Abstract
Gliomas are highly invasive tumors and the pronounced invasive features of gliomas prevent radical surgical resection. In the search for new therapeutics targeting invasive glioma cells, in vivo-like in vitro models are of great interest. We developed and evaluated an in vivo-like in vitro model preserving the invasive features and stem cell features of glioma cells. Fluorescently labelled primary glioma spheroids and U87MG cell line-derived spheroids were implanted into organotypic rat corticostriatal slice cultures and the invasion was followed over time by confocal microscopy. The invasion was validated immunohistochemically with paraffin sections using a human-specific vimentin antibody. Moreover, the preservation of immature stem cell features was evaluated immunohistochemically using the stem cell markers CD133, Sox2, Bmi-1 and nestin. The confocal and immunohistochemical results showed that the primary glioma spheroid area was constant or decreasing after implantation, with a clear increase in the number of invading cells over time. In contrast, the U87MG spheroid area increased after implantation, with no convincing tumor cell invasion. High levels of Bmi-1 and nestin were found in all spheroids, whereas high levels of Sox2 and low to moderate levels of CD133 were only found in the primary spheroids. In conclusion, the invasion of gliomas is preserved using primary glioma spheroids. Some stem cell features are preserved as well, making this model useful in drug development elucidating both invasion and cancer stemness at the early in vitro level.
Keywords: Glioma, invasion, organotypic slice cultures, spheroids
Introduction
Gliomas are known for being highly invasive making them incurable by surgery. Furthermore, these tumors are known to be resistant towards radiation and chemotherapy. Despite aggressive surgery, radiation and chemotherapy, the median survival of the most frequent and most malignant type of glioma - the glioblastoma - is only 14.6 months [1]. Tumor cell invasion plays a crucial role for the poor prognosis by infiltrating the brain parenchyma.
In the standard treatment strategies of glioblastomas, the focus has primarily been on the bulk of the tumor mass consisting of proliferating tumor cells and not on the invading tumor cells. However, the importance of the invasive cells has become even more evident with results suggesting that treatment with the VEGF antibody bevacizumab significantly enhances invasion [2]. The aim of the present study was to develop and evaluate an in vivo-like in vitro model preserving the invasive features of glioma cells in order to facilitate research using models reflecting the clinical reality better.
In the present study we developed and evaluated an invasion model where primary organotypic spheroids derived from human gliomas as well as U87MG cell line-derived spheroids were implanted into organotypic rat brain slice cultures. We hypothesised that invasion models based on organotypic tumor tissue would be more exact models than invasion models using tumor cell lines such as the U87MG cell line and invasion substrates such as artificial gels. In the present study, we used three-dimensional primary glioma spheroids. The spheroids were obtained from one anaplastic astrocytoma (WHO grade III) and two glioblastomas (WHO grade IV). The advantage of using primary spheroids is the preservation of the three-dimensional tissue structure with the specific brain tumor cytoarchitecture and complexity including the unique extracellular matrix, blood vessels, and macrophages [3]. Thereby, cellular heterogeneity, differentiation and proliferation patterns for the individual tumors are better preserved. These primary spheroids have been proved to be a valid model providing a biological system that mimics the original tumor [3-5]. Furthermore, the close resemblance to the original tumor in situ has already led to the use of primary glioma spheroids in investigations regarding radio- and chemotherapy responsiveness [6-9]. Moreover, three-dimensional spheroids can be obtained in vitro using cell lines cultured as homogeneous spheroids, although cell line spheroids are deficient in specific brain tumor cytoarchitecture, unique extracellular matrix, blood vessels and macrophages [3,5,10,11]. Since our aim was to improve existing invasion models, U87MG spheroids were included for comparison with primary glioma spheroids. The frequently used U87MG cell line, established by Pontén and Macintyre in 1968, originates from an astrocytic tumor with necrosis [12], which corresponds to a glioblastoma multiforme according to the WHO 2007 guidelines [13]. The U87MG cell line is one of the most well-characterized cell lines with a wide range of biological information available. However, several in vivo studies indicate that the U87MG cell line have lost the invasive features characteristic for glioblastomas [14-17].
In the present study, organotypic corticostriatal rat brain slice cultures were used for implantation of spheroids as an alternative to artificial gels, hypothesising organotypic brain slice cultures to be an ideal in vivo-like matrix for studying glioma invasion in vitro. Corticostriatal slice cultures have previously been used in glioma invasion studies, since most gliomas arise in this area of the brain [18]. Furthermore, it has been suggested that glioma cells from fresh tumor fragments as well as commercial cell lines, are able to infiltrate these cultures in an in vivo-like manner [19-22]. This has been suggested studying fluorescently labelled or transfected spheroids, after the supposed invasion has occurred and without histological confirmation of the invasion. However, when using vital dyes such as DiI, cross-diffusion between cells and host cells can occur [23] and it is not known to what extend DiI cross-diffusion occurs in invasion models using organotypic brain slice cultures. In order to overcome the possible pitfall using DiI, we added a new level to this model by following the invasion over time in the same co-cultures using confocal microscopy. Furthermore, we were able to verify the invasion and characterize the tumor cells immunohistochemically by paraffin embedding and histologically sectioning of the co-cultures.
In recent years, the cancer stem cell hypothesis has come into focus in glioma research. Since the cancer stem cells are known to be highly resistant toward chemotherapy and radiation [24-29], these cells are thought to be left behind after the standard treatment. The cell surface marker CD133 has been used as one of the most important stem cell markers [27,30-37]. We therefore investigated the immunohistochemical expression of CD133 together with the well known general stem cell markers nestin [38-40], Sox2 [41] and Bmi-1 [33,42,43] in the co-cultures. We aimed to elucidate whether spheroids in this invasion model preserve these markers. Preservation of the important features of stemness, makes the model developed in the present study suitable for investigation of two of the most challenging aspects in the treatment of glioblastomas: the resistant tumor stem cells and the invading tumor cells left behind after surgery.
Materials and methods
Collection of tumor tissue
Fresh tumor tissue from three patients, who underwent initial surgery of astrocytomas WHO grade III-IV from May 2007 to June 2007 at the Department of Neurosurgery, Odense University Hospital, Denmark, was included in the study. Informed consent was obtained before surgery. All tissue samples were divided into two parts. One part of the tissue was prepared for establishing of the diagnosis according to the WHO classification of tumors of the central nervous system [13]. Moreover, this assured that the tissue was vital tumor tissue. The second part of the tissue was used for culturing in the present study. Tumor tissue from one anaplastic astrocytoma as well as two glioblastomas was obtained. The two glioblastomas will further on be referred to as glioblastoma-1 and glioblastoma-2. The Regional Scientific Ethical Committee approved the use of human tissue in the present study (approval number S-VF-20040102).
