the social brain supports social cognition, which then gives rise to social behavior, thus comprising social functioning when integrated over time and context. Although the causal relationships between these levels are complex, this schematic is intended to suggest the concepts associated with each level of description.
The relationships between these different levels (social functioning, social behavior, social cognition, social brain) are not unidirectional: for instance, altered social functioning over time results in changes in brain and cognition [32]. Several recent findings showcase these surprising links: immune responses in the body modulate BOLD response in the amygdala [33] and ventral striatum [34], as well as social behavior in disease [35]. Stressors resulting from living in large cities (particularly the alienation certain individuals experience [36]) fuel the high incidence of psychiatric illnesses seen in modern metropolises, where the incidence of schizophrenia, for instance, is twice that of rural environments [37] – and result in specific effects on regional brain activation [38,39]. One recent set of observed associations are correlations between the volume of specific brain structures (notably including the amygdala) and the size and complexity of the social network in which an individual functions: this has been found for human social networks [40] (even when they are instantiated over the internet [41]), as well as in monkeys [42]. Finally, it is important to note that, of course, genetic effects play a significant role – and are being vigorously investigated. However, that is a topic outside the scope of this review (Box 3).
Box 3. Genetic considerations.
One important effect that the study of psychiatric disorders has had on social neuroscience is the incorporation of genetic data to supplement the phenotypic and neurobiological components. There is now a plethora of genes implicated in human social behavior, together with mouse model counterparts. Two developments have driven this research: one is entirely methodological and consists simply in the ready and ever cheaper availability of sequencing methods; a second is that many psychiatric illnesses show considerable heritability.
But have these premises resulted in the discovery of genes for social behavior? There are a few examples of such discoveries in animals. For instance, there are genes encoding specific pheromone receptors that, when silenced, result in specific impairments. A receptor for the chemical cis-vaccenyl acetate serves such a function in flies [149]. There are also genes coding for central molecules involved in some model systems of social behavior; for instance, mouse knockouts of the oxytocin gene show impaired social memory for the odors of other mice [150]. However, the vast majority of examples in humans show nothing like this tight link between genes and behavior. Instead, individual genes are now thought to contribute a very small amount of the variance in brain and behavior, requiring the collective profile of hundreds or thousands of genes to explain pathology [151] and making the search for specific gene-behavior or gene-brain correlations with small sample sizes difficult [152,153]. These challenges are now being addressed through large research consortia providing much larger sample sizes than were possible even a decade ago.
In many ways, genetic information has a similar status with respect to informing social cognition as does neurobiological information. Just like neurobiological information bereft of the large background of accumulated neuroscience data ends up being meaningless and phrenological, so does genetic information in isolation. But once a large amount of such information has accrued, each new piece of information becomes more meaningful, because it can be situated within that larger context and an overall network. For instance, knowing that autism is associated, in a tiny fraction of people, with mutations to genes coding for neuroligins is relatively uninformative by itself. Knowing that there are hundreds of genes that all contribute to autism, and many are involved in gene networks for synaptic guidance and plasticity tells us a lot about autism as a disorder of brain connectivity (in turn informing process models) [80,154,155]. It is this accrual of data that will provide a rich background against which future findings can be interpreted and that will take the level of explanatory understanding beyond individual genes or brain structures to functional networks.
The view we advocate in relation to social neuroscience in this review is, in many ways, similar to the recommendations recently made regarding investigations of the genetic basis for psychiatric disorders [156]. Just as we advocate concentrating on a well-delineated set of structures (‘the social brain’, which is an evolving set), psychiatric genetics may see the quickest progress by focusing on a small set of clearly implicated genes. This does not mean that most of the rest of the brain does not contribute to social cognition or that findings from genome-wide association studies are all false positives. Of course, there is an significantly larger set of brain structures and genes that contribute to social cognition. But perhaps the place to start in order to understand the mechanisms should be that smaller set of structures and genes that show the clearest and largest effects, results from which will serve as a scaffold to understanding the rest.
Some examples
It is important to distinguish those cases of impaired social behavior that are primarily social (in that they arise from dysfunction at the level of social cognition and social brain) from those that are secondarily social (through incidental effects arising from nonsocial processes). Two examples illustrate this point. Someone with blindness due to damage to visual cortex would be unable to respond appropriately to visually presented social cues. If one attempted to interpret this case as the result of a primary dysfunction in social brain networks, it would quickly become apparent that the person does not have a problem with social cognition nor damage in any component of the social brain, and that the impairment is in no way specific to social behavior. Given all these reasons, once the reason for the apparent social dysfunction is understood, this would no longer be considered a fundamentally social problem. Interpreting it as such would do little to advance understanding of the patient's impairment and would do little to inform social neuroscience.
In a more contentious second example, consider a patient who suffered a stroke in left frontal operculum and became severely aphasic. The patient's interaction with other people would be severely disrupted as a consequence and social functioning impaired. We would again argue that, once the cause in this case is understood, this should not be considered to be a fundamentally social impairment, precisely because the apparent social dysfunction is not caused by impairment in social cognition as such or by damage to the components of the social brain. Of course, the patient's social behavior and functioning has changed (and it is useful and important clinically to describe this as such), but interpreting this change as explained by a primarily ‘social’ deficit must carry the presumption that its cause is to be traced to the level of social cognition and the social brain. Just as one would not say that a person who is blind has a memory problem (despite the fact that they will not ‘remember’ written words because they cannot see them), non-social causes that incidentally disrupt social behavior do not constitute a primary social impairment. In this latter example, it is also important to point out again that, over time, the secondary social dysfunction due to the aphasia may very well impact social brain networks and result in abnormal social cognition – with the implication that a nonspecific impairment in social functioning can give rise to a primary impairment in social cognition. Certainly, there are aspects of language, namely, pragmatics and prosody, that do fall under the purview of social cognition. We therefore fully acknowledge that examples such as this may not be unequivocal, but hope that the conceptual point is clear enough.
