Abstract
Background: Vascular endothelial growth factor (VEGF)-A and VEGF-C are two important molecules involving in tumor development and metastasis via angiogenesis and lymphangiogenesis. However, the combined effect of VEGF-A and VEGF-C on the growth of gastric cancer (GC) is not clear. Methods: The correlations of VEGF-A and VEGF-C expressions with clinicopathologic parameters and prognosis were evaluated in patients with GC. Furthermore, lentivirus-mediated RNA interfering (RNAi) targeting VEGF-A and/or VEGF-C was employed to silence their expressions in SGC7901 GC cell line. Cell proliferation and apoptosis were measured in vitro. Suppressive effect lentivirus-mediated VEGF-A and/or VEGF-C silencing on GC growth was evaluated in GC bearing mice. Results: The patients with high expression of both VEGF-A and VEGF-C (A+C+) had larger tumor size, higher peritumoral lymphatic vessel density(P-LVD), microvessel density(MVD), lymphatic vessel invasion (LVI), lymph node(LN) metastasis, and worse prognosis than those with low expression of both VEGF-A and VEGF-C (P<0.05). Lentivirus-mediated RNAi significantly reduced the mRNA and protein expression of VEGF-A and VEGF-C in the SGC7901 cells. The Lenti-miRNA-VEGF-A+VEGF-C significantly inhibited the cell proliferation and tumor growth, compared with Lenti-miRNA-VEGF-A or Lenti-miRNA-VEGF-C (P<0.05). In addition, Lenti-miRNA- VEGF-A+VEGF-C markedly lowered the tumor size in vivo in comparison with Lenti-miRNA-VEGF-A or Lenti-miRNA–VEGF-C (P<0.05). Conclusion: Expressions of both VEGF-A and VEGF-C predict worse prognosis of GC patients. Combined silencing of VEGF-A and VEGF-C markedly suppresses cancer growth than silencing of VEGF-A or VEGF-C. Thus, to inhibit the expressions of VEGF-A and VEGF-C may become a novel strategy for the treatment of GC.
Keywords: Vascular endothelial growth factor-A, vascular endothelial growth factor-C, tumor growth, prognosis, gastric cancer
Introduction
Although the incidence of gastric cancer (GC) is decreasing, it is still a leading cause of cancer related death in China, which is usually attributed to its metastasis via lymph vessels and/or blood vessels.
Tumor-related angiogenesis has been proved to be a prerequisite for the growth and progression of solid malignancies. Vascular endothelial growth factor (VEGF)-A is considered as a most potent factor in the angiogenesis through activating receptor tyrosine kinases, VEGF receptor-1 (VEGFR-1) and VEGFR-2 [1]. In GC patients, over-expression of VEGF-A is correlated with increase in microvessel density (MVD), hematogenous metastasis, peritoneal dissemination and poor prognosis [2,3]. However, although VEGF-A has been found to induce the lymphatic growth in the avascular cornea and promote the lymph node metastasis via VEGF-C/-D/VEGFR-3-independent pathway in animals [4], its role in the lymphangiogenesis still remains undetermined in human cancers.
In the past few years, many studies have shown that tumor-induced lymphangiogenesis, driven by the lymphangiogenic growth factors (such as VEGF-C and/or VEGF-D) via VEGFR-3 signaling, promotes the regional lymph node metastasis [5-7]. The increase in VEGF-C expression has the positive correlation with lymphatic invasion, lymphatic vessel density, lymph node metastasis, and prognosis of many human cancers including GC [8-10]. However, a study showed the expression of VEGF-A or VEGF-C alone is not an independent prognostic marker for patients with surgically resected gastric adenocarcinoma [11].
Recently, the anti-angiogenesis therapy (bevacizumab) as an adjunct to chemotherapy has been applied in patients with advanced GC [12]. Therefore, we speculate that simultaneous inhibition of VEGF-A and VEGF-C may reduce cancer growth, progression and lymphatic metastasis. However, most of previous studies in GC just focused the role of either VEGF-A or VEGF-C in the biological behaviors of GC. Our previous study showed that some of the GC patients have high expressions of both VEGF-A and VEGF-C, who present with higher potential to induce lymph node metastasis, lymphatic vessel invasion (LVI), vascular invasion (VI), high MVD and poorer survival, compared with those having high expressions of both VEGF-C and VEGF-D, or both VEGF-A and VEGF-D [13]. However, the significance of the different expression status of VEGF-A and VEGF-C expression, i.e. both expressions of VEGF-A and VEGF-C, compared with only VEGF-A expression, or VEGF-C expression, in the GC is still unknown.
In this study, the correlations of VEGF-A and VEGF-C expressions with clinicopathologic parameters and prognosis were evaluated in patients with GC. Furthermore, lentivirus-mediated RNA interfering (RNAi) targeting VEGF-A and/or VEGF-C was employed to silence their expressions in SGC7901 GC cell line. The influence of VEGF-A and/or VEGF-C on the biological behaviors of GC cells was assessed.
Materials and methods
Patients and sample collection
Cancer specimens were obtained from 123 patients with primary GC who received gastrectomy in the Department of Surgery, Tongji Hospital of Tongji University from January 2000 to December 2003. None of them had received preoperative chemotherapy or radiotherapy. There were 80 men (65%) and 43 women (35%) with an average age of 65 years (range: 28-87 years) at the time of diagnosis. Thirty-one patients were diagnosed with early GC (EGC) and 92 with advanced GC (AGC). Histological staging was done based on UICC TNM classification system. Other clinical features are summarized in Table 1. All patients were followed up for at least 5 years after surgery. The average follow-up period was 56 months (range: 6-85 months). Overall survival (OS) was calculated from the date of surgery to the last follow up. Eleven patients were diagnosed with peritoneal dissemination, 26 with liver metastasis, and 19 with recurrence after operation. Forty patients died of GC. The current study was approved by the Ethics Committee of Tongji Hospital of Tongji University. The work was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all the patients in this study. All the results were accomplished by two pathologists independently, and the means were calculated for each case based on the data obtained.