Culturing of primary spheroids
The glioma tissue was transported to the laboratory aseptically immersed in Hanks Balanced Salt Solution (Sigma Aldrich) supplemented with 0.9% D-glucose. The tumor tissue was sectioned into 2-3 mm small pieces and each piece was processed individually. Each piece was divided into two parts, as described in the section above. The tissue was sectioned manually using two scalpels to obtain small fragments of approximately 200-400 μm in diameter. The fragments were cultured in 0.75% agar-coated 12-well plates containing Dulbecco modified Eagle medium (Sigma Aldrich, Denmark) supplemented with 10% fetal calf serum (Fisher Scientific), 2% L-glutamine (Cambrex), 4% nonessential amino acids (Cambrex) and 2% penicillin/streptomycin (Cambrex) using a standard tissue culture incubator (95% humidity, 95% air, and 5% CO2). The cultured tumor fragments, confirmed to be vital, were pooled the following day and cultured for 7-10 days, until spheroids were formed. Spheroids obtained from the human glioma cell line U87MG (ECACC, UK) were cultured under the same conditions for 5 days. Medium was changed twice a week for all spheroids.
Preparation of organotypic brain slice cultures
Organotypic corticostriatal slice cultures were prepared and cultured by the interface method [44], slightly modified from Stoppini et al. [45]. The rats used in the present study were treated according to the Animal Experiments Inspectorate guidelines. In brief, newborn Wistar rats (Taconic Europe, Denmark) were decapitated. The brains were transferred to petri dishes and the meninges were removed. The brains were cut into 400 μm thick slices using a McIIwain tissue chopper. The slices were separated, divided into the two hemispheric parts, trimmed and washed in Hanks Balanced Salt Solution (Sigma Aldrich) supplemented with 0.9% D-glucose. The corticostriatal slice cultures were placed on sterile porous (0.4 μm) insert membranes (Millipore), transferred to six well plates (NUNC) with 1 ml prewarmed culture medium consisting of 50% Optimem 1 (GIBCO), 25% horse serum (GIBCO), 25% Hanks Balaced Salt Solution (HBSS, GIBCO), supplemented with 25 mM D-glucose, 1% penicillin/streptomycin (Cambrex), 3% non-essential amino acids (Cambrex) and 2% glutamine (Cambrex). The cultures were incubated using a standard tissue culture incubator (95% humidity, 95% air, and 5% CO2) at 36°C. The medium was changed twice a week.
Invasion assay
The day prior to implantation, all spheroids were labelled with the fluorescent dye DiI (1,1’ - Dioctadecyl - 3,3,3’,3’ - tetramethylindocarbocyanine iodide, Sigma-Aldrich) for 24 h. At the day of implantation the spheroids were washed in culturing medium to remove excessive DiI. Using a denudation pipette small spheroids of approximately 200-300 μm were implanted into 4 days old brain slice cultures between the cortex and striatum into or close to the corpus callosum. The co-cultures, each containing one spheroid per slice culture, were incubated using a standard tissue culture incubator (95% humidity, 95% air, and 5% CO2) at 36°C. The medium was changed twice a week.
Assessment of tumor cell invasion using confocal microscopy
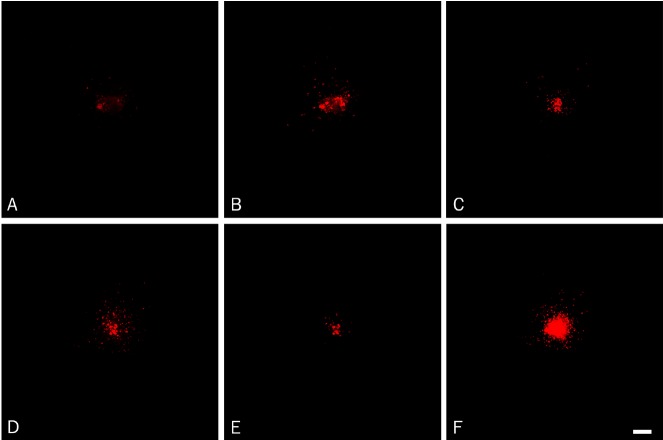
The tumor cell invasion was followed using a Nikon Eclipse TE2000-E inverted confocal microscope with perfect focus system one hour after implantation as well as three and six days after implantation. The software EZ-C1 (Nikon) was used for obtaining confocal z-stacks. Images were taken every 20 μm down through the co-culture, visualizing invasive tumor cells in the individual layers (Figure 1A-E). The images in the z-stack were superimposed into one image (Figure 1F). Hereafter the total tumor cell invasion was assessed using the program EZ-C-1 FreeViewer (Nikon). Invasive tumor cells were counted in a zone of 200 μm around the spheroid, and the diameter of the spheroids was estimated (Figure 2).
Figure 1.
Tumor cell invasion was followed over time using confocal microscopy. Images were taken every 20 μm down through the co-culture, visualizing invasive tumor cells in the individual layers (A-E). When investigating the individual images in the z-stack obtained from glioblastoma-1 at day 6, invading cells were seen in almost all layers (A-E) with only few invading cells at the top (A) and at the bottom (E) of the spheroids suggesting pronounced tumor cell invasion inside the brain slice cultures (B-D). The images in the z-stack were superimposed into one image (F), visualizing all invasive tumor cells. Scalebar 100 μm.
Figure 2.
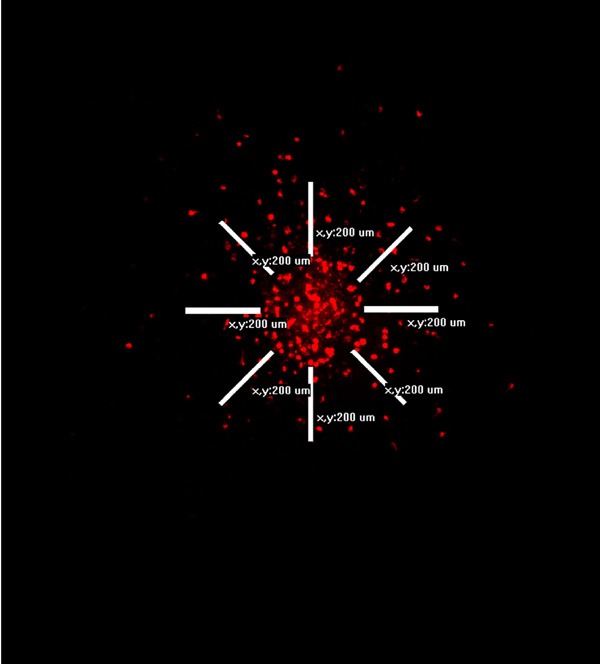
The images in the confocal z-stacks were superimposed into one image, visualizing all invasive tumor cells. Hereafter the total tumor cell invasion was assessed using the program EZ-C1 FreeViewer (Nikon). Invasive tumor cells were counted in a 200 μm zone around the spheroid, and the diameter of the spheroids was estimated.