Processing features of social cognition
We have already described two characteristics by which social cognition can be circumscribed: social cognition is implemented by ‘social brain’ networks and social cognition causes social behavior. Below, we detail a third characteristic: social cognition exhibits particular processing features, dictated by the processing requirements of social stimuli. It is important to stress that none of these features in isolation is unique to social cognition; yet in aggregate they are more prominent in social than in nonsocial cognition. Similarly, not every example of social cognition speaks to the points we will present below; yet social cognition in general does. Rather than specifying necessary or sufficient conditions (which is a futile exercise), we highlight salient aspects of social cognition. These salient aspects raise the (empirical) question of the extent to which these features also pertain, or pertain to the same degree, to nonsocial processes:
Social cognition draws on a large number of different brain structures and their connectivity. Moreover, this network function often depends on rapid, efficient, and interactive processing (thus, even mild dysfunction in any structure, diffuse dysfunction that is not neuroanatomically specific, or white-matter damage can result in impairment).
The distributed nature of social cognition makes it vulnerable to insult, but also leaves open the possibility of compensation and recovery through spared components of the network (as well as through other intact networks).
Social cognition often involves a deep level of abstraction, inference, and counterfactual thinking (thus, any compromise in these processes will result in impaired social behavior, often disproportionately so). This also renders social cognition often highly context-dependent.
Social cognition requires extended tuning during development, within a particular social context and culture (and this is perhaps one reason why developmental disorders often feature pronounced and pervasive impairments in social behavior and functioning).
Social cognition is highly variable and communal (thus, there are large individual differences even in healthy individuals and compromised social functioning can to some extent be compensated for by the behavior of other people in a supportive environment).
Social cognition and neurological disease
The classic approach to identify structure-function associations relies on so-called ‘double-dissociations’: a dissociation in one patient with brain damage (e.g., impaired recognition of faces, but not other objects [5]) and the mirror image of that dissociation in another patient (e.g., impaired recognition of other objects, but spared recognition of faces [43]). In this example, face perception is shown to rely on partly distinct processes, a finding that, together with other evidence, has been used to argue for domain-specificity [44]: face processing relies on specialized processes, which appear to be implemented by specialized regions in temporal cortex [20,45,46].
Even relatively diffuse brain damage generally provides more anatomical information than do psychiatric disorders and can present process dissociations that are informative. For instance, patients with the behavioral variant of the neurodegenerative disorder frontotemporal dementia (bvFTD), exhibit disproportionate impairments in aspects of social behavior in early stages of the disease. These patients have impairments in their ability to understand other people's intentions and beliefs (‘mentalizing’) that depend on frontal, insular, and temporal cortex damaged in the disorder [47,48]. These findings have motivated network models of social cognition that implicate these regions particularly in the ability to incorporate context into the control of social behavior [49].
Face perception is probably the best known case of a highly specific dissociation, but there are several others, ranging from social aspects of reasoning to judgment, attention, and decision-making. Several modern lesion studies have also begun to use statistical mapping in large samples of a hundred or more participants [50], going well beyond the single- or multiple case-study approach of the past. Two notable neurological dissociations are the social impairments seen after damage to the prefrontal cortex or the amygdala. It is interesting to note that the most severe social deficits are often observed when damage to these components of the social brain occur during early development [51–53], a finding in line with the observation based on neurodevelopmental disorders that social behavior depends critically on a protracted period of development in a social context [see processing feature (iv) above]. In addition, damage that is bilateral, with both left and right structures affected, will result in a more profound social impairment than unilateral damage, since the homologous structure is unable to compensate for the damage [see processing features (i) and (ii) above].
Far from simply providing phrenological correlates of a social impairment, lesions of the prefrontal cortex and amygdala have informed detailed processing models, in particular about the role of emotions in social cognition. In the case of the ventromedial prefrontal cortex, this region has now been identified as necessary for the acquisition and storage of associations between stimuli and their value [54] – especially value related to social emotions [55,56] that motivate and guide complex social behaviors [57]. In the case of the amygdala, there is now a large corpus of data from humans and animals documenting its role in receiving input about faces (from temporal cortex), orchestrating emotional responses (via projections to hypothalamus and brainstem), and modulating attention and perception (via projections to basal forebrain and feedback to sensory cortices). These components work in concert and inform models of how amygdala lesions impair aspects of social cognition and behavior (Figure 4). Together with other findings about the amygdala's broader role in valuation [58,59] and saliency detection [60,61], they also rekindle the old question about the domain-specificity of social cognition: is the amygdala ‘specialized’ for social cognition or is its role in social cognition derivative of a broader function [62]? This question remains an open challenge, in part because it is becoming clear that the original question was ill-posed. The amygdala, by itself, does nothing; instead, it is important to begin asking questions about the networks within which the amygdala participates – and of these there are many. Indeed, even the conceptualization of the amygdala as a single structure is problematic, especially when one considers that the amygdala consists of a collection of approximately 13 individual nuclei, each exhibiting its own connectivity (both intra- and extra-amygdalar) and distinct response patterns [63–65].
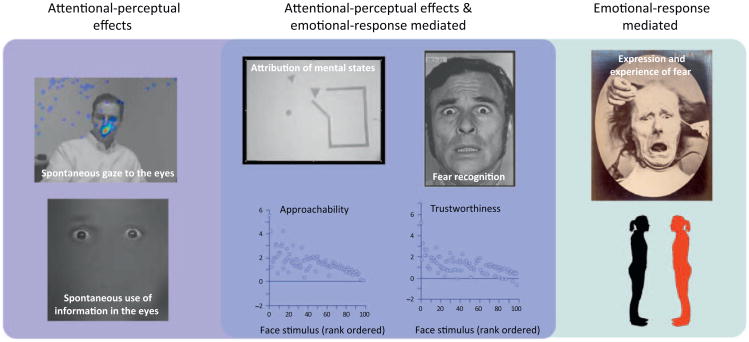
Figure 4.