Table 1.
Correlations of different expression status of VEGF-A and VEGF-C with clinicopathologic parameters
| Factors | N | A+C+ | A-C- | P | N | A+C- | P | N | A-C+ | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.143 | 0.222 | 0.045 | |||||||
| <65 | 51 | 32 | 19 | 24 | 5 | 24 | 5 | |||
| ≥65 | 45 | 35 | 10 | 17 | 7 | 20 | 10 | |||
| Size | 0.049 | 0.056 | 0.334 | |||||||
| <3.2cm | 44 | 25 | 19 | 23 | 4 | 26 | 7 | |||
| ≥3.2cm | 52 | 42 | 10 | 18 | 8 | 18 | 8 | |||
| P-LVD | 0.000 | 0.219 | 0.029 | |||||||
| <14 | 46 | 23 | 23 | 30 | 7 | 30 | 7 | |||
| ≥14 | 50 | 44 | 6 | 11 | 5 | 14 | 8 | |||
| Histological differentiation | 0.303 | 0.017 | 0.967 | |||||||
| Well-differentiated | 9 | 5 | 4 | 4 | 0 | 6 | 2 | |||
| Moderate-poor differentiated | 87 | 62 | 25 | 37 | 12 | 38 | 13 | |||
| Depth of invasion | 0.925 | 0.145 | 0.931 | |||||||
| pT1-2 | 56 | 39 | 17 | 21 | 4 | 26 | 9 | |||
| pT3-4 | 40 | 28 | 12 | 20 | 8 | 18 | 6 | |||
| LVI | 0.024 | 0.173 | 0.364 | |||||||
| Negative | 59 | 36 | 23 | 30 | 7 | 33 | 10 | |||
| Positive | 37 | 31 | 6 | 11 | 5 | 11 | 5 | |||
| LNM | 0.037 | 0.082 | 0.909 | |||||||
| Negative | 44 | 25 | 19 | 19 | 3 | 24 | 8 | |||
| Positive | 52 | 42 | 10 | 22 | 9 | 20 | 7 | |||
| Liver metastasis | 0.264 | 0.391 | 0.598 | |||||||
| Negative | 76 | 51 | 25 | 34 | 9 | 37 | 12 | |||
| Positive | 20 | 16 | 4 | 7 | 3 | 7 | 3 | |||
| Clinical stage | 0.164 | 0.107 | 0.878 | |||||||
| I-II | 56 | 36 | 20 | 25 | 5 | 30 | 10 | |||
| III-IV | 40 | 31 | 9 | 16 | 7 | 14 | 5 | |||
| MVD | 0.009 | 0.384 | 0.211 | |||||||
| <8 | 50 | 29 | 21 | 28 | 7 | 29 | 8 | |||
| ≥8 | 46 | 38 | 8 | 13 | 5 | 15 | 7 | |||
| VI | 0.286 | 0.023 | 0.553 | |||||||
| Negative | 69 | 46 | 23 | 28 | 5 | 34 | 11 | |||
| Positive | 27 | 21 | 6 | 13 | 7 | 10 | 4 | |||
| VEGFR-3 | 0.005 | 0.181 | 0.211 | |||||||
| Negative | 48 | 27 | 21 | 32 | 11 | 29 | 8 | |||
| Positive | 47 | 39 | 8 | 9 | 1 | 15 | 7 |
Note: LVD: lymphatic vessel density; LVI: lymphatic vessel invasion; VI: venous invasion; MVD: microvessel density. Lymph node metastasis (LNM).
Immunohistochemistry for VEGF-A and VEGF-C
For immunohistochemical staining, 4-μm-thick paraffin-embedded sections were obtained. Sections were treated with 0.3% h2O2 for 10 min at room temperature. For antigen retrieval, the sections were heated in 0.01 mmol/L sodium citrate (pH 6.0) in a microwave oven. Sections were then incubated at 4°C overnight in a humidity environment with primary antibodies (mouse monoclonal VEGF-A antibody [1:100, DAKO, Carpentaria, CA] and goat polyclonal VEGF-C antibody [1:100, Santa Cruz Biotechnology, Santa Cruz, USA]). Slides were rinsed thrice in 0.1 mmol/L PBS for 2 min, and incubated for 30 min at room temperature with horseradish peroxidase (Envision, DAKO) conjugated goat anti-rabbit/mouse secondary antibody. Development was done with 3’ 3-diaminobenzidine. The normal goat IgG served as a negative control for VEGF-C detection, and the normal rabbit IgG served as a negative control for VEGF-A detection.
Assessment of VEGF-A and VEGF-C expressions
Assessment of VEGF-A and VEGF-C expressions were done according to previously described [9]. The VEGF-A and VEGF-C expressions were semi-quantitatively determined according to the percentage of positive cancer cells. Staining intensity was classified as four grades: none (0), weak (1), moderate (2) and strong (3). The percentage of positive cancer cells was classified as 4 grades: 0 (0%), 1 (1%-10%), 2 (11%-49%) and 3 (50%-100%). The total score was a product of two scores. The median score was determined, according to which cancers were categorized into low- (score 0-3) and high-expression (score 4-6) cancers [9].