Immunohistochemistry
At the end of the experiment, the co-cultures were fixed, paraffin-embedded and sections of 3 μm were cut using a microtome. One section was used for hematoxylin eosine staining and the adjacent sections were used for immunohistochemical (IHC) staining. The IHC staining was performed on a Dako autostainer, Universal Staining System. Paraffin sections were deparaffinised and heat-induced epitope retrieval was performed in a T-EG buffer (10 mmol/L Trisbase and 0.5 mmol/L EGTA). After blocking of endogenous peroxidase using 1.5% hydrogen peroxide (H2O2), the sections were incubated for 60 minutes with antibodies against Vimentin (Nordic Biosite, 1 + 200), Ki67 (Dako, 1 + 200), Sox2 (R&D Systems, 1 + 400), Bmi-1 (Upstate, 1 + 400), and nestin (R&D Systems, 1 + 3000). Detection was performed using EnVision (Dako) and diaminobenzidine (DAB) as chromogene. CD133 staining was performed as described in Christensen et al. [37]. Finally, the sections were counterstained with Mayer’s hematoxylin and cover slips were mounted with Aquatex.
Statistical analysis
Data are expressed as mean + standard error of mean (SEM). Mean values of spheroid diameter and density of invading cells were compared using analysis of variance (ANOVA) with Bonferroni correction. Statistical analyses were performed using GraphPad Instat (GraphPad Software, San Diego, CA, USA). Differences were considered significant at P < 0.05.
Results
Invasion model
We successfully implanted spheroids from one anaplastic astrocytoma (WHO grade III) and two glioblastomas (WHO grade IV), as well as U87MG spheroids into corticostriatal brain slice cultures. Confocal z-stacks were obtained at the day of implantation as well as day three and day six after implantation (Figures 1 and 3). The images in each z-stack (Figure 1A-E) were superimposed into one image representing one spheroid (Figure 1F).
Figure 3.
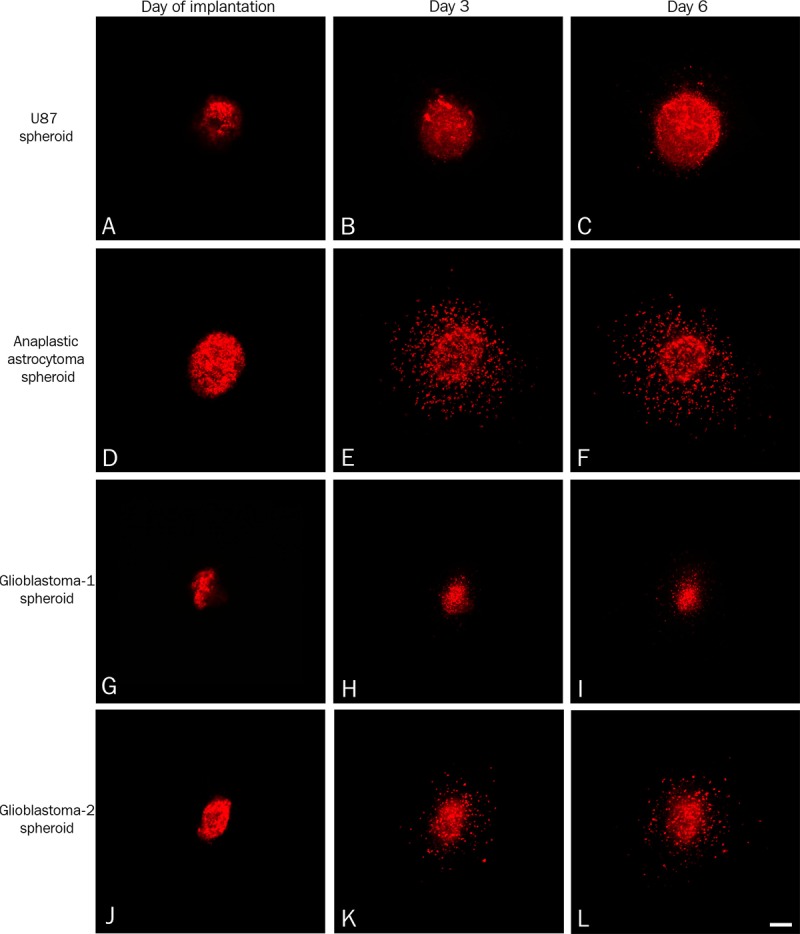
The U87MG cell line-derived spheroids (A-C) and primary spheroids (D-L) were labelled with the vital fluorescent dye DiI and implanted into 4 days old brain slice cultures between the cortex and striatum into or close to the corpus callosum. The tumor cell invasion was followed using confocal microscopy at the day of implantation (A, D, G, J), after three days (B, E, H, K) and after six days (C, F, I, L). For the U87MG cell line-derived spheroids only a few fluorescent cells were detected close to the spheroid at day three (B) and day six (C). In contrast to U87MG spheroids pronounced invasion was observed for the primary spheroids derived from the anaplastic astrocytoma (E, F) and glioblastoma-2 (K, L), whereas invasion appeared to be limited for glioblastoma-1 (H, I). Scalebar 100 μm.
The area of the U87MG cell line-derived spheroids (n = 16) increased rapidly over time (Figures 3A-C and 4A). When implanting primary spheroids derived from an anaplastic astrocytoma (n = 23) and from glioblastoma-1 (n = 13) and glioblastoma-2 (n = 5), the spheroid area did not increase significantly (Figure 4C, 4E and 4G). In fact, a small decrease was seen for glioblastoma-1 spheroids (Figure 4E).
Figure 4.