Social impairments following bilateral amygdala damage. At the simplest level, the accrued data on the consequences of bilateral amygdala lesions support a dual-process model of sorts. Some of the impairments arise from reduced social attention/perception, likely based on the basolateral amygdale's connections with structures such as basal forebrain and feedback to visual cortices (light blue box on far left: impaired fixations onto and utilization of the eye region in faces). Others arise from reduced emotional response to stimuli, likely based on the central amygdale's connections to hypothalamus, brainstem, and other regions (green box on far right: impaired expression and experience of fear, impaired social distancing). Many social impairments probably arise from both of these mechanisms (dark blue box in middle: anthropomorphizing to shapes, recognizing fear in facial expressions, trustworthiness and approachability judgments from faces). See [62] for a review of these findings.
Considerable progress is now being made by integrating lesion studies with diffusion imaging, in order to infer damage to specific white matter tracts that are invariably involved in the accidents of nature that human neurological lesions provide. For instance, the classic case of Phineas Gage, who sustained frontal lobe damage due to the passage of a tamping iron through his head, was initially construed with a focus on a local lesion [66], but has now been reconstructed in terms of damage to white matter pathways [67]. In the same vein, an earlier finding that lesions in right somatosensory-related cortices impair recognition of emotional facial expressions [50] has been supplemented by the finding that damage to connections between this cortical region and posterior visual areas also produces such an impairment [68]. Modeling-based work has begun to shed light on how lesions of particular network nodes impact network function, with a generally more severe consequence for lesions to more medial structures than to lateral structures [69].
Future directions in lesion studies of social cognition thus return to the first points we made and also set the stage for our discussion of psychiatric disorders: they can only be understood in the context of anatomically distributed networks comprised of many structures. As such, they feature compensation by other structures when a single structure is damaged and they can be compromised by damage to connecting white matter [68,70] (Box 2). Rather than thinking of the ‘social brain’ as an independent collection of structure-function relationships, as Figure 1a might naively suggest, it will eventually need to be understood as a complex, integrated network (Figure 1b) – one that can also be dynamically reconfigured and depends on normal social development for its emergence.
Box 2. Agenesis of the corpus callosum (AgCC).
A fascinating developmental disorder, AgCC occurs in approximately 1:4000 live births and in the clearest cases manifests as complete absence of the corpus callosum with otherwise minimal developmental malformations and normal intellect (Figure I) [70]. Instead of crossing the midline to form the corpus callosum, axons form abnormal bundles of white matter that course along the medial wall of each hemisphere. The consequences of this disorder are striking, because they resemble those of high functioning autism: like people with autism, people with AgCC have the greatest difficulty in social functioning in the real world and show impaired ability to mentalize. Indeed, approximately 30% of people with AgCC meet formal research criteria for an autism spectrum disorder [147]. There is even an inbred mouse, the BTBR strain, that shows pronounced deficits in social behavior and is being studied as a murine model of autism – and turns out to have agenesis of the corpus callosum [148]. Although the majority of human AgCC is idiopathic, several cases run in families and some of the genes have been identified. Notably, a reduced cross-sectional area of the corpus callosum is also observed in autism spectrum disorders.
These examples emphasize not only the importance of development in social cognition, but also the importance of white matter connectivity. One appealing hypothesis is that social cognition requires rapid communication between neural processing components that are spatially separate, such as language-related processing in the left hemisphere and emotion-related processing in the right hemisphere. Given the highly interactive, real-time Nature of social behavior, there is substantial pressure to integrate processing amongst these components as rapidly as possible: they require considerable amounts of myelinated connections, which are reduced in autism and entirely lacking in AgCC. A recent puzzling finding has been that direct structural connectivity between the hemispheres is obviously eliminated (although the anterior and posterior commissures remain normal), there is intact functional connectivity, and an intact set of normal resting-state networks with bilateral symmetry in AgCC [122]. How such normal functional networks can emerge despite the absence of the brain's largest white matter bundle remains a mystery.
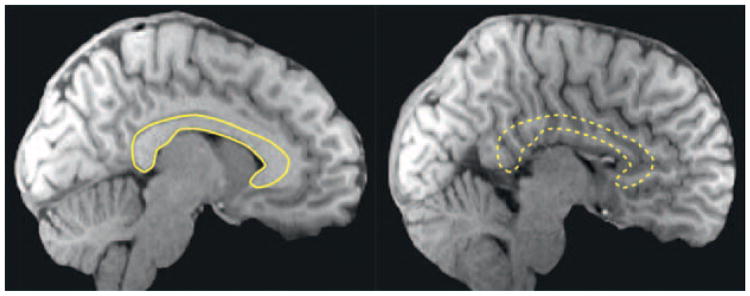
Figure I.
Agenesis of the corpus callosum (AgCC). Saggital structural MRI scans of a typically developed brain (left; outline of the corpus callosum in yellow) and of the brain of a patient with AgCC (right). Images courtesy of Lynn Paul and Mike Tyszka.
Social cognition and psychiatric disorders
Unlike neurological disorders, which often feature more precise neuroanatomical structure-function relationships, neuroanatomical descriptions of psychiatric disorders are often lacking. However, specific neuroanatomical information does not need to be available in order to inform models of social cognition (Box 1; Table 3). For instance, we described above cases of acquired impairment in processing faces and spared processing of objects following localized brain damage, and vice versa. Similarly, informative double dissociations, but without clear anatomical specificity, can also be observed in developmental disorders (e.g., developmental prosopagnosia [71], as compared to developmental non-face visual object agnosia [72]). Together, these cases demonstrate that face and object processing are not only dissociable in adulthood but that they can also develop independently of one another (i.e., the ability to process objects is not a prerequisite to process faces, and vice versa). This example highlights the usefulness of social process dissociations in studying disorders, even though the dissociations may not implicate particular brain structures.