Double immunohistochemical staining for D2-40/CD34
The double immunohistochemical staining for D2-40/CD34 was done to evaluate the lymph vessels (D2-40) and blood vessels (CD34) according to the manufacturer’s instructions (No: 95-9999, Histostain-DS Kit, Zymed, CA). The sections were treated with peroxidase quenching solution for 10 min. After incubation overnight at 4°C with primary antibody against CD34 (mouse monoclonal antibody, 1:100, DAKO), the sections were incubated with the biotinylated secondary antibody (DAKO). Subsequently, sections were treated with alkaline phosphatase conjugated secondary antibody for 10 min and with the substrate chromogen mixture and double staining enhancer. Then, the sections were incubated with primary antibody against D2-40 (mouse monoclonal antibody, 1:200, GM36190, Gene Tech Company Limited, Shanghai, China) for 60 min and with the biotinylated secondary anti-immunoglobulin (Ig) (DAKO). After incubation with enzyme conjugate for 10 min, the sections were incubated with the mixture of substrate buffer, chromogen solution and 0.6% hydrogen peroxide for hypothalamic regulatory peptide (HRP) and then observed under a microscope. Tap water containing 0.05% Tween-20 was used to stop the reaction. For negative controls, the sections were treated with a non-immune serum instead of primary antibody. The CD34 positive blood vessels were intense red, and the D2-40 positive lymphatic vessels were dark purple.
Quantitative analysis of LVD and MVD was performed in sections after immunohistochemistry for D2-40 and CD34 [14]. The mean number of lymphatic vessels was determined as LVD, and the mean number of blood vessels as MVD. Lymphatic vessel invasion (LVI) was defined if at least one tumor cell cluster was in D2-40 positive vessels, and microvessel invasion (MVI) in CD34 positive vessels [14]. The mean LVD and MVD were calculated for each case. Scoring and counting were performed independently by two investigators who were blind to the study.
Cell line and cell culture
The human GC cell line SGC7901 was purchased from THE Type Culture Collection of Chinese Academy of Sciences (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences). The cancer cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) in flasks at 37°C in an environment with 5% CO2.
Recombinant plasmid construction, lentivirus production and transduction
All the procedures were performed with BLOCK-iT™ Pol II miR RNAi Expression Vector Kit and the Lentiviral Pol II miR RNAi Expression System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Four pairs of miR-155-based VEGF-A or VEGF-C sequences were designed using the Invitrogen RNAi Designer (www.invitrogen.com/rnaiexpress). The engineered pre-miRNA sequence is designed based on the murine miR-155 sequence, and synthesized double oligonucleotides were cloned into the pcDNA6.2–GW/EmGFP/miR plasmid (Invitrogen) to produce the recombinant plasmids containing VEGF-A miRNA (pCMV-VEGF-AmiRNA-1-4) or VEGF-C miRNA (pCMV-VEGF-CmiRNA-1-4).The pc DNA6.2 -GW/EmGFP-miR-neg control plasmid (Invitrogen) supplied by the Kit was used as a negative control. Each purified expression plasmid was transfected into the SGC7901 cells. Real-time quantitative–PCR (qPCR) and western blot assay were performed to assess the silencing efficiency of VEGF-A and VEGF-C at 48 h post-transfection.
The lentiviral miRNA system based on the above vectors was generated. The third generation self-inactivating lentivirus vector, pLenti6/V5-DESTcontaining a CMV-driven EGFP reporter, a SV40 promoter upstream of the cloning sites and high-level expression of miRNA was used. The constructed functional pre-miRNA expression cassette targeting VEGF-A or VEGF-C was transferred into the destination vector (pLenti6/V5-DEST) to generate the lentiviral expression vectors (Lenti-VEGF-AmiRNA, Lenti-VEGF-CmiRNA and Lenti-miRNA-neg). The recombinant lentivirus and the control lentivirus were produced by co-transfecting 293FT cells with the transfer vector and the three packaging vectors. The virus-containing supernatant was harvested 72 h post-transduction. The prepared SGC7901 cells were transduced with the lentiviral expression vectors. At 48 h after transduction, the expression of VEGF-A and VEGF-C was evaluated by western blot assay. Cells with stable expression were obtained after culture in medium containing 4 μg/ml Blasticidin for 12 days.
Real-time quantitative–PCR (qPCR)
Real-time quantitative PCR was performed on a Bio-Rad iCycler & iQ Real-Time PCR Systems (BIO-RAD, HERCULES, CA, USA) according to the manufacturer’s instructions. Total RNA were extracted with Trizol reagent (Invitrogen) from VEGF-A-knockdown cells, VEGF-C-knockdown cells, negative control cells and SGC7901 cells, respectively. Two micrograms of RNA was used as a template for first-strand DNA synthesis using the SuperScript III first-strand synthesis system for RT (Invitrogen). PCR amplification was performed using Taq DNA polymerase (Invitrogen). Real-time quantitative PCR mixture contained 27.5 μl of Real Time PCR Master Mix, 0.3 μl 2× SYBR Premix Ex Taq (TOYOBO Biotech Co.Ltd., Osaka, Japan), 1.2 μl of primer mix and 1 μl of cDNA (total: 30 μl). The primers for VEGF-A were 5’-ATAAGTCCTGGAGCGTGTACGTT-3’ (forward), 5’-CAGGAACATTTACACGTCTGCG-3’ (reverse) and 5’-FAMCCCGCTGCTGTCTAATGCCCTGGAGTAMRA-3’ (probe); the primers for VEGF-C were 5’-ACAGAAATGCTTGTTAAAAGGAAAG-3’ (forward), 5’-TATGAAGGGACACAACGACACACT-3’ (reverse) and 5’-FAM-TTCCACCACCAAA CATGCAG CTGTTATAMRA-3’ (probe). All the primers and probes were designed with Primer Express software (version 2.0; Applied Biosystems). The relative amount of specific mRNA was normalized to human β-actin using the following primers: 5’-CAACTGGG ACGACATGGAGAAA-3’ (forward), 5’-GATAGCAACGTACATGGCTGGG-3’ (reverse) and 5′-Fam-TCTGGCACCACACCTTCTACAATGAGCTAMRA-3′ (probe). All primers spanned an intron to ensure the discrimination between cDNA and genomic DNA. All PCRs were run in duplicate as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 20 s, 60°C for 20 s and 72°C for 10 s. The relative mRNA expressions of VEGF-A and VEGF-C were calculated using the 2-ΔΔCt method and analyzed with the Icycler version3.1.7050 software (BIO-RAD).