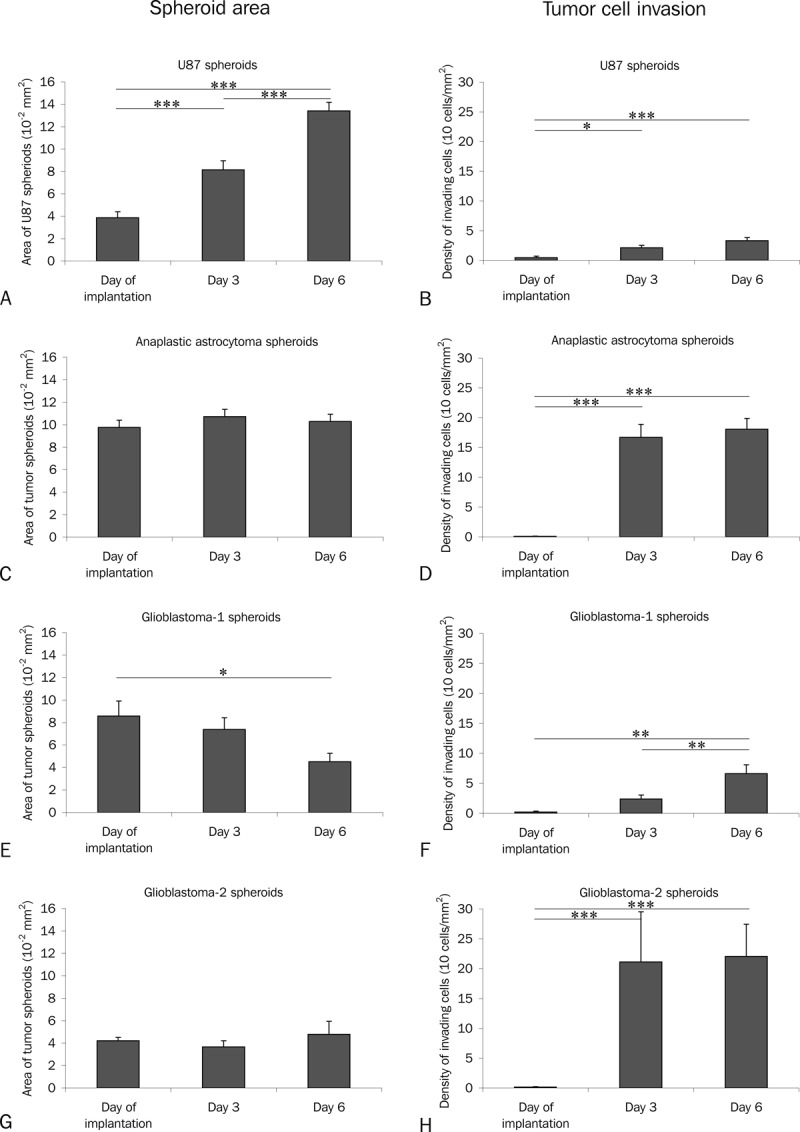
Spheroid area was measured (A, C, E, G) and invasive cells were counted (B, D, F, H) on superimposed confocal z-stack images of U87MG spheroids (A, B) and primary spheroids (C-H). The area of the U87MG spheroids increased rapidly over time (A). When implanting primary spheroids derived from an anaplastic astrocytoma (C) and from two glioblastomas (E, G) the area of the spheroids did not increase significantly (C, E, G). In fact, a small decrease was seen for glioblastoma-1 spheroids (E). For the U87MG spheroids only a few invading cells were detected (B). In contrast to U87MG pronounced invasion was observed for the anaplastic astrocytoma (D) and glioblastoma-2 (H), whereas invasion appearently was limited for glioblastoma-1 (H, I). Scalebar 100 μm.
When investigating the individual images in the z-stacks, invading cells were seen in almost all layers (Figure 1A-E) with only few invading cells at the top (Figure 1A) and the bottom (Figure 1E) of primary spheroids suggesting the presence of tumor cell invasion inside the brain slice cultures (Figure 1B-D). The individual images in the z-stack were superimposed into one image (Figure 1F), where the total tumor cell invasion was estimated in a zone of 200 μm around the spheroid (Figure 2). For the U87MG spheroids only a few invading cells were detected (Figure 4B). In contrast to U87MG spheroids (Figure 4B) pronounced invasion was observed for the primary spheroids derived from the anaplastic astrocytoma and glioblastoma-2 (Figure 4D and 4H). The invasion observed for glioblastoma-1 derived spheroids was partly increased compared to U87 (Figure 4F).
Detection of the invading tumor cells in paraffin sections of co-cultures
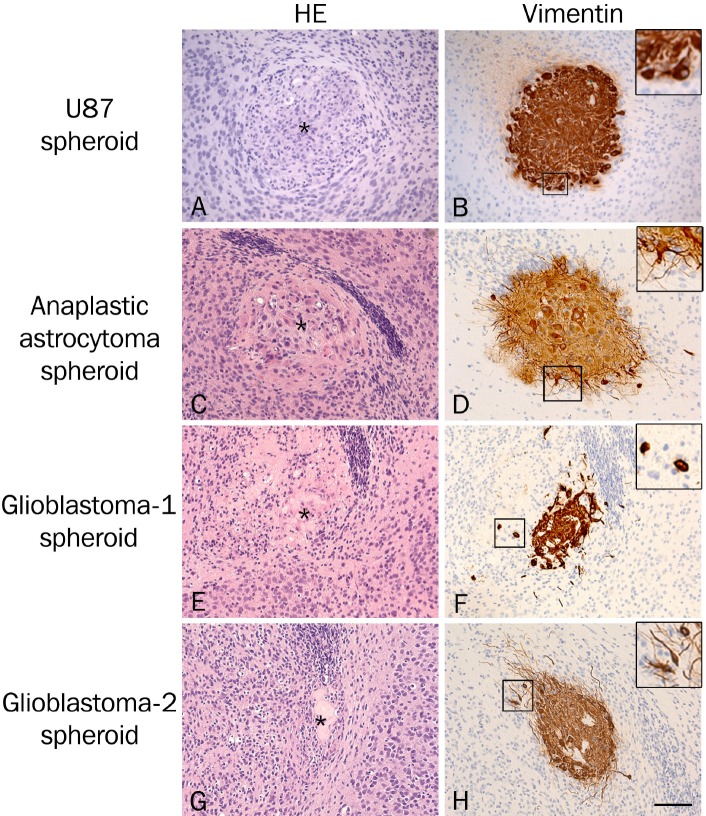
Hematoxylin and eosin-stained paraffin sections of the co-cultures revealed viable brain slice cultures with a well preserved cytoarchitecture and viable tumor spheroids with a high tumor-like cell density (Figure 5A, 5C, 5E and 5G). In general, the border between the brain tissue and the U87MG spheroids (Figure 5A) was more well-defined than the border between the brain tissue and the primary spheroids (Figure 5C, 5E and 5G). Immunohistochemical staining with a human specific vimentin antibody was performed in order to visualize and confirm the invasion observed by confocal microscopy (Figure 5B, 5D, 5F and 5H). All tumor cells in the spheroids appeared to express high levels of vimentin suggesting this marker to be suitable for identifying human cells in the spheroids as well as the invasive tumor cells. Only few invasive tumor cells were seen close to the implanted U87MG spheroids (Figure 5B) confirming the results obtained by confocal microscopy (Figure 3A-C). The invasion observed with confocal microscopy when implanting primary spheroids (Figure 3D-L) was confirmed for the anaplastic astrocytoma (Figure 5D) and for glioblastoma-1 and glioblastoma-2 (Figure 5F and 5H). Two types of invasive cells were seen surrounding the primary spheroids including small round tumor cells (Figure 5F) as well as long fusiform tumor cells (Figure 5H). The majority of the invading cells were seen near the spheroids, but some tumor cells were also seen several mm from the implanted spheroids. It is important to keep in mind that the invasion seen in one tissue section corresponds to the invasion seen in the individual confocal layers and not the invasion seen in the superimposed images (Figure 1).
