Abstract
Expression of MUC apomucins has rarely been investigated in the signet-ring cell carcinoma (SRCC) of the stomach and colorectum. The author examined immunohistochemically the expression status of MUC1, MUC2, MUC5AC, and MUC6 in 30 cases of gastric SRCC and 12 cases of colorectal SRCC. The normal distribution of these MUC apomucins was also examined in the non-tumorous parts of the stomach and colorectum. In normal tissues, the stomach epithelial cells consistently expressed MUC2, MUC5AC, MUC6, but consistently not MUC1. In colorectum, cryptal epithelial cells consistently expressed MUC2, but consistently not MUC1, MUC5AC, and MUC6. The expression pattern of the gastric SRCC was as follows: MUC1, 3/30 (10%); MUC2, 4/30 (13%); MUC5AC, 20/30 (67%), and MUC6 21/30 (70%). The expression pattern of the colorectal SRCC was as follows: MUC1, 5/12 (42%); MUC2, 11/12 (92%); MUC5AC, 4/12 (33%); and MUC6, 0/12 (0%). Significant differences (p<0.05) were found in the expression of MUC1 (stomach SRCC 10% vs colorectal SRCC 42%), MUC2 (13% vs 92%), MUC5AC (67% vs 33%), and MUC6 (70% vs 0%). Thus, there was a significant tendency that primary gastric SRCC express MUC5AC and MUC6 but not MUC1 and MUC2, while primary colorectal SRCC express MUC1, MUC2 and MUC5A, but not MUC6. These different expressions of these MUC apomucins in gastric and colorectal SRCC seem useful to determine the primary site of metastatic SRCC and for differential diagnosis of SRCC of other sites. In the gastric SRCC, the up-regulation of MUC1 and the down-regulation of MUC2, MUC5AC and MUC6 appear to be associated with carcinogenesis, malignant potential, progression, and clinical behaviors in gastric SRCC. In the colorectal SRCC, the up-regulation of MUC1 and MUC5AC may be associated with carcinogenesis, malignant potential, progression, and clinical behaviors in colorectal SRCC. A comparative review of the present SRCC and presently reported ordinary adenocarcinoma and SRCC cases of the stomach and colorectum was performed.
Keywords: Signet-ring cell carcinoma, MUC, mucins, stomach, colorectum, histopathology, immunohistochemistry
Introduction
Mucins are high-molecular-weight glycoproteins, which are heavily decorated with a large number of O-linked oligosaccharides and a few N-glycan chains, linked to a protein backbone [1]. The protein backbone is called mucin core protein or MUC [1]. At present, at least 20 MUC have been identified [2] in humans. Mucins are now classified into secreted mucins and transmembrane mucins [1,2]. Secreted mucins form mucin gels, and are composed of MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9 and MUC19, while transmembrane mucins consist of MUC1, MUC3A, MUC3B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20 and MUC21. These transmembranous MUCs are located in the cell membrane and do not form mucin gels [1,2]. These MUC protein are encoded by various MUC genes [1,2]. Mucins play an important role in the protection, local innate immunity, and lubrication of mucosal surface of various organs. Mucins are also involved in the pathogenesis of benign and malignant diseases of secretory epithelial cells [1,2]. It is well known that MUC expression is down-regulated or up-regulated in most malignant neoplasms of various organs [1,2]. These alterations of MUC apomucins, which are regulated by MUC genes, are thought to be associated with carcinogenesis and malignant potentials of cancer, though the mechanisms remain unclear [3-5].
Among the MUC apomucins, MUC1, MUC2, MUC5AC and MUC6 are representative. MUC1 is a transmembranous apomucin, and present dominantly in pancreatic and preset epithelium [1,2]. MUC1 is also called “polymorphic epithelial mucin (PEM)”. MUC2 is a secretory apomucin, and present mainly in goblet cells of small intestine, large intestine, and bronchus. MUC2 is also called “goblet cell mucin”. MUC5AC is a secretory apomucin, and is seen mainly in gastric foveolar cells. MUC5AC is also called “gastric foveolar mucin”. MUC6 is also a secretory mucin, and is found largely in pyloric glands of the stomach, duodenal Brunner’s glands, and esophageal glands. MUC6 is also termed “pyloric gland-type mucin” [1-5].