Table 3.
The pattern of social impairments found across select neurological and psychiatric disorders. It should be noted that this table attempts to synthesize a large amount of information and present it in a concise manner. It does not claim to accurately capture the heterogeneity that is found across individuals and subgroups, or include all the domains on which groups may differ. The down arrow refers to a decrease compared to typical, the up arrow refers to an increase compared to typical, and the dash means that there is either no difference from typical, or that it is unknown.
| Disorder | Face processing (broadly construed) | Mentalizing (broadly construed) | Sociability | Eye contact | Intellectual functioning |
|---|---|---|---|---|---|
| Autism | ↓ | ↓ | ↓ | ↓ | —/↓ |
| Williams syndrome | — | ↓ | ↑ | ↑ | ↓ |
| Behavioral-variant frontotemporal dementia | ↓ | ↓ | ↓ | ↓ | — |
| Fragile X | —/↓ | ↓ | ↓ | ↓ | ↓ |
| Developmental Prosopagnosia | ↓ | — | — | — | — |
| Capgras | ↓ | ↓ | — | — | — |
| Psychopathy | ↓ | ↓ | ↑ | ↓ | — |
Some of the clearest examples of psychiatric illnesses that feature putative social dissociations are developmental disorders: Williams syndrome and autism spectrum disorders, to name the two most prominent (Table 4). We will not review these disorders in any detail here (for detailed recent reviews, see [9,73–76]), but will instead use them as examples to support the conceptual points we made earlier. There is a large and rich literature from healthy development that informs current understanding of the social brain: certain social abilities develop before others do and several social competencies have to unfold in a particular sequence. Moreover, developmental perturbations have shed light on how social abilities depend on particular sources of environmental input. For instance, infants who were blind from birth nonetheless develop, in most cases, normal abilities to attribute intentions and beliefs to others, and show normal regional brain activation when they do so [77], whereas abnormal social experience during development [78], just like abnormal development due to a neurodevelopmental disorder such as autism [73,79], can result in disproportionate impairments in such ‘mentalizing’ abilities.
Table 4.
Autism and Williams syndrome. A more detailed comparison of the spared and compromised abilities in these two disorders highlights their dissociation.
| Autism | Williams syndrome | |
|---|---|---|
| Social overtures directed toward others | Decreased | Increased |
| Eye contact | Decreased | Increased |
| Social communication | Decreased | Increased |
| Face recognition | Decreased | Normal |
| Emotion recognition | Decreased | Mixed findings |
| Use of communicative gestures | Decreased | Decreased |
| Joint attention | Decreased | Decreased |
| Facial expressions | Decreased | Mixed findings |
| Prosody | Decreased | Increased |
Autism spectrum disorders
Autism spectrum disorders (ASD) are a collection of neurodevelopmental disorders [80], with estimated prevalence of approximately 1:100, a substantial genetic component, and impairments in social interaction and communication, together with restricted interests and often stereotyped and repetitive behaviors [74]. In high-functioning individuals with autism or Asperger's syndrome, social dysfunction is often the most significant complaint in everyday life and altered social cognition has been demonstrated in many studies [73,79,81–83].
One insight from ASD has been that general intellectual functioning can be dissociated from social behavior and social functioning, often in very dramatic ways (mental retardation and the severity of autistic symptoms are generally correlated, but not necessarily because they have a common cause [84]). There are many examples of people with ASD who have an Intelligence Quotient (IQ) well above average, yet still have severe difficulties in social interactions. This intriguing observation corroborates the fundamental distinction between social and nonsocial cognition and suggests the hypothesis that the brains of people with ASD should reveal pathology or abnormal activation in components of the ‘social brain’ (Figure 1). Indeed, this is in large part what has been found [85], although the conclusion needs to be tempered by the observation that this is also mostly what researchers have looked for.
Primary insights into social cognition more generally have come from the entire body of neuroscience studies in ASD, rather than from any single finding. Taken together, these studies argue for the importance of early development in social cognition, perhaps more than in other cognitive domains; for the partial independence of social abilities from general intellectual functioning; and for the importance of white matter connectivity [80], especially in a developmental context, in sculpting the social brain (Box 2). Further insight has been gained from recent studies of high-functioning adults with ASD that have attempted to dissect some very specific components of social cognition. For instance, selective impairments have been found in the ability to incorporate social reputation effects into altruistic behavior (people with ASD are insensitive to the effects of being observed while making charitable donations, controlling for their ability simply to register the presence of another person) [81]. Moreover, people with ASD were found to be impaired when needing to combine a person's intentions and actual outcomes in making moral judgments, even though they are able to process each of the two components individually [82]. However, just as the strong hypothesis that face processing is domain-specific [44] remains contested [86], so does the hypothesis that individuals with ASD exhibit an impairment to represent false beliefs specifically in the social domain [87]. In both of these cases, when social and nonsocial versions of stimuli or tasks are matched as closely as possible, the initially reported dissociations are typically reduced. However, this does not show that the impairment is not disproportionately social under most circumstances; it just identifies those specific features that distinguish the processing of social stimuli.
The investigation of single structures responsible for abnormal social cognition in ASD has given way to the view of ASD as a disorder of brain connectivity [80,88]. In particular, several recent studies have shown abnormal connectivity precisely between the components of the social brain rather than everywhere the brain [89,90]. For instance, there is abnormal functional coupling between amygdala and temporal cortex when processing faces [91], as well as reduced long-range connectivity with the amygdala [92].