Figure 5.
Hematoxylin and eosin-stained paraffin sections of co-cultures revealed viable brain slice cultures with a well preserved cytoarchitecture and viable tumor spheroids with a high tumor-like cell density (A, C, E, G). Immunohistochemical staining with a human specific vimentin antibody (B, D, F, H) confirmed the invasion observed by confocal microscopy. Only few invasive tumor cells were seen around the implanted U87MG cell line-derived spheroids (B) in contrast to a more pronounced invasion seen around the primary spheroids (D, F, H), where small round tumor cells (F) as well as long fusiform tumor cells (H) were seen. Spheroids are marked with *(A, C, E, G). Scalebar 200 μm.
Proliferation in the spheroids
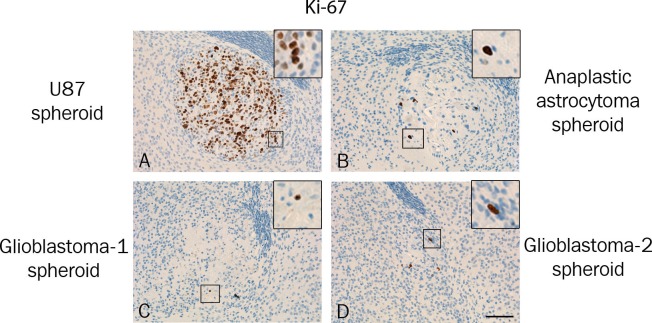
Ki-67 immunohistochemical staining identified several proliferating cells in all U87MG spheroids (Figure 6A), whereas much fewer proliferating cells were identified in the primary spheroids (Figure 6B-D). Positive cells were observed at the border of some spheroids, but very few Ki-67 positive cells were detected in the invasion zone of the primary spheroids.
Figure 6.
Ki-67 immunohistochemically stained paraffin sections revealed a high level of proliferating cells in all U87MG spheroids (A). The Ki-67 expression in the primary spheroids (B-D) was considerably lower compared to U87MG (A). Scalebar 200 μm.
Expression of stem cell markers in the co-cultures
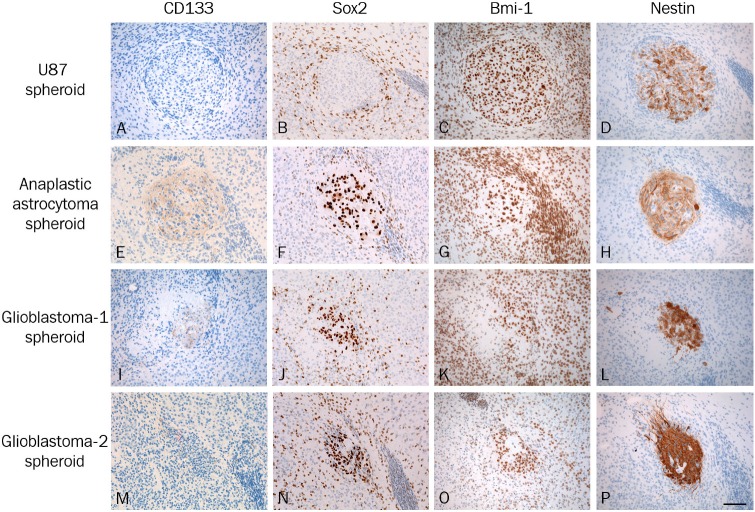
We further evaluated the co-cultures immunohistochemically for the expression of CD133, the early transcription factors Sox2 and Bmi-1 as well as nestin. The expression of CD133 was in general not detected in U87MG spheroids (Figure 7A) whereas some CD133 staining was detected in the primary spheroids (Figure 7E, 7I and 7M).
Figure 7.
The co-cultures were investigated immunohistochemically using the putative cancer stem cell marker CD133 (A, E, I, M), the early transcription factors Sox2 (B, F, J, N) and Bmi-1 (C, G, K, O) as well as nestin (D, H, L, P). Using paraffin sections the expression of CD133 was in general not detected in U87MG spheroids (A) whereas some CD133 staining was detected in the primary spheroids (E, I, M). Sox2 was not detected in the U87MG spheroids (B), whereas high levels of Sox2 were seen in all primary spheroids (F, J, N). All tumor cells expressed Bmi-1 in the U87MG cell line-derived spheroids (C) as well as in the primary spheroids (G, K, O). The majority of the tumor cells in all spheroids were nestin positive (D, H, L, P), though differences in staining intensity were seen between spheroids from the same tumor. In general, the lowest staining intensity was seen in U87MG derived spheroids (D), whereas higher staining intensities were seen in the primary glioblastoma spheroids (H, L, P). Scalebar 200 μm.
The early transcription factor Sox2 was not detected in the U87MG spheroids (Figure 7B), whereas high levels of Sox2 were seen in all primary spheroids (Figure 7F, 7J and 7N). In addition, Sox2 positive cells were detected in the rat brain tissue as well as in the neuroepithelial cells lining the ventricles, but the staining intensity was lower than the intensity detected in the spheroids. Accordingly, Sox2 expression could not be allocated with certainty to invasive tumor cells.
All tumor cells expressed Bmi-1 in the U87MG spheroids as well as in the primary spheroids (Figure 7C, 7G, 7K and 7O). Furthermore, Bmi-1 was widely expressed in the slice cultures including the neuroepithelial cells making possible allocation of Bmi-1 to invasive cells impossible.