Western blot assay
For of the detection of protein expression of VEGF-A and VEGF-C, cancer cells were collected at 48 h after transduction, and lysed in cold PBS containing 0.5% NP-40 and a protease inhibitor cocktail (Novogen, Aus). The whole-cell proteins were extracted using the Whole Cell Extraction Kit (Chemicon, Temecula, CA, USA). Protein concentrations were determined according to the manufacturer’s instructions (Bicinchoninic acid [BCA] Protein Assay Kit, Pierce, Rockford, USA). Then, 40 mg of proteins were loaded onto 12% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred onto an nitrocellulose membrane (Invitrogen, Carlsbad, CA, USA). Western blotting was done using antibodies against VEGF-A and VEGF-C (Santa Cruz Biotechnology, CA, USA). All the bands were visualized with the enhanced chemiluminescence (Pierce, Rockford, IL).
Proliferation assay
Cell proliferation was evaluated using 3-(4,5-dimethylthiazol -2-yl)-2,5–diphenylte trazolium bromide (MTT, Sigma, USA) assay. SGC7901 cells (2x105 cells/ml) were seeded in 96-well plates in DMEM containing 10% FCS (100 μl/well). After incubation for 18 h, the cell confluence reached about 85% and then these cells were transduced with different lentiviral supernatants (50μl/well) at 24 h, 48 h, 60 h and 96 h before MTT assay. SGC7901 cells without transduction served as a normal control. MTT solution (5g/L) was added (20 μl/well) followed by incubation for 4 h at 37°C. The absorbance of product was determined at 490 nm by using a microplate reader (Multiskan MK3, Thermo Labsystems, Finland).
Apoptosis assay
The apoptosis-mediated alteration of membrane phospholipids was monitored by annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining and quantified with a fluorescence-activated cell sorter (FACS) (Becton Dickinson, San Jose, CA). In brief, 5×105 cells were harvested and suspended in 400 μl of Binding Buffer. After addition of 5 μl of Annexin V-FITC and 5 μl of PI, cells were incubated at room temperature for 10 min. Then, these cells were subjected to FACS Calibur flow cytometry within 1 h. A total of 10,000 cells were recorded and analyzed with the Cell Quest software (BD Biosciences).
Animals
Forty-five 4-week-old athymic female BALB/c nude mice were purchased from the Shanghai Experimental Animal Center (Shanghai, China). The mice were housed under a specific pathogen-free condition in Tongji Hospital. All animals were given ad libitum access to food and water. The animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Tongji University. Mice were euthanized by CO2 inhalation, followed by cervical dislocation.
Lentivirus-mediated VEGF-A and/or VEGF-C gene silencing in vivo
To investigate whether Lenti-miRNA-VEGF-A+VEGF-C can more effectively suppress the growth of GC in nude mice in vivo, nude mice were divided into 5 groups (n=5 per group): SGC7901 group, Lenti-miRNA-neg control group, Lenti-miRNA-VEGF-A group, Lenti-miRNA-VEGF-C group, and Lenti-miRNA-VEGFA+VEGF-C group. The cells (5×106 in 0.3 ml of phosphate-buffered saline [PBS]) transduced with lentivirus expression vectors or SGC7901 cells were injected subcutaneously into the dorsal midline of nude mice and the cancer growth was observed.
To further evaluate the effect of Lenti-miRNA-VEGF-A+VEGF-C on cancer growth, SGC7901 cells (5×106 cells in 0.3 ml of PBS) were injected subcutaneously into the dorsal midline of another 20 nude mice. Two weeks later, when the cancer size reached 0.3-0.4 cm in diameter, 15 μl of Lenti-miRNA-neg, Lenti-miRNA-VEGF-A, Lenti-miRNA-VEGF-C, or Lenti-miRNA-VEGF-A+VEGF-C solution (0.5 μg/μl) was directly injected into the cancers. Injection was performed once weekly for 8 weeks.
Cancer size was detected every 2-3 days using a caliper. On day 56, all the mice were sacrificed, and the cancers were collected. Cancer volume (mm3) was estimated by measuring the longest and shortest diameter and calculated as follows: volume = (shortest diameter)2 × (longest diameter) ×0.5.
Statistical analysis
Statistical analysis was performed using the Statistics Package for the Social Science software (version 11.5; SPSS Inc, Chicago, IL). The correlations of VEGF-A / VEGF-C expression with clinicopathologic factors were analyzed with independent t test or Mann-Whitney U test. Survival curves were delineated using the Kaplan-Meier method and compared with log-rank test. A value of P < 0.05 was considered statistically significant.
Results
Expressions of VEGF-A and VEGF-C in GC
Cells positive for VEGF-A or VEGF-C had cytoplasmic granules (Figure 1). The lymphatic vessels (D2-40) and blood vessels (CD34) were clearly distinguished (Figure 1C). As shown in Table 1, 67 patients had high expressions of both VEGF-A and VEGF-C (A+C+, 54.5%, 67/123); 12 patients had high expression of VEGF-A but low expression of VEGF-C (A+C-, 9.8%, 12/123); 15 patients had high expression of VEGF-C but low expression of VEGF-A (A-C+, 12.2%, 15/123); 29 patients had low expressions of both VEGF-A and VEGF-C (A-C-, 23.6%, 29/123). The core region of GC was strong positive for VEGF-A and VEGF-C when compared with superficial area.
Figure 1.