Signet-ring cell carcinoma (SRCC) is characterized by an adenocarcinoma whose carcinoma cells were composed predominantly of SRCC cells [6,7]. SRCC cells are characterized by abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins [6,7]. SRCC can occur in any organs, but is most prevalent in the stomach, followed in order by colorectum and lung [6,7]. According to the current WHO blue book, SRCC is defined as an adenocarcinoma with the presence of >50% of tumor cells (signet-ring cells) with prominent intracytoplasmic mucins [7]. The author has examined SRCC in the extra-gastric and extra-colorectal SRCC [8-18], and found that the expression of MUC apomucins was markedly different among SRCC of extra-gastric and extra-colorectal organs. Several studies on the expression of MUC of ordinary adenocarcinoma of the stomach [19-21] and colorectum [22,23] have been performed, but there have been no definite conclusions although it has been suggested that MUC expression is associated with carcinogenesis, malignant behavior, tumor progression, metastasis, and prognosis of the patients [1-5,19-23]. In the present study, the author reports a study of primary SRCC in the stomach and colorectum. Although SRCC is characterized by mucins, there have been only one study of MUC profile in gastric and colorectal SRCC [24] and only one study of MUC profile in pulmonary SRCC [25]. In contrast, there have been several studies on the MUC profiles of ordinary adenocarcinomas of the stomach, colorectum, lung and other organs.
The author herein examined the expression pattern of MUC1, MUC2, MUC5AC and MUC6 molecules in 42 cases of primary SRCC of the stomach and colorectum. The normal distribution of these MUC apomucins in the stomach and colorectum was also examined.
Materials and methods
The author retrieved primary adenocarcinomas with signet-ring cells phenotypes of the stomach and colon in the author’s computer database files of primary SRCC of the digestive organs in the recent 15 years. The computer survey identified 68 cases of primary adenocarcinoma of the stomach and colorectum with signet-ring cell phenotype. The author reviewed these 68 cases under the microscopy. The author confirmed the signet-ring cell phenotype of these adenocarcinomas, and excluded cases of adenocarcinoma with SRCC cells whose percentage was less than 50% of the tumor cells. As the results, 42 cases of SRCC fulfilling the WHO criteria [6,7] remained. The primary nature of these 42 cases of SRCC was confirmed by the clinical and pathological findings. Of the 42 cases, 30 were primary gastric SRCC and the remaining 12 were primary colorectal SRCC. Of the 42 cases, 26 cases were biopsies and the remaining 16 cases were surgically resected cases. In the 30 gastric SRCC cases, 21 were male and 9 were female. The mean age and standard deviation was 74 years ±14 years. In the 12 colorectal SRCC cases, 7 were male and 5 were female. The mean age and standard deviation was 68 years ±12 years.
An immunohistochemical study was performed by the Dako EnVision method (Dako Corp, Glostrup, Denmark), as previously described [26-30]. The antigens examined included a panel of monoclonal antibodies; MUC1 (clone Ma695, Novocastra Laboratories, NewCastle Upon Tyne, UK; diluation=1: 100) MUC2 (clone Ccp58, Novocastra; diluation=1: 100, MUC5AC (clone CLH2, Novocastra; dilution=1: 200) and MUC6 (clone CLH5, Novocastra, dilution=1: 200). Microwave pretreatment was performed in each immunohistochemical run.
A histochemical investigation was also performed by mucicarmine stain and by combined periodic acid-Schiff after diastase digestion (d-PAS) and Alcian blue (AB) at pH2.5. Statistical analysis was performed by Chi-square test.
Results
The SRCC was composed of medullary proliferation of large clear cells with much intracytoplasmic mucin (neutral mucin and acidic mucin) (Figure 1A-C), which was confirmed by the combined d-PAS/AB technique (Figure 1C) and mucicarmine stains.
Figure 1.