The majority of the tumor cells in all spheroids were nestin positive, though differences in staining intensity were seen between spheroids from the same tumor. In general, the lowest staining intensity was seen in U87MG spheroids (Figure 7D), whereas the highest staining intensities were seen in the primary glioblastoma spheroids (Figure 7L and 7P). The tumor cells at the border of the U87 spheroids were nestin negative, although the tumor cells were positive using vimentin (Figures 5B and 7D). For the primary spheroids, all tumor cells in the spheroids as well as invasive tumor cells were nestin positive.
Discussion
In the present study we developed and evaluated an in vivo-like in vitro invasion model, where human primary glioma spheroids were implanted into corticostriatal rat brain slice cultures. We added new levels to this model by following tumor cell invasion over time using confocal microscopy and by verifying the invasion in paraffin sections using immunohistochemistry, which has not been done before. We showed that only the primary tumor cells invade the brain tissue mimicking the tumor in situ, in contrast to the cell line-derived tumor cells. Moreover, we showed that the invasion model can be used in cancer stem cell research.
Assessment of invasion
In the present study we were able to follow and quantify the spheroid area and tumor cell invasion into living brain slice cultures over time using confocal microscopy, which has not been done before. In most other similar invasion studies, the tumor cell invasion has to our knowledge been investigated at the end of the experiments on fixed co-cultures, and not at different time points during the process of invasion in the same individual co-cultures [19,21,22,46]. The increases in spheroid area over time have therefore not been observed before. To our knowledge one similar study with confocal microscopy has been performed, where T98G glioblastoma cell line spheroids were placed on top of organotypic brain slice cultures prepared from two days old rats [20]. The authors found tumor cell migration on top of the culture as well as diffuse invasion into the slice cultures using confocal microscopy. After following the invasion for 72 hours, the cultures were fixed and the immunohistochemical expression of matrix matalloproteinase-2 and -9 were investigated on whole mounts.
In the present study we added a new level to the model, since we were able to paraffin-embed the co-cultures and investigate the co-cultures immunohistochemically using thin histological sections. This procedure allows us to further investigate the spheroids and invasive tumor cells in terms of both morphology and expression of several markers using adjacent histological sections. An important point was confirmation of the invasion detected by confocal microscopy, since it is known that the fluorescent dye DiI, used for spheroid labelling in invasion assays, can diffuse into adjacent cells [23] possibly resulting in false positively labelled rat brain cells, which can then be mistaken for being invasive tumor cells. In order to identify invasive tumor cells we established the human specific vimentin staining - a strategy which has not been used before. The immunohistochemical results confirmed the results obtained by confocal microscopy. For the U87MG spheroids, the presence of invasive cells located far from the spheroids in the confocal scannings (Figure 3B and 3C) could not be confirmed by immunohistochemistry (Figure 5B), suggesting that this may be an artefact induced e.g. by the implantation procedure. Interestingly, the vimentin staining could be used to visualize cell morphology of the invading tumor cells revealing the presence of fusiform cells as well as small round cells. The U87MG cells at the spheroid border zone only showed the round morphology. Another human specific marker, nestin, was used as part of the cancer stem cell marker panel. However, nestin was not expressed by all U87MG cells thereby suggesting vimentin to be a better marker for tumor cell identification.
In contrast to primary spheroids, the U87MG spheroids seem to have lost the invasive features characterising the glioblastomas in patients. However, our results are in line with several in vivo studies using tumor biopsies as well as the U87MG cell line for invasion studies [14-17]. In a study by Engebraaten et al. primary spheroids prepared from glioblastoma biopsies were implanted into the brains of nude rats and in this model they behaved as in the patients with varying degrees of invasion. However, when implanting U87MG spheroids, the authors observed that the spheroids maintained their localization at the injection site and showed expanding growth with minimal invasion [14]. Whether U87MG cells in general have lost the ability to invade healthy brain tissue is controversial. In contrast to our findings Jung et al. [46] showed that U87MG cells transfected with a GFP plasmid were able to invade brain slice cultures. However, similar to the present study, the study by Jung et al. would have benefitted from immunohistochemical confirmation of the results since green auto-fluorescence could be a problem in some in vitro assays.
Proliferation in the spheroids
In the present study we evaluated the proliferation of the tumor cells using Ki-67 immunohistochemistry. Not surprisingly, we found pronounced proliferation in U87MG spheroids corresponding to the increase in diameter over time observed for these spheroids. The high level of proliferating cells may be closely associated with the low number of invasive cells as suggested by the ‘Go or Grow’ hypothesis, which has been proposed in a study based on observations of astrocytoma cells rarely dividing while moving [47]. The hypothesis suggests that cell division and cell migration are temporally exclusive phenomena, and tumor cells defer proliferation for cell migration. The hypothesis is based on the concept that a cell is unlikely to commit its cytoskeletal and genetic machinery to both cell division and migration concurrently [47]. Since the U87MG cells undergo more frequent mitosis, they are therefore not supposed to invade the slice culture as fast as the tumor cells in the primary spheroids undergoing fewer divisions. Moreover, the low proliferation and pronounced invasion observed for the primary spheroids could explain the rather constant or decreasing spheroid diameter observed in the present study. In general, the proliferative activity in glioblastomas is usually prominent (15-20%), but varies significantly between tumors [13]. Furthermore, glioblastomas are known for being widely heterogeneous and regional heterogeneity in terms of proliferation is also observed. The low proliferation observed in the primary spheroids in the present study, could be partly explained by this heterogeneity. However, a previous study performed in our laboratory demonstrated that culturing of primary spheroids in conventional serum-containing medium, causes decreased proliferation in the spheroids compared to the original biopsies [48]. All together the results points to primary spheroids having a reduced proliferative capacity, but on the other hand, they have the ability to invade organotypic brain slice cultures.