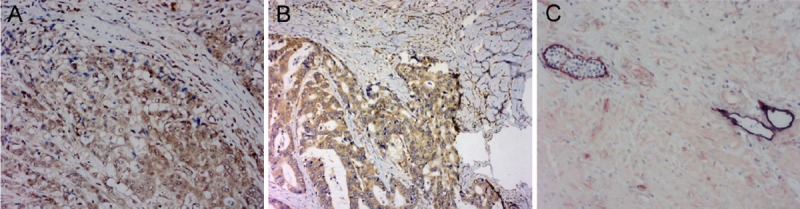
Detection of expressions of VEGF-A and VEGF-C in GC by immunohistochemistry and double immunohistochemical staining for D2-40 and CD34. A. VEGF-A expression in the cytoplasm (×200). B. VEGF-C expression in the cytoplasm (×400). C. The lymphatic vessels were clearly distinguished from blood vessels after double immunohistochemical staining for D2-40 (dark purple) and CD34 (intense red) (×400).
Correlations of clinicopathological factors with expression of VEGF-A and VEGF-C
The correlation of VEGF-A and VEGF-C expressions with clinicopathological factors are shown in Table 1. When compared with the A-C- patients, the A+C+ patients had larger tumor size (≥3.2cm, P=.049), higher P-LVD (P=.000), higher MVD (P=.009), LVI (P=.024), LN metastasis (P=.037) and higher VEGFR-3 expression (P=.005). When compared with A+C+ patients, the A+C- patients had worse histological differentiation (P=.017) and more VI (P=.023); the A-C+ patients were older (P=.045) and had higher P-LVD (P=.029).
Prognostic significance of VEGF-A and VEGF-C expressions in GC
Kaplan-Meier analysis of OS was performed to investigate whether the VEGF-A and VEGF-C expressions had prognostic significance. The univariate survival analysis showed that, in comparison with A-C-, only A+C+ was associated with poor OS (Figure 2, P=.0438), and no significant relationship was observed between OS and A+C- or A-C+ (P >.05).
Figure 2.
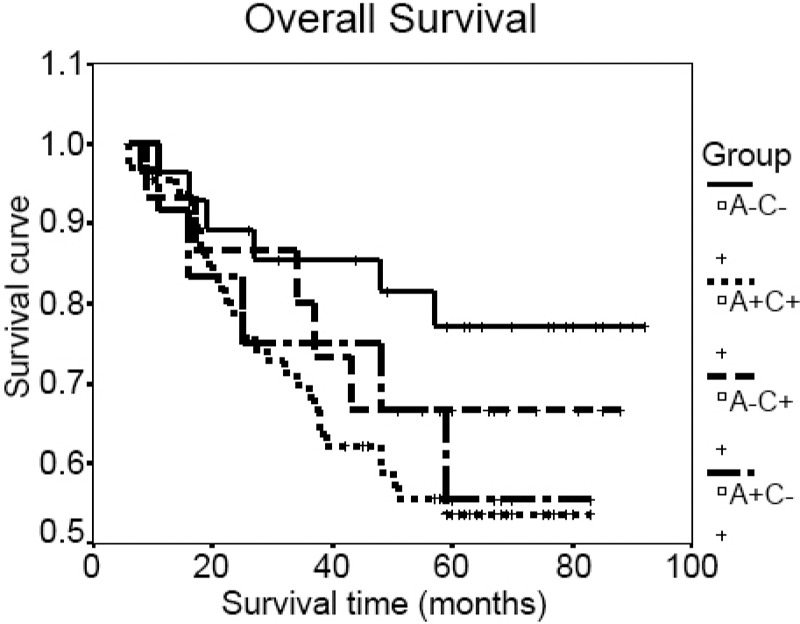
Relationship between expressions of VEGF-A and VEGF-C and overall survival.
Silencing of VEGF-A and VEGF-C expression in SGC7901 cells
In order to detect the silencing efficiency of VEGF-A and VEGF-C genes, real-time PCR was performed to detect their mRNA expressions, and Western blot assay to measure their protein expressions in the transduced cells and their parental cells. When compared with the parental cells, the four pCMV-VEGF-AmiRNA transfected cells showed dramatic decreases in the mRNA and protein expressions of VEGF-A. In particular, the silencing efficiency of pCMV-VEGF-AmiRNA-4 was the highest, with the reduction of VEGF-A mRNA expression by 85.6% and protein expression by 66.35% (Figure 3). Similarly, the four pCMV-VEGF-C miRNAs significantly inhibited the expression of VEGF-C at both mRNA and protein levels, in comparison with the parental cells. The pCMV-VEGF-C miRNA-1 showed the most potent silencing efficiency, with the reduction of VEGF-C mRNA expression by 89.8% and protein expression by 60.4% (Figure 3).
Figure 3.
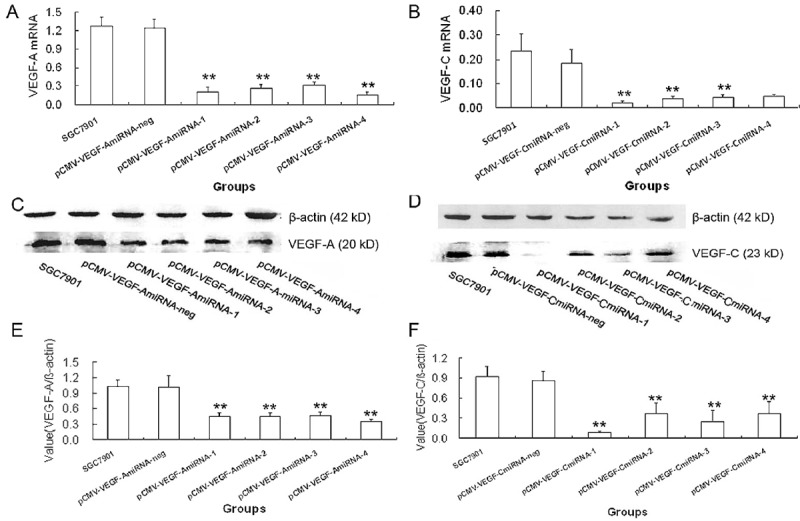
Expression of VEGF-A or VEGF-C in SGC7901 GC cells transduced with pCMV-VEGF-A miRNA or pCMV-VEGF-C miRNA expression plasmids at 48 h (*P<.05, **P<.01). mRNA and protein expressions of VEGF-A in SGC7901 cells transduced with pCMV-VEGF-A miRNA expression plasmids. A. Real-time qPCR showed VEGF-A mRNA expression in pCMV-VEGF-A miRNA-4 transduced cells was significantly reduced by 85.6% when compared with parental SGC7901 cells. C. Western blot assay. E. VEGF-A protein expression in pCMV-VEGF-A miRNA-4 transduced cells was significantly reduced by 89.8% when compared with parental SGC7901 cells. mRNA and protein expressions of VEGF-C in SGC7901 cells transduced with pCMV-VEGF-C miRNA expression plasmids. B. Real-time qPCR assay showed VEGF-C mRNA expression in pCMV-VEGF-C miRNA-1 transduced cells was significantly reduced by 89.8% when compared with parental SGC7901 cells. D. Western blot assay. F. VEGF-C protein expression in pCMV-VEGF-C miRNA-1 transduced cells was significantly reduced by 60.4% when compared with parental SGC7901 cells.