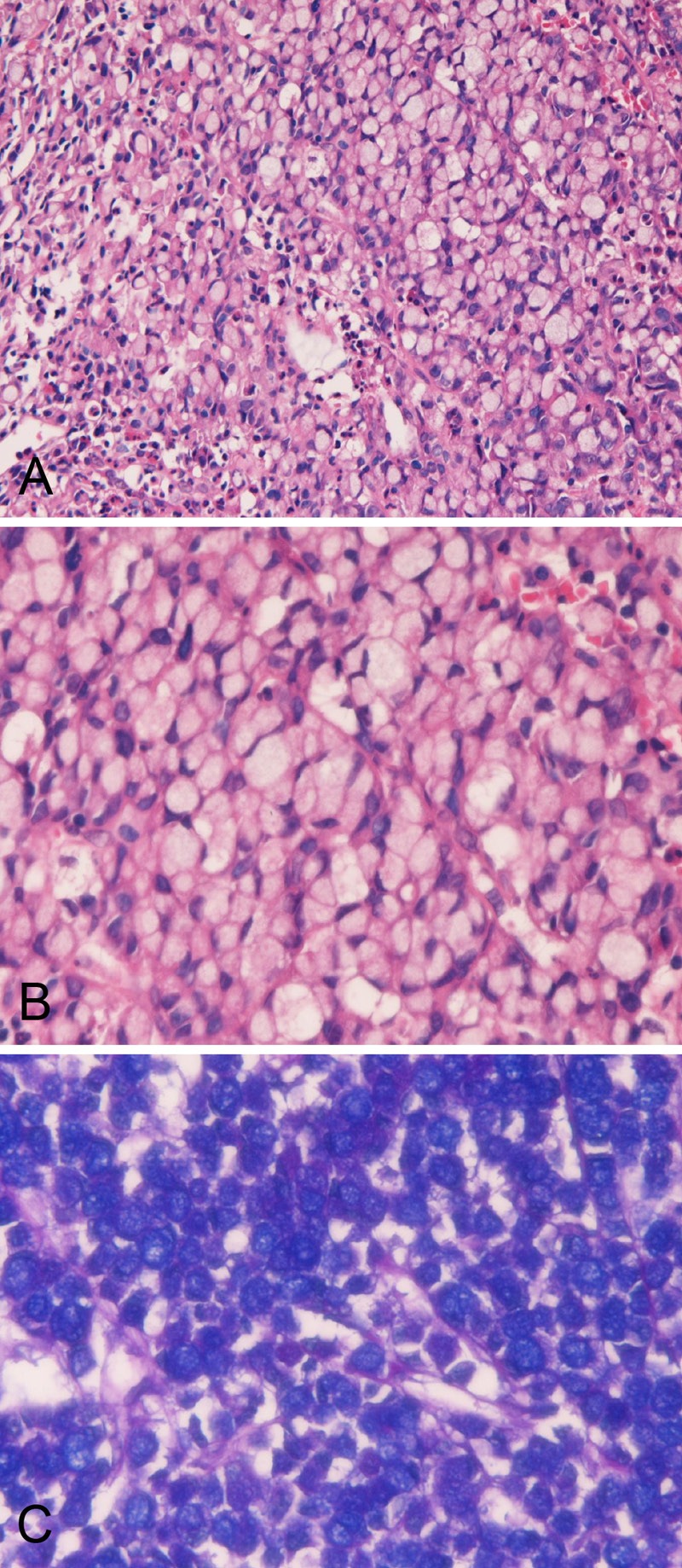
Histology and histochemistry of primary signet-ring cell carcinoma of the stomach. A. Lower power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. The signet-ring cell carcinoma is medullary and the stroma is scant in amount. HE: x100. B. High power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. HE: x400. C. Combined d-PAS and AB stains revealed abundant intracytoplasmic mucins composed of neutral (Mazenta color) and acidic (blue color) mucins. Combined d-PAS/AB double staining: x200.
The proportion of signet ring cells in SRCC ranged from 60% to 100%. In most cases, the SRCC contained areas of other histologies such as mucinous adenocarcinoma, and tubular adenocarcinoma.