Expression of cancer stem cell-related markers
We further evaluated the co-cultures immunohistochemically using putative cancer stem cell markers including CD133, Sox2 and Bmi-1 as well as nestin, since several studies have demonstrated these markers to be important in glioma tumor stem cell biology [24,31,32,41,49]. The expression of CD133 varied between spheroids from the same tumor as well as between tumors, demonstrating the heterogeneity of the spheroids. The heterogenic expression of CD133 is in line with previous findings describing CD133 positive cells to be present in tumor cell niches of various sizes as well as dispersed single cells [36,37]. The three markers Sox2, Bmi-1 and nestin were expressed abundantly in all primary spheroids suggesting that the cancer stem cell phenotype is preserved in implanted spheroids. These results are in line with an earlier study performed in our laboratory, where the expression of CD133, Sox2, Bmi-1 and nestin in primary organotypic glioma spheroids was found to be preserved in serum-containing medium, although a small decrease in CD133 and Sox2 levels was seen in spheroids cultured in serum-containing medium compared to serum-free medium. The high levels of Sox2, Bmi-1 and nestin are in contrast to a study by Lee et al. [16], where a dramatic decrease in Sox2, Bmi-1 and nestin was seen, when primary glioma single cells, obtained from dissociated glioma biopsies, were cultured in conventional serum-containing medium compared to serum-free medium. The differences could be partly explained by the fact that Lee et al. cultured the tissue as single cells and not as small organotypic tumor fragments, which due to the presence of intercellular connections and blood vessels may be capable of preserving the cancer stem cell phenotype even in serum-containing medium. Taken together, the results presented in the present study suggest that preservation of the cancer stem cell phenotype together with the invasive properties, qualify this model for being used in studies focussing on these important aspects in the development of new anti-cancer strategies.
Immunohistochemical expression of Sox2 and Bmi-1 was in addition detected in cells of the rat cortex, suggesting developmental immaturity of the brain tissue. In the present study, we prepared brain slice cultures from newborn rats and cultured the slice cultures for four days prior to implantation. In order to obtain more mature and in vivo-like conditions, it could be considered to culture the brain slice cultures longer before implantation of spheroids or to use brain slice cultures from adult rats [50-52].
Viability of spheroids
Before the present invasion study, we performed a pilot study where we collected, dissected and implanted fresh tumor tissue directly into the brain slice cultures without prior culturing. However, this protocol led to a very high variability between co-cultures from the same tumor. Speculating that a main reason for this variability was due to necrosis, we there fore moved on to free floating primary organotypic spheroids, which have been shown to be a valid tumor model providing a biological system that mimics the original glioma in patients [3]. The advantage of using this method is that only vital fragments form spheroids, thereby leaving only vital spheroids for implantation. Moreover, in the present study we dissected the tumor into small pieces of a maximum of 5 mm. One part of these pieces was prepared for hematoxylin eosine staining in order to confirm the diagnosis and make it possible to distinguish between viable tumor tissue, necrotic areas or tissue obtained from the tumor invasion zone. Jung et al. [22] and Palfi et al. [19] did not culture the tissue prior to implantation but labelled freshly removed glioma fragments with vital fluorescent dye for 24-48 h and implanted them directly into brain slice cultures. However, in our hands the use of primary organotypic spheroids led to a more reproducible assay and a more flexible procedure not being dependent on having a whole slice culture setup ready at the time of surgery.
Conclusion
In conclusion, we demonstrated that implantation of primary glioma spheroids into rat brain slice cultures is a valid and feasible in vivo-like in vitro model for glioma invasion studies. For the first time, we added new levels to this model by following tumor cell invasion over time and by confirming the invasion using immunohistochemistry. As an in vivo-like model of invasion preserving stem cell features, implantation of primary spheroids into rat brain slice cultures seems to be a model suitable for drug development incorporating both invasion and cancer stemness at the early in vitro level.
Acknowledgments
The excellent laboratory work of Tanja Dreehsen Højgaard and Helle Wohlleben is gratefully acknowledged. This work was supported by the Danish Cancer Society, Danish Medical Research Council, Kathrine and Vigo Skovgaard’s Foundation, The Cancer Foundation, Johs. Clemmesen’s Research Foundation, Hørslev Foundation, Memorial Foundation for Alice Brenaa, Merchant M. Kristian Kjær and wife Margrethe Kjær born la Cour-Holmen’s Foundation, Eva and Henry Frænkel’s Memorial Foundation, and Merchant M. Brogaard and wife’s Memorial Foundation.
Declaration of conflict of interest
All authors declare that no conflicts of interest exist.
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, Miletic H, Wang J, Stieber D, Stuhr L, Moen I, Rygh CB, Bjerkvig R, Niclou SP. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerkvig R, Tonnesen A, Laerum OD, Backlund EO. Multicellular tumor spheroids from human gliomas maintained in organ culture. J Neurosurg. 1990;72:463–475. doi: 10.3171/jns.1990.72.3.0463. [DOI] [PubMed] [Google Scholar]
- 4.De Witt Hamer PC, Jonker A, Leenstra S, Ruijter JM, Van Noorden CJ. Quantification of viability in organotypic multicellular spheroids of human malignant glioma using lactate dehydrogenase activity: a rapid and reliable automated assay. J Histochem Cytochem. 2005;53:23–34. doi: 10.1177/002215540505300104. [DOI] [PubMed] [Google Scholar]
- 5.Kaaijk P, Troost D, Das PK, Leenstra S, Bosch DA. Long-term culture of organotypic multicellular glioma spheroids: a good culture model for studying gliomas. Neuropathol Appl Neurobiol. 1995;21:386–391. doi: 10.1111/j.1365-2990.1995.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaaijk P, Troost D, Sminia P, Hulshof MC, van der Kracht AH, Leenstra S, Bosch DA. Hypofractionated radiation induces a decrease in cell proliferation but no histological damage to organotypic multicellular spheroids of human glioblastomas. Eur J Cancer. 1997;33:645–651. doi: 10.1016/s0959-8049(96)00503-5. [DOI] [PubMed] [Google Scholar]
- 7.Sminia P, Acker H, Eikesdal HP, Kaaijk P, Enger P, Slotman B, Bjerkvig R. Oxygenation and response to irradiation of organotypic multicellular spheroids of human glioma. Anticancer Res. 2003;23:1461–1466. [PubMed] [Google Scholar]
- 8.Fehlauer F, Muench M, Rades D, Stalpers LJ, Leenstra S, van der Valk P, Slotman B, Smid EJ, Sminia P. Effects of irradiation and cisplatin on human glioma spheroids: inhibition of cell proliferation and cell migration. J Cancer Res Clin Oncol. 2005;131:723–732. doi: 10.1007/s00432-005-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehlauer F, Muench M, Smid EJ, Slotman B, Richter E, Van der Valk P, Sminia P. Combined modality therapy of gemcitabine and irradiation on human glioma spheroids derived from cell lines and biopsy tissue. Oncol Rep. 2006;15:97–105. [PubMed] [Google Scholar]