Williams syndrome
A second psychiatric disorder of great interest to social neuroscience is Williams syndrome (or Williams-Beuren syndrome), a genetic disorder caused by a discrete hemideletion of a set of approximately 20-25 contiguous genes on chromosome seven [8]. It presents in some respects as a social phenotypic opposite of autism (Table 4). People with Williams syndrome tend to approach strangers, whereas people with ASD often do not [6,7]; they tend to rate faces as abnormally trustworthy, whereas people with ASD do not [93]; and they spend more time looking at social stimuli in scenes than do people with ASD [94]. On the other hand, Williams syndrome also features severely impaired cognitive functioning in other domains, notably visuospatial functioning [76,95]. Viewed in conjunction with what is observed in people with ASD, these patterns of impaired and spared functions have sometimes been taken as evidence for the modularity of social cognition [76,96]. Yet, as with evidence from lesion studies, the data are ultimately insufficiently unequivocal to support any strong claim of modularity [75] that presumes that social processes are highly encapsulated or innately specified; instead, the ‘modularity’ typically found and discussed is a matter of degree, both functionally and anatomically.
The data also again highlight the importance of considering developmental aspects. The precise profile of abilities and disabilities that is revealed in these disorders varies with age [97]. Some of the most valuable insights regarding social abilities and their dissociations have come from careful comparisons of people with Williams syndrome to those with other disorders, such as autism [94], Down syndrome [7], or Prader-Willi syndrome [98], which, together with neurological illnesses such as prosopagnosia [99], have provided evidence that representing other people's mental states and recognizing their faces may be two distinct and dissociable processes [100].
Compensation and recovery: towards a network-view of the social brain
Whereas functional neuroimaging in healthy brains reveals regions involved in, or perhaps sufficient for, a particular function, lesion studies reveal which nodes of the network compromise function when damaged and hence are necessary. The methods together can map out the degree of necessity and redundancy, and yield the concept of ‘degeneracy’ [101]: damage to any single component is insufficient to abolish the function; damage to multiple components is required instead. This observation sets the stage for thinking about two fundamental aspects of recovery: the nearly instantaneous residual function following focal brain damage that is possible with the remaining, intact anatomical components (e.g., contralateral homologues [102]; spared tissue immediately adjacent to a partial lesion [103,104]), and the typically considerably greater residual function after some time has elapsed, which includes not only functional reorganization [105], but also actual structural change [106], along with compensatory cognitive strategies [107]. The majority of these studies, however, have been in the domain of motor function and language, with few, as yet, focusing on recovery of social functioning.
Functional and anatomical reorganization can also be used as a tool to reveal the network structure of the brain. Changes in one region of the brain will, over time, affect the function and structure of other regions that are functionally or anatomically connected to it, with distal effects long documented in the peripheral as well as the central nervous system [108]. With the development of more sensitive neuroimaging methods, more subtle changes can now be detected, making the combination of neuroimaging and patient studies particularly fruitful for studying network-level anatomy, functioning, plasticity, and compensation. For instance, morphometric changes in distal cortex following bilateral amygdala lesions have been observed [109], as have structural changes in white matter following damage to the visual cortex (which may account in part for the striking abilities of patients with visual blindsight [110]).
Network perspectives are now being widely applied to the study of neurological and psychiatric patients [111,112], representing a shift in emphasis from specific brain regions to specific brain networks. The majority of this work currently relies on resting-state networks, on the one hand obviating the need to equate task performance between clinical populations and healthy individuals, but on the other hand leaving vague exactly what participants are doing ‘at rest’. Considerable advances have been made in dissecting the brain into functional network components from such resting-state data [113], and in particular in identifying networks that are also revealed during the performance of specific social tasks [114]. A recent focus of social neuroscience has been on dissecting the default-mode network into subcomponents [31,115], in part because the individual components overlap with those assigned to the ‘social brain’ and in part because abnormal default-mode networks have been implicated in many psychiatric and neurological illnesses [116,117].
Some examples of these network approaches in psychiatric disorders come from functional imaging studies in ASD, which have pointed towards specific compensatory components for processing biological motion, a domain in which this population typically shows specific behavioral impairments [118]. One study found differences in networks that subserve biological motion processing in ASD compared to healthy controls, even when the task was carefully matched [119]. Even more intriguing was a study of children with autism and their unaffected siblings: biological motion activated regions in unaffected siblings that were distinct from the regions activated in both the affected siblings (with ASD) and in healthy children with no family history of ASD, which suggests that such activation reflects compensatory processing [120].
Whereas ASD is entirely a developmental disorder, other important examples come from contrasting the effects of developmental versus adult-onset lesions. One striking example is absence of the corpus callosum: whereas bilaterally coupled functional networks are abolished immediately after acute transection of the corpus callosum [121], developmental agenesis (Box 2) results in completely spared bilateral networks [122]. In another example, bilateral amygdala damage in adulthood results in deficits in rapid or non-conscious detection of salient emotional stimuli [123], whereas developmental-onset lesions appear not to [124,125].
An intriguing finding that highlights the importance of environmental interactions comes from a recent study of identical twins with Urbach-Wiethe disease, who both had developmental-onset bilateral amygdala lesions [126]. Whereas impaired fear recognition has been associated with amygdala lesions across several patients [127], only one of the twins in that study [126] showed such a deficit, whereas the other was normal. A similar pattern emerged for modulation of acoustic startle responses and social network size. This discordant phenotype of social functions generally linked to the amygdala suggests that very similar brain damage can produce different effects depending on compensatory abilities. In fact, this study [126] found differential brain activation in the two twins that might reflect such compensatory processing.
Some key mechanisms that should be further explored are compensation through contralateral homologues, as well as through top-down strategies. Both instances suggest fairly specific hypotheses (changes in lateralized activation and in recruitment of prefrontal regions, for instance). These mechanisms may also be related to the finding that brain activation in normal aging tends to become less lateralized [128] and more dependent on prefrontal regions [129]. It is interesting to note that initial findings in people with ASD have also pointed to compensatory activations within the prefrontal cortex [120] and that impulsive behavior has been associated with decoupling of prefrontal networks from subcortical ones [130]. One important future direction for understanding compensatory processing within specific neural regions is to demonstrate their causal role. For instance, a recent study on Parkinson's Disease first used fMRI to identify a compensatory brain region, and then transiently inactivated this region (using theta-burst transcranial magnetic stimulation) to show that this resulted in a behavioral deficit in Parkinson's Disease, but not in healthy individuals [131].