The normal expression pattern of these MUC apomucins in non-tumorous normal mucosa was investigated in all the sections of SRCC. In the stomach, no expression of MUC1 was seen in any cell types of the normal stomach. Expression of MUC2 (Figure 2A), MUC5AC (Figure 2B), and MUC6 (Figure 2C and 2D) were consistently recognized in the normal stomach. The expression of MUC2 was mainly seen in the goblet cells of the intestinal metaplasia of the stomach, but it was also noted in non-goblet cells of foveolar epithelium in the stomach (Figure 2A). MUC2 was not seen in other cells types including the fundic glands in the stomach. The expression of MUC5AC was seen only in the foveolar cells of the stomach (Figure 2B), but not in the fundic glands and pyloric glands. The expression of MUC 6 was seen in the foveolar epithelial cells (Figure 2C) and in the pyloric glands (Figure 2D) in the stomach, but not in the fundic glands. In the colorectum, no expression of MUC1, MUC5AC, and MUC6 was seen in any cell types of the colorectum. In contrast, expression of MUC2 was consistently recognized (Figure 3) in the cryptal epithelial cells, particularly in the deep parts of the crypts.
Figure 2.
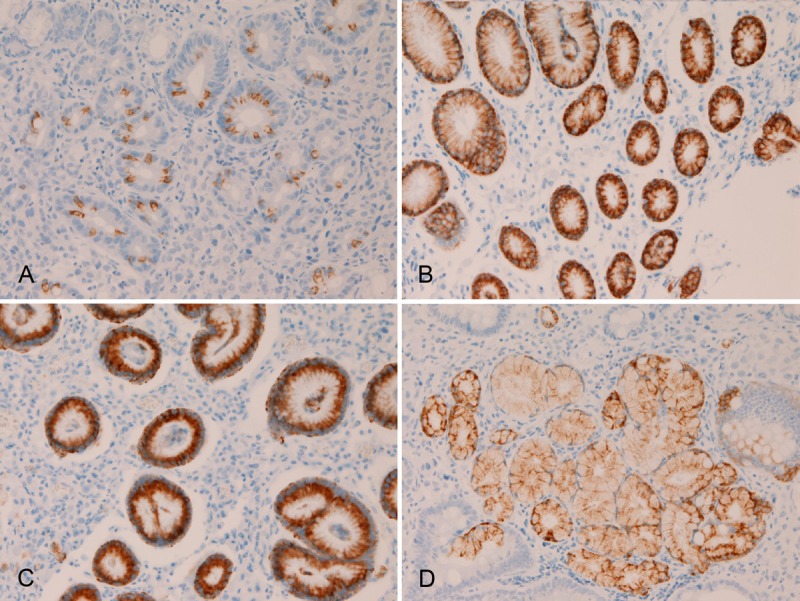
Immunohistochemical findings of the normal stomach. No expression of MUC1 was seen in any cell types of the normal stomach. Expression of MUC2 (A), MUC5AC (B), and MUC6 (C and D) were consistently recognized in the normal stomach. The expression of MUC2 was mainly seen in the goblet cells of the intestinal metaplasia of the stomach, but it is also noted in non-goblet cells of foveolar epithelium in the stomach (A). MUC5AC expression is seen only in the foveolar cells of the normal stomach (B). MUC6 expression is seen in the foveolar epithelial cells (C) and in the pyloric glands (D) in the normal stomach. Immunostaining, x300.
Figure 3.