Then, the pCMV-VEGF-AmiRNA-4 and pCMV-VEGF-C miRNA-1 were independently inserted into pLenti6/V5-DEST vector to prepare the Lenti-miRNA-VEGF-A or Lenti-miRNA-VEGF-C expression vectors. After infection of SGC7901 cells with the lentivirus carrying Lenti-miRNA-VEGF-A, Lenti-miRNA-VEGF-C or pCMV-miRNA-neg, cells with stable expression were harvested after Blasticidin selection.
Combined silencing of VEGF-A and VEGF-C significantly suppressed cell proliferation and induced apoptosis
MTT assay showed that the cell proliferation was inhibited in the Lenti-miRNA-VEGF-A infected cells, Lenti-miRNA-VEGF-C infected cells, and Lenti-miRNA-VEGF-A+VEGF-C infected cells at 48 h, 60 h and 96 h, in comparison with their parental cells (P<.001, Figure 4A). Moreover, the cells infected by Lenti-miRNA-VEGF-A+VEGF-C had the highest proliferation inhibitory rate (76.24%±8.23%) at 96 h post-infection, and the highest apoptosis rate (64%±10.2%) (P<.001, Figure 4B).
Figure 4.
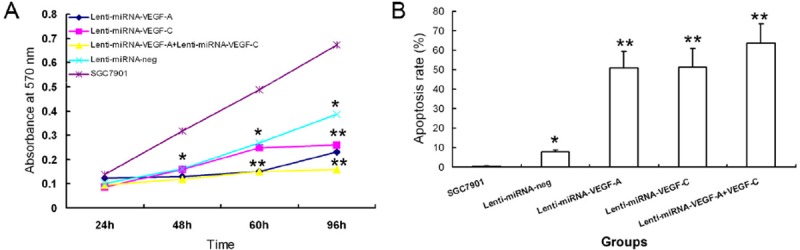
Combined silencing of both VEGF-A and VEGF-C significantly suppressed the cell proliferation and induced the apoptosis of GA cells. A. Cell proliferation was significantly inhibited in SGC7901 cells after infection with Lenti-miRNA-VEGF-A + Lenti-miRNA-VEGF-C vectors as measured by MTT assay, compared with control groups (*P<.05, **P<.01). B. Cells infected with Lenti-miRNA-VEGF-A + Lenti-miRNA-VEGF-C vectors had the highest apoptosis rate detected by apoptosis assay, compared with control groups (*P<.05, **P<.01).
Combined silencing of VEGF-A and VEGF-C significantly inhibited cancer growth in vivo
The cancer growth curves in nude mice after injection of Lenti-miRNA-VEGF-A and/or VEGF-C infected cells and the control cells are shown in Figure 5. The inhibition of cancer growth was the most obvious in the Lenti-miRNA- VEGF-A+VEGF-C mice than in Lenti-miRNA-VEGF-A mice, Lenti-miRNA-VEGF-C mice Lenti-miRNA-neg mice or SGC7901 mice (P<.05). These results suggest that combined silencing of VEGF-A and VEGF-C is more effectively to suppress the GC growth than silencing of VEGF-A or VEGF-C alone.
Figure 5.
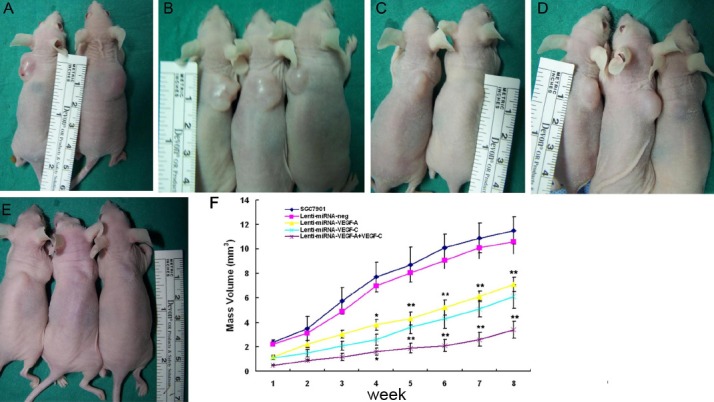
Combined silencing of VEGF-A and VEGF-C significantly inhibited cancer growth in vivo. A. SGC7901; B. Lenti-miRNA-neg; C. Lenti-miRNA-VEGF-A; D. Lenti-miRNA- VEGF-C; E. Lenti-miRNA-VEGF-A + Lenti-miRNA-VEGF-C. F. Silencing of VEGF-A or VEGF-C in SGC7901 cells could significantly inhibit the cancer growth, when compared with the parental cells or cells infected with Lenti-miRNA-neg (*P<.05, **P<.01). The combined silencing of VEGF-A and VEGF-C could more effectively suppress the GC growth than the silencing of VEGF-A or VEGF-C alone (*P<.05, **P<.01).