Conclusions and challenges
Neurological and psychiatric disorders have traditionally demonstrated that some aspects of cognition and behavior could be disproportionately impaired, informing processing architectures (Box 1). The most crucial insight was the finding that, across disorders ranging from frontal lobe damage [66] to amygdala lesions [132], autism [79] and Williams Syndrome [76], social behavior could be disproportionately affected relative to nonsocial behavior. Additional, more specific dissociations made distinctions among a variety of social processes, but still left the genetic and neurobiological details rather obscure and often irrelevant.
This picture has changed in the past few years – and the trend is likely to continue, especially in light of the vast amount of data (particularly from neuroimaging) now available in individual articles and in shared databases. Knowing which specific brain regions, when lesioned, can result in a given impairment now informs conceptualizations of the impairment, as does knowing which brain networks are activated during a particular task [and even which genes are associated with a particular process (Box 3)]. The accrual of neuroimaging data, in particular, has generated a wealth of priors on how to interpret regional brain function. Although in the extreme case this can spawn so-called ‘reverse inferences’ (the activation of a particular brain region is interpreted in terms of the putative function of that structure as gleaned from the prior literature [133]), this is slowly becoming a valuable background against which to interpret new results as more data are accrued [134]. More than that, this body of work is beginning to identify sets of brain structures that constitute networks and systems – the level at which we will need to understand the social brain (Box 4).
Box 4. Outstanding questions.
How can lesion studies and fMRI in healthy individuals best be combined to map out degeneracy in brain networks? This approach requires a systematic effort on both fronts and has typically been done piecemeal up to the present.
How can patterns of activation obtained with fMRI in patients with focal brain lesions be interpreted? Although the approach has significant potential to reveal network function and compensation, it is fraught with problems in accounting for performance impairments and regional changes in perfusion and BOLD response.
How can compensatory networks be investigated? In principle, the approach requires three groups: healthy controls, patients with a specific neurological or psychiatric illness who are impaired on a process, and patients with the same illness who are less impaired and somehow able to compensate. Differences in cognitive activation would be informative of which brain regions are involved in compensation; experimental inactivation with techniques such as TMS could then test their causal role.
How many ‘social networks’ are there? Although we sketched a few of the most popular ones in Figure 1b, undoubtedly several more will be discovered. Moving from social brain structures to social brain networks is in the right direction; however, principles for categorizing or relating the various networks are needed.
What is the best route to discovery and to formulating subsequent hypotheses? The first part of this question is being addressed with consortia and public databases (e.g, The Autism Brain Imaging Data Exchange – ABIDE – http://fcon_1000.projects.nitrc.org/indi/abide/index.html); the second part will require focus on a subset of networks and on the domain of social phenomena we discussed at the beginning of this review.
Although essentially most of the brain will to some extent participate in social cognition, we argue that, at least for the time being, research into the social dysfunctions of neurological and psychiatric disorders should focus on the core set of brain structures that constitute the ‘social brain’ and their connectivity (Figure 1). There is no question that many structures and networks participate in social behavior, but a return to the core theme of what it means for a level of description to pertain to ‘social’ phenomena, as we discussed at the outset of this review, will help to anchor current research. In the end, the approach that may be most productive in understanding the social impairments seen in neurological and psychiatric disorders is likely to be a mixture of discovery science and hypothesis-driven investigation. Resting-state networks, mining large databases, and exploratory network visualization all provide the base from which we can start asking constrained questions. Careful design and contrast of tasks to isolate social processing, and establishing links across the levels of social brain, cognition, behavior, and functioning will help to keep social neuroscience domain-specific to some extent. Finally, cautious addition to the inventory of structures and networks that comprise the social brain will keep the study of the neural basis of social cognition a clearly defined, systematic task.
Acknowledgments
We thank Jed Elison, John Constantino, Bob Spunt, and three anonymous reviewers for comments. Supported by grants from the NIMH to R.A. (R01MH080721; P50MH094258) and D.P.K. (K99 MH094409).