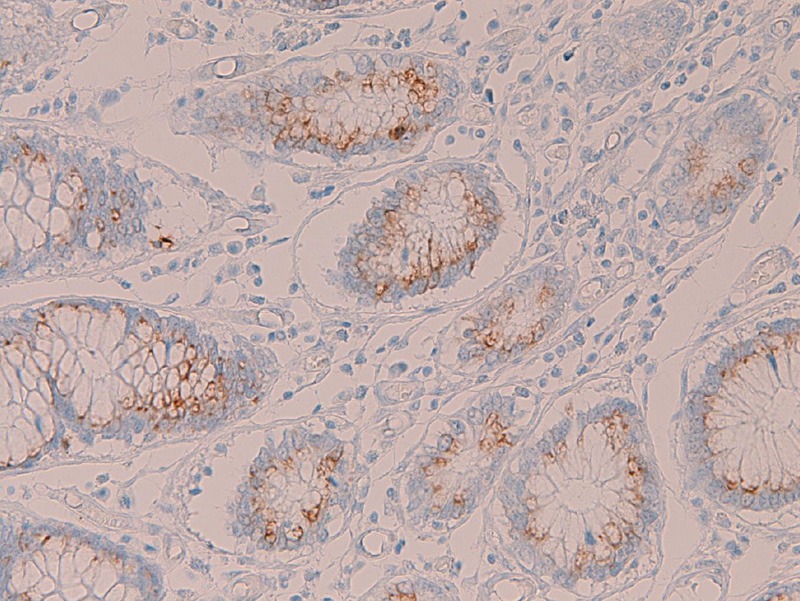
Immunohistochemical findings of the normal colon. In the colorectum, no expression of MUC1, MUC5AC, and MUC6 was seen in any cell types of the colorectum. In contrast, MUC2 expression is consistently recognized in the cryptal epithelial cells. Immunostaining, x300.
In primary gastric SRCC, the expression of MUC1 was seen in 3/30 (10%) (Figure 4A), MUC2 in 4/30 (13%) (Figure 4B), MUC5AC in 20/30 (67%) (Figure 4C), and MUC6 in 21/30 (70%) (Figure 4D). There were significant differences in the positive percentage; the expression percentage of MUC5AC and MUC6 was significantly (p<0.05) higher than that of MUC1 and MUC2. There was a tendency that the MUC immunoreactivity was strong and diffuse in cases with high expression percentage (MUC5AC and MUC6), and that the MUC immunoreactivity was weak and focal in cases with low expression percentage of (MUC1 and MUC2).
Figure 4.
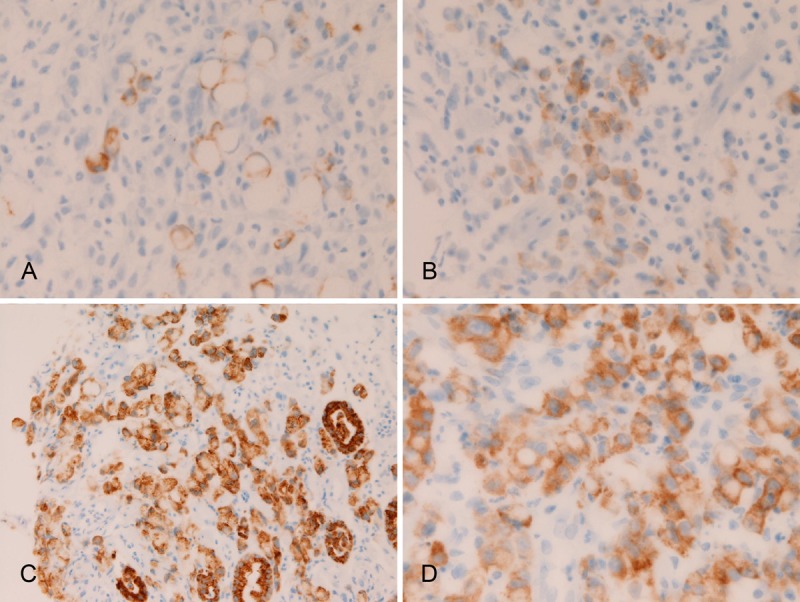
Immunohistochemical findings of primary gastric signet ring cell carcinoma. There are positive expressions of MUC1 (A), MUC2 in (B), MUC5AC (C), and MUC6 (D). Immunostaining, x400.