- 10.Fehlauer F, Stalpers LJ, Panayiotides J, Kaaijk P, Gonzalez Gonzalez D, Leenstra S, van der Valk P, Sminia P. Effect of single dose irradiation on human glioblastoma spheroids in vitro. Oncol Rep. 2004;11:477–485. [PubMed] [Google Scholar]
- 11.Lund-Johansen M, Engebraaten O, Bjerkvig R, Laerum OD. Invasive glioma cells in tissue culture. Anticancer Res. 1990;10:1135–1151. [PubMed] [Google Scholar]
- 12.Ponten J, Macintyre EH. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74:465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 13.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumors of the Central Nervous System. International Agency for Research on Cancer (IARC); 2007. [Google Scholar]
- 14.Engebraaten O, Hjortland GO, Hirschberg H, Fodstad O. Growth of precultured human glioma specimens in nude rat brain. J Neurosurg. 1999;90:125–132. doi: 10.3171/jns.1999.90.1.0125. [DOI] [PubMed] [Google Scholar]
- 15.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, Gao L, Wang JM, Bian XW. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008;265:124–134. doi: 10.1016/j.canlet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Ironside JW, Moss TH. Astrocytic tumors. 2002. [Google Scholar]
- 19.Palfi S, Swanson KR, De Bouard S, Chretien F, Oliveira R, Gherardi RK, Kros JM, Peschanski M, Christov C. Correlation of in vitro infiltration with glioma histological type in organotypic brain slices. Br J Cancer. 2004;91:745–752. doi: 10.1038/sj.bjc.6602048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura H, Ohnishi T, Kanemura Y, Maruno M, Yoshimine T. Quantitative analysis of glioma cell invasion by confocal laser scanning microscopy in a novel brain slice model. Biochem Biophys Res Commun. 2000;269:513–520. doi: 10.1006/bbrc.2000.2332. [DOI] [PubMed] [Google Scholar]
- 21.de Bouard S, Christov C, Guillamo JS, Kassar-Duchossoy L, Palfi S, Leguerinel C, Masset M, Cohen-Hagenauer O, Peschanski M, Lefrancois T. Invasion of human glioma biopsy specimens in cultures of rodent brain slices: a quantitative analysis. J Neurosurg. 2002;97:169–176. doi: 10.3171/jns.2002.97.1.0169. [DOI] [PubMed] [Google Scholar]
- 22.Jung S, Kim HW, Lee JH, Kang SS, Rhu HH, Jeong YI, Yang SY, Chung HY, Bae CS, Choi C, Shin BA, Kim KK, Ahn KY. Brain tumor invasion model system using organotypic brainslice culture as an alternative to in vivo model. J Cancer Res Clin Oncol. 2002;128:469–476. doi: 10.1007/s00432-002-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoshyomn S, Penar PL, McBride WJ, Taatjes DJ. Four-dimensional analysis of human brain tumor spheroid invasion into fetal rat brain aggregates using confocal scanning laser microscopy. J Neurooncol. 1998;38:1–10. doi: 10.1023/a:1005758626348. [DOI] [PubMed] [Google Scholar]
- 24.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 25.Chargari C, Moncharmont C, Levy A, Guy JB, Bertrand G, Guilbert M, Rousseau C, Vedrine L, Alphonse G, Toillon RA, Rodriguez-Lafrasse C, Deutsch E, Magne N. Cancer stem cells, cornerstone of radioresistance and perspectives for radiosensitization: glioblastoma as an example. Bull Cancer. 2012;99:1153–60. doi: 10.1684/bdc.2012.1666. [DOI] [PubMed] [Google Scholar]
- 26.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–456. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven MC, Hainfellner JA, Heppner FL, Dietrich PY, Zimmer Y, Cairncross JG, Janzer RC, Domany E, Delorenzi M, Stupp R, Hegi ME. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 30.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 31.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 32.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 33.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 35.Kang MK, Hur BI, Ko MH, Kim CH, Cha SH, Kang SK. Potential identity of multi-potential cancer stem-like subpopulation after radiation of cultured brain glioma. BMC Neurosci. 2008;9:15. doi: 10.1186/1471-2202-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen K, Schrøder HD, Kristensen BW. CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol. 2011;104:129–143. doi: 10.1007/s11060-010-0488-y. [DOI] [PubMed] [Google Scholar]
- 37.Christensen K, Schrøder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol. 2008;90:157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 38.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 39.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. discussion 143-134. [DOI] [PubMed] [Google Scholar]
- 40.Maderna E, Salmaggi A, Calatozzolo C, Limido L, Pollo B. Nestin, PDGFRbeta, CXCL12 and VEGF in glioma patients: different profiles of (pro-angiogenic) molecule expression are related with tumor grade and may provide prognostic information. Cancer Biol Ther. 2007;6:1018–1024. doi: 10.4161/cbt.6.7.4362. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, Bachmann M, Rieber EP, Weigle B. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007;96:1293–1301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, van Lohuizen M, Betticher DC. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–1376. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristensen BW, Noraberg J, Jakobsen B, Gramsbergen JB, Ebert B, Zimmer J. Excitotoxic effects of non-NMDA receptor agonists in organotypic corticostriatal slice cultures. Brain Res. 1999;841:143–159. doi: 10.1016/s0006-8993(99)01833-8. [DOI] [PubMed] [Google Scholar]
- 45.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 46.Jung S, Ackerley C, Ivanchuk S, Mondal S, Becker LE, Rutka JT. Tracking the invasiveness of human astrocytoma cells by using green fluorescent protein in an organotypical brain slice model. J Neurosurg. 2001;94:80–89. doi: 10.3171/jns.2001.94.1.0080. [DOI] [PubMed] [Google Scholar]
- 47.Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67:275–282. doi: 10.1002/(SICI)1097-0215(19960717)67:2<275::AID-IJC20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Christensen K, Aaberg-Jessen C, Andersen C, Goplen D, Bjerkvig R, Kristensen BW. Immunohistochemical expression of stem cell, endothelial cell, and chemosensitivity markers in primary glioma spheroids cultured in serumcontaining and serum-free medium. Neurosurgery. 2010;66:933–947. doi: 10.1227/01.NEU.0000368393.45935.46. [DOI] [PubMed] [Google Scholar]
- 49.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 50.Noraberg J, Kristensen BW, Zimmer J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res Brain Res Protoc. 1999;3:278–290. doi: 10.1016/s1385-299x(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 51.Benardete EA, Bergold PJ. Genomic analysis of ischemic preconditioning in adult rat hippocampal slice cultures. Brain Res. 2009;1292:107–122. doi: 10.1016/j.brainres.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 52.Hassen GW, Tian D, Ding D, Bergold PJ. A new model of ischemic preconditioning using young adult hippocampal slice cultures. Brain Res Brain Res Protoc. 2004;13:135–143. doi: 10.1016/j.brainresprot.2004.03.004. [DOI] [PubMed] [Google Scholar]