References
- 1.Dalton KM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klin A, et al. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 3.Pelphrey KA, et al. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 4.Spezio ML, et al. Analysis of face gaze in autism using ‘Bubbles’. Neuropsychologia. 2007;45:144–151. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Damasio AR, et al. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- 6.Järvinin-Pasley A, et al. Affiliative behavior in Williams Syndrome: social perception and real-life social behavior. Neuropsychologia. 2010;48:2110–2119. doi: 10.1016/j.neuropsychologia.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter MA, et al. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. 2007;45:2839–2849. doi: 10.1016/j.neuropsychologia.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 9.Ellis HD, Lewis MB. Capgras delusion: a window on face recognition. Trends Cogn Sci. 2001;5:149–156. doi: 10.1016/s1364-6613(00)01620-x. [DOI] [PubMed] [Google Scholar]
- 10.Ellis HD, et al. Reduced autonomic responses to faces in Capgras delusion. Proc R Soc Lond B: Biol Sci. 1997;264:1085–1092. doi: 10.1098/rspb.1997.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adolphs R. Investigating the cognitive neuroscience of social behavior. Neuropsychologia. 2003;41:119–126. doi: 10.1016/s0028-3932(02)00142-2. [DOI] [PubMed] [Google Scholar]
- 12.Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fodor JA. The Modularity of Mind. MIT Press; 1983. [Google Scholar]
- 14.Coltheart M. Modularity and cognition. Trends Cogn Sci. 1999;3:115–119. doi: 10.1016/s1364-6613(99)01289-9. [DOI] [PubMed] [Google Scholar]
- 15.Barrett HC, Kurzban R. Modularity in cognition: framing the debate. Psychol Rev. 2006;113:628–647. doi: 10.1037/0033-295X.113.3.628. [DOI] [PubMed] [Google Scholar]
- 16.Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frith CD. The social brain? Philos Trans R Soc Lond B: Biol Sci. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- 19.Tsao DY, Livingstone MS. Neural mechanisms for face perception. Annu Rev Neurosci. 2008;31:411–438. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanwisher N, et al. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B: Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz J, et al. Distinct regions of right temporoparietal junction are selective for theory of mind and exogenous attention. PLoS ONE. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- 24.Gallager HL, Frith C. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 26.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Spunt RP, et al. Dissociable neural systems support retrieval of how and why action knowledge. Psychol Sci. 2010;21:1593–1598. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- 30.Spunt RP, Lieberman MD. An integrative model of the neural systems supporting the comprehension of observed emotional behavior. Neuroimage. 2012;59:3050–3059. doi: 10.1016/j.neuroimage.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Andrews-Hanna JR, et al. Functional-Anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki TK, et al. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberger NI, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson PH. Infectious Behavior: Brain-Immune Connections in Autism, Schizophrenia, and Depression. MIT Press; 2011. [Google Scholar]
- 36.Zammit S, et al. Individuals, schools, and neighborhood: a multilevel longitudinal study of variation in incidence of psychotic disorders. Arch Gen Psychiatry. 2010;67:914–922. doi: 10.1001/archgenpsychiatry.2010.101. [DOI] [PubMed] [Google Scholar]
- 37.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence– conditional on genetic risk. Schizophr Bull. 2005;31:795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 38.Lederbogen F, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 40.Bickart KC, et al. Amygdala volume and social network size in humans. Nat Neurosci. 2010;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanai R, et al. Online social network size is reflected in human brain structure. Proc R Soc Lond B: Biol Sci. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallet J, et al. Social network size affects neural circuits in Macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 43.Moscovitch M, et al. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but normal normal face recognition. J Cogn Neurosci. 1997;9:555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- 44.Yovel G, Kanwisher N. Face perception: domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Tsao DY, et al. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao DY, et al. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci U S A. 2009;49:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adenzato M, et al. Theory of mind ability in the behavioral variant of frontotemporal dementia: An analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48:2–12. doi: 10.1016/j.neuropsychologia.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Lough S, et al. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78:1354–1362. doi: 10.1212/WNL.0b013e3182518375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adolphs R, et al. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson SW, et al. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. 2000;18:281–296. doi: 10.1207/S1532694202Anderson. [DOI] [PubMed] [Google Scholar]
- 52.Anderson SW, et al. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 53.Shaw P, et al. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- 54.Chib VS, et al. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krajbich I, et al. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shamay-Tsoory SG, et al. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- 57.Koenigs M, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 59.Paton JJ, et al. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitkanen A, et al. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–532. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 64.Amaral DG, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- 65.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 66.Damasio H, et al. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1104. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 67.Van Horn J, et al. Mapping connectivity damage in the case of Phineas Gage. PLoS ONE. 2012;7:e37454. doi: 10.1371/journal.pone.0037454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Philippi CL, et al. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alstott J, et al. Modeling the impact of lesions in the human brain. PLoS Comput Biol. 2009;5:e1000408. doi: 10.1371/journal.pcbi.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paul LK, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 71.Duchaine B, Nakayama K. Dissociations of face and object recognition in developmental prosopagnosia. J Cogn Neurosci. 2005;17:249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- 72.Germine L, et al. A new selective developmental deficit: impaired object recognition with normal face recognition. Cortex. 2011;47:598–607. doi: 10.1016/j.cortex.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- 74.Frith U. Autism: Explaining the Enigma. Blackwell; 2003. [Google Scholar]
- 75.Meyer-Lindenberg A, et al. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behavior. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- 76.Bellugi U, et al. Bridging cognition, the brain and molecular genetics: evidence from Williams syndrome. Trends Neurosci. 1999;22:197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- 77.Bedny M, et al. Growing up blind does not change the neural bases of theory of mind. Proc Natl Acad Sci U S A. 2009;106:11312–11317. doi: 10.1073/pnas.0900010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yagmurlu B, et al. The role of institution and home contexts in theory of mind development. App Dev Psychol. 2005;26:521–537. [Google Scholar]
- 79.Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. MIT Press; 1997. [Google Scholar]
- 80.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 81.Izuma K, et al. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011;108:17302–17307. doi: 10.1073/pnas.1107038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moran JM, et al. Impaired theory of mind for moral judgment in high-functikoning autism. Proc Natl Acad Sci U S A. 2011;108:2688–2692. doi: 10.1073/pnas.1011734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Losh M, et al. Neuropsychological profile of autism and broad autism phenotype. Arch Gen Psychiatry. 2009;66:518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skuse DH. Rethinking the Nature of genetic vulnerability to autistic spectrum disorders. Trends Genet. 2007;23:387–395. doi: 10.1016/j.tig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Pelphrey KA, et al. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2005;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 86.Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- 87.Perner J, Leekam S. The curious incident of the photo that was accused of being false: Issues of domain specificity in development, autism, and brain imaging. Q J Exp Psychol. 2008;61:76–89. doi: 10.1080/17470210701508756. [DOI] [PubMed] [Google Scholar]