In primary colorectal SRCC, the expression of MUC1 was seen in 5/12 (42%) (Figure 5A), MUC2 in 11/12 (92%) (Figure 5B), MUC5AC in 4/12 (33%) (Figure 5C), and MUC6 in 0/12 (0%). There were significant differences in the positive percentage; the expression percentage of MUC5AC and MUC6 was significantly (p<0.05) higher than that of MUC1 and MUC2. There was a tendency that the MUC immunoreactivity was strong and diffuse in cases with high expression percentage (MUC1 and MUC2), and that the MUC immunoreactivity was weak and focal in cases with low expression percentage of (MUC 5AC).
Figure 5.
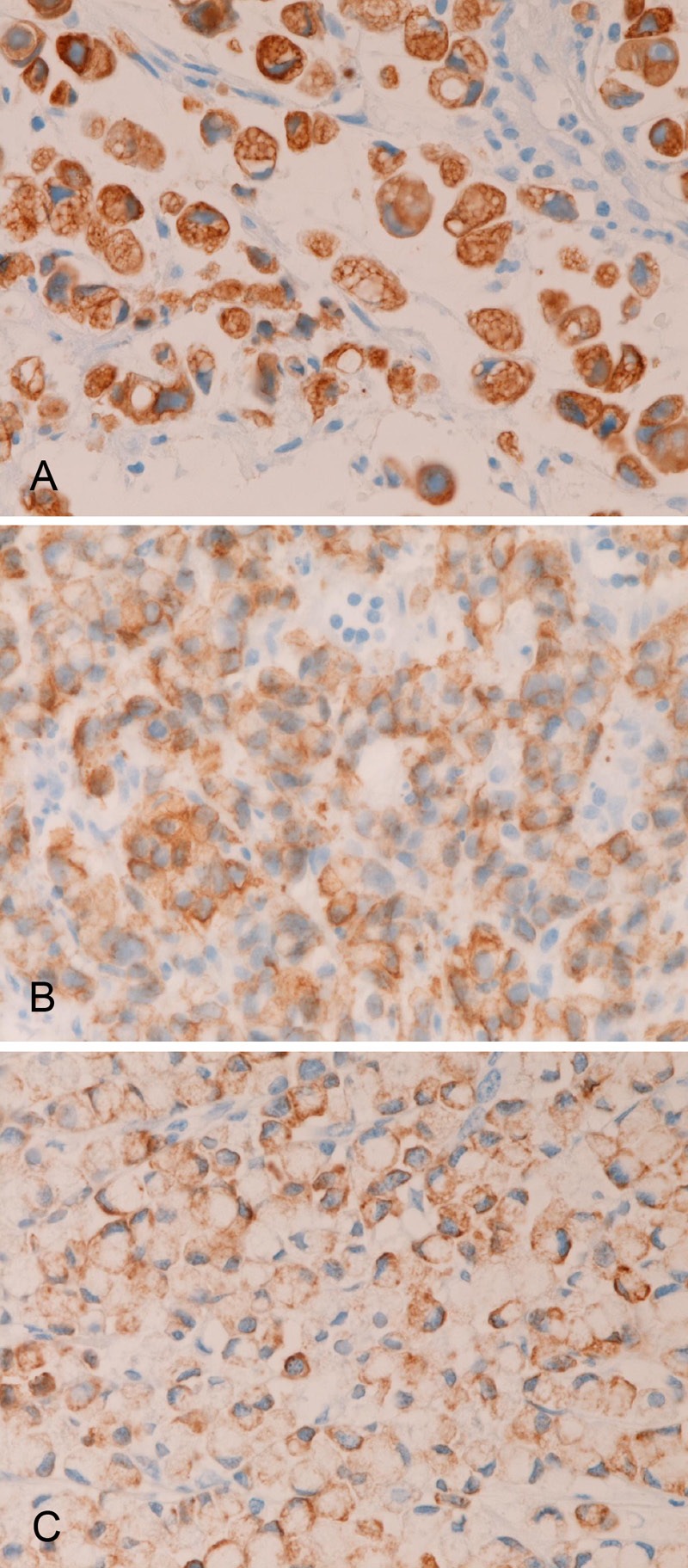
Immunohistochemical findings of primary colorectal signet ring cell carcinoma. There are positive expressions of MUC1 (A), MUC2