Cancer growth in nude mice after treatment with Lenti-miRNA-VEGF-A and/or VEGF-C
To further study the therapeutic efficacy of Lenti-miRNA-VEGF-A and/or VEGF-C in GC, 15 μl of lentivirus solutions (0.5 μg/μl) or PBS was injected into the cancers of nude mice. As shown in Figure 6, the cancer volumes were significantly reduced in the Lenti-miRNA-VEGF-A mice, Lenti-miRNA-VEGF-C mice and Lenti-miRNA-VEGF-A+VEGF-C mice from day 3 to week 8, as compared with the Lenti-miRNA-neg treated mice or PBS treated mice (P<.05). Moreover, the cancers in Lenti-miRNA-VEGFA+VEGF-C mice were the smallest than in the Lenti-miRNA-VEGF-A mice and Lenti-miRNA-VEGF-C mice (P<.05). However, no significant difference was found in the cancer growth between the Lenti-miRNA-VEGF-A mice and Lenti-miRNA-VEGF-C mice (P >.05).
Figure 6.
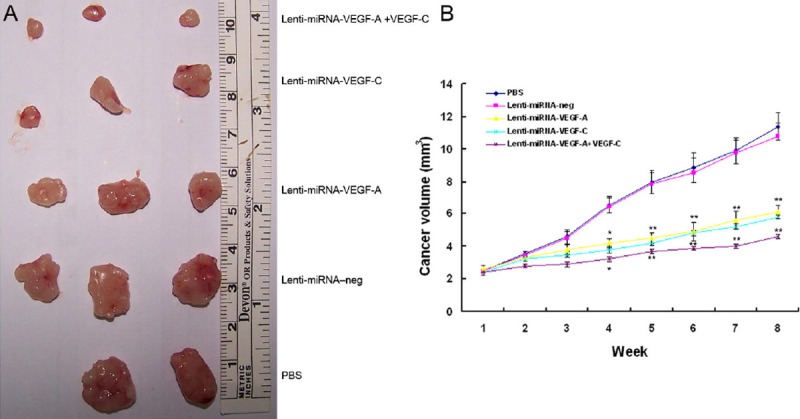
Effects of Lenti-miRNA-VEGF-A and/or Lenti-miRNA-VEGF-C on cancer growth in GC bearing nude mice. A. Cancer volumes were significantly reduced in mice treated with Lenti-miRNA-VEGF-A, Lenti-miRNA-VEGF-C, or Lenti-miRNA-VEGF-A +VEGF-C, as compared to those treated with Lenti-miRNA–neg or PBS (*P<.05, **P<.01). B. Cancers in the mice treated with Lenti-miRNA-VEGF-A + VEGF-C had the smallest volumes than those treated with Lenti-miRNA-VEGF-A or Lenti-miRNA–VEGF-C (*P<.05, **P<.01).
Discussion
The significance of VEGF-A and VEGF-C in GC has been investigated in a variety of clinical studies. Both factors play complicated roles in the development and metastasis of GC due to their angiogenic and lymphangiogenic effects. VEGF-A can induce angiogenesis via the VEGFR-1 and VEGFR-2 leading to the cancer growth and metastasis. It has been suggested that VEGF-A is also helpful for lymphangiogenesis and lymphatic enlargement through the VEGFR-2 signaling pathway in experiments [1].
VEGF-C is a key lymphangiogenic growth factor and can activate the VEGFR-3 signaling pathway in human solid tumors. Moreover, mature VEGF-C can activate the VEGFR-2 signaling pathway to induce the lymphatic enlargement and lymphangiogenesis. In GC, the distinct roles of VEGF-A and VEGF-C suggest that VEGF-A and VEGF-C may play distinct roles: VEGF-A is more likely to be associated with hematogenous metastasis, while VEGF-C is indicative of lymphatic metastasis [15]. However, for the signaling pathways activated by VEGF-A and VEGF-C, the overlapping biological effects of these two factors have not been clarified clearly in human cancers. A majority of previous studies focused the effect of VEGF-A or VEGF-C alone. A study of Kondo et al showed that VEGF-A and VEGF-C could synergistically enhance the lymph node metastasis, which means that the patients with expressions of both VEGF-A and VEGF-C have higher risk for lymph node metastasis [16]. However, their prognostic significance has not been studied in this study.
In the breast cancer, the patients with high expressions of both VEGF-A and VEGF-C have been shown to possess a worst prognosis, compared with those having low expressions of both VEGF-A and VEGF-C, and those with high expression of VEGF-C or VEGF-A alone. However, the expressions of VEGF-A and VEGF-C with clinicopathologic factors have not been investigated to date [9]. In our previous study, findings revealed that the patients with high expressions of both VEGF-A and VEGF-C had a poorer outcome in comparison with those with high expression of VEGF-A or VEGF-C alone [13]. These results imply that cancers with expression of two factors at different levels have distinct biological behaviors and clinical significance.
In the present study, a cohort of patients was classified into 4 groups according to the expressions of VEGF-A and VEGF-C in the same patient. In comparison with patients having low expression of both VEGF-A and VEGF-C (A-C-), the patients with high expressions of both VEGF-A and VEGF-C (A+C+) had larger cancer size, higher P-LVD, MVD and frequency of LVI, and LN metastasis. Furthermore, these patients presented with poorer OS. Compared with A+C+ patients, the patients with high expression of VEGF-A but low expression of VEGF-C (A+C-) had higher incidence of VI, and those with high expression of VEGF-C but low expression of VEGF-A (A-C+) had higher P-LVD. However, both A+C- and A-C+ had no correlation with cancer size, LN metastasis and prognosis.
These results suggest that the GC expressing both VEGF-A and VEGF-C has more rapid cancer growth, higher level of angiogenesis and lymphangiogenesis, and higher risk for invasion and metastasis. Therefore, the clinical significance of one factor (VEGF-A or VEGF-C alone) may be affected by the expression of the other factor in the same patient. The combined analysis of related factors may be of clinical significance for patients with gastric cancer.
To further investigate the biological effects of both VEGF-A and VEGF-C in GC, the mRNA and protein expressions of VEGF-A and VEGF-C in human GC cell line SGC7901. Both VEGF-A and VEGF-C were highly expressed at mRNA and protein levels. The proliferation and invasion of GC cells in vitro were significantly suppressed after silencing of both VEGF-A and VEGF-C by the lentivirus expressing vectors, when compared with that after silencing of VEGF-A or VEGF-C alone. The cancer growth in the Lenti-miRNA-VEGF-A+VEGF-C nude mice was significantly suppressed as compared to nude mice treated with cells undergoing silencing of VEGF-A or VEGF-C alone. Treatment with both lentivirus vectors targeting VEGF-A and VEGF-C respectively also significantly inhibited the cancer growth in vivo when compared with treatment with lentivirus vector targeting VEGF-A or VEGF-C alone. Lymph node metastasis was not observed in the GC animal model. However, in the mouse immunocompetent mammary cancer model, combined siRNA therapy targeting both VEGF-A and VEGF-C inhibited both lymph node metastasis and lung metastasis and showed higher therapeutic efficacy than siRNA therapy targeting VEGF-A or VEGF-C alone. Therefore, combined siRNA therapy targeting both VEGF-A and VEGF-C may become a promising strategy for the treatment of metastatic breast cancer [17].
Although the cancer metastasis was not found in this study, the cancer growth and invasion were significantly inhibited. These results were consistent with those found in clinical studies. Inhibition of expressions of both VEGF-A and VEGF-C in GC led to the decrease in angiogenesis resulting in reduction in the nutrition supply to the cancer. The inhibited cancer growth also leads the compromised cancer invasion, and subsequent metastasis via the blood vessel and/or lymphatic vessels. On the contrary, once GC cells have high expression of both VEGF-A and VEGF-C, they may present with more rapid growth and higher potential for invasion due to their angiogenic and lymphangio genic effects, eventually leading to distant metastasis and lymph node metastasis.
Treatment with Bevacizumab, a humanized monoclonal antibody that inhibits VEGF-A, in combination with chemotherapy has been used as a first-line therapy in patients with AGC [12]. Based on the expressions of VEGF-A and VEGF-C, we speculate the therapy targeting VEGF-C expression in combination with bevacizumab (targeting VEGF-A) may be more effective to control the cancer growth in patients with GC having high expression of both VEGF-A and VEGF-C.
References
- 1.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34:1785–1788. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 2.Fondevila C, Metges JP, Fuster J, Grau JJ, Palacin A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer. 2004;90:206–215. doi: 10.1038/sj.bjc.6601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyagi K, Kouhuji K, Yano S, Miyagi M, Imaizumi T, Takeda J, Shirouzu K. VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer. 2005;8:155–163. doi: 10.1007/s10120-005-0329-4. [DOI] [PubMed] [Google Scholar]
- 4.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 5.Shida A, Fujioka S, Ishibashi Y, Kobayashi K, Nimura H, Mitsumori N, Suzuki Y, Kawakami M, Urashima M, Yanaga K. Prognostic significance of vascular endothelial growth factor D in gastric carcinoma. World J Surg. 2005;29:1600–1607. doi: 10.1007/s00268-005-0076-z. [DOI] [PubMed] [Google Scholar]
- 6.Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Hocker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J. Clin. Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 7.Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, Sasaki T, Miura M. Role of VEGF-C and VEGF-D in lymphangiogenesis ingastric cancer. Int J Clin Oncol. 2005;10:318–27. doi: 10.1007/s10147-005-0508-7. [DOI] [PubMed] [Google Scholar]
- 8.Gao P, Zhou GY, Zhang QH, Su ZX, Zhang TG, Xiang L, Wang Y, Zhang SL, Mu K. Lymphangiogenesis in gastric carcinoma correlates with prognosis. J Pathol. 2009;218:192–200. doi: 10.1002/path.2523. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer. 2007;96:1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachisuka T, Narikiyo M, Yamada Y, Ishikawa H, Ueno M, Uchida H, Yoriki R, Ohigashi Y, Miki K, Tamaki H, Mizuno T, Nakajima Y. High lymphatic vessel density correlates with overexpression of VEGF-C in gastric cancer. Oncol Rep. 2005;13:733–737. [PubMed] [Google Scholar]
- 11.Lee SJ, Kim JG, Sohn SK, Chae YS, Moon JH, Kim SN, Bae HI, Chung HY, Yu W. No association of vascular endothelial growth factor-A (VEGF-A) and VEGF-C expression with survival in patients with gastric cancer. Cancer Res Treat. 2009;41:218–223. doi: 10.4143/crt.2009.41.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, Shah MA. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012;30:119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 13.Wang XL, Ai ZS, Fang JP, Tang RY, Chen XM. [Expression of vascular endothelial growth factors (VEGF)-A, -C and -D and their prognostic significance and relationship with angio- and lymphangiogenesis in gastric cancer] . Zhonghua Zhong Liu Za Zhi. 2008;30:837–843. [PubMed] [Google Scholar]
- 14.Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:193–1199. doi: 10.1016/j.humpath.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Ding S, Li C, Lin S, Han Y, Yang Y, Zhang Y, Li L, Zhou L, Kumar S. Distinct roles of VEGF-A and VEGF-C in tumour metastasis of gastric carcinoma. Oncol Rep. 2007;17:369–375. [PubMed] [Google Scholar]
- 16.Kondo K, Kaneko T, Baba M, Konno H. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol Pharm Bull. 2007;30:633–637. doi: 10.1248/bpb.30.633. [DOI] [PubMed] [Google Scholar]
- 17.Shibata MA, Morimoto J, Shibata E, Otsuki Y. Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther. 2008;15:776–786. doi: 10.1038/cgt.2008.43. [DOI] [PubMed] [Google Scholar]