- 88.Anderson JS, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2010;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gotts SJ, et al. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012 doi: 10.1093/brain/aws160. http://dx.doi.org/10.1093/brain/aws1160. [DOI] [PMC free article] [PubMed]
- 90.von dem Hagen EAH, et al. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss053. http://dx.doi.org/10.1093/scan/nss1053. [DOI] [PMC free article] [PubMed]
- 91.Kleinhans NM, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 92.Rudie JD, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22:1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellugi U, et al. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport. 1999;10:1653–1659. doi: 10.1097/00001756-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 94.Riby DM, Hancock PJB. Viewing it differently: social scene perception in Williams Syndrome and autism. Neuropsychologia. 2008;46:2855–2860. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 95.Meyer-Lindenberg A, et al. Neural basis of genetically determined visuospatial construction deficit in Williams Syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 96.Karmiloff-Smith A, et al. Is there a social module? Language, face processing, and theory of mind in individuals with Williams Syndrome. J Cogn Neurosci. 1995;7:196–208. doi: 10.1162/jocn.1995.7.2.196. [DOI] [PubMed] [Google Scholar]
- 97.Paterson SJ, et al. Cognitive modularity and genetic disorders. Science. 1999;286:2355–2357. doi: 10.1126/science.286.5448.2355. [DOI] [PubMed] [Google Scholar]
- 98.Rosner BA, et al. Social competence in persons with Prader-Willi, Williams, and Down's syndromes. J App Res Intell Disabil. 2004;17:209–217. [Google Scholar]
- 99.Duchaine B, et al. Normal social cognition in developmental prosopagnosia. Cogn Neuropsychol. 2010;26:620–634. doi: 10.1080/02643291003616145. [DOI] [PubMed] [Google Scholar]
- 100.Tager-Flusberg H, Sullivan K. A componential view of theory of mind: evidence from Williams syndrome. Cognition. 2000;76:59–89. doi: 10.1016/s0010-0277(00)00069-x. [DOI] [PubMed] [Google Scholar]
- 101.Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–420. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 102.Chollet F, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- 103.Cramer SC, Bastings EP. Mapping clinically relevant plasticity after stroke. Neuropharmacology. 2000;39:842–851. doi: 10.1016/s0028-3908(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 104.Warburton E, et al. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marsh EB, Hillis AE. Recovery from aphasia following brain injury: the role of reorganization. Prog Brain Res. 2006;157:143–156. doi: 10.1016/S0079-6123(06)57009-8. [DOI] [PubMed] [Google Scholar]
- 106.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 107.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 108.Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibre. Philos Trans R Soc Lond B: Biol Sci. 1850;140:423–429. [Google Scholar]
- 109.Boes AD, et al. Changes in cortical morphology resulting from long-term amygdala damage. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr047. http://dx.doi.org/10.1093/scan/nsr047. [DOI] [PMC free article] [PubMed]
- 110.Leh SE, et al. Unconscious vision: new insights into the neural correlate of blindsight using diffusion tractography. Brain. 2006;129:1822–1832. doi: 10.1093/brain/awl111. [DOI] [PubMed] [Google Scholar]
- 111.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 112.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simmons WK, Martin A. Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci. 2012;7:467–475. doi: 10.1093/scan/nsr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mars RB, et al. On the relationship between the ‘default mode network’ and the ‘social brain’. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buckner R, et al. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 117.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Klin A, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;123:1383–1391. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McKay LS, et al. Do distinct atypical cortical networks process biological motion information in adults with Autism Spectrum Disorders? Neuroimage. 2012;59:1524–1533. doi: 10.1016/j.neuroimage.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 120.Kaiser MD, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Johnston JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tyszka JM, et al. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31:15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 124.Bach DR, et al. Automatic relevance detection in the absence of a functional amygdala. Neuropsychologia. 2011;49:1302–1305. doi: 10.1016/j.neuropsychologia.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsuchiya N, et al. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Becker B, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72:70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 127.Adolphs R, et al. Recognition of facial emotion in nine subjects with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 128.Cabeza R, et al. Task-independent and task-specific age effects on brain activity during working memory, visual attention, and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 129.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davis FC, et al. Impulsitivity and the modular organization of resting-state neural networks. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs126. http://dx.doi.org/10.1093/cercor/bhs1126. [DOI] [PMC free article] [PubMed]
- 131.van Nuenen BFL, et al. Compensatory activity in the extrastriate body area of Parkinson's disease patients. J Neurosci. 2012;32:9546–9553. doi: 10.1523/JNEUROSCI.0335-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adolphs R, et al. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 133.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–64. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 134.Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 136.Dodell-Feder D, et al. fMRI item analysis in a theory of mind task. Neuroimage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 137.Saxe R. Why and how to study Theory of Mind with fMRI. Brain Res. 2006;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 138.de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:436–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 139.Lamm C, et al. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 140.Molenberghs P, et al. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 141.Rizzolatti G, Craighero L. The mirror-Neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 142.Coltheart M, Caramazza A, editors. Cognitive Neuropsychology Twenty Years On. Psychology Press; 2006. [DOI] [PubMed] [Google Scholar]
- 143.Shallice T. From Neuropsychology to Mental Structure. Cambridge University Press; 1988. [Google Scholar]
- 144.Coltheart M. Lessons from cognitive neuropsychology for cognitive Science: a reply to Patterson and Plaut. Topics Cogn Sci. 2010;2:3–11. doi: 10.1111/j.1756-8765.2009.01067.x. [DOI] [PubMed] [Google Scholar]
- 145.Barkow JH, et al., editors. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. Oxford University Press; 1992. [Google Scholar]
- 146.Pinker S. How the Mind Works. Norton; 1997. [DOI] [PubMed] [Google Scholar]
- 147.Booth R, et al. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. In: Braddick O, et al., editors. Progress in Brain Research. Academic Press; 2011. pp. 303–317. [DOI] [PubMed] [Google Scholar]
- 148.Silverman JL, et al. Behavioral phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 151.Bishop DVM. Genes, cognition and communication: insights from neurodevelopmental disorders. Ann N Y Acad Sci. 2009;1156:1–18. doi: 10.1111/j.1749-6632.2009.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Green AE, et al. Using genetic data in cognitive neuroscience: from growing pains to genuine insight. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 153.Munafo MR, et al. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathways. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Karayiorgou M, et al. The best of times, the worst of times for psychiatric disease. Nat Neurosci. 2012;15:811–812. doi: 10.1038/nn.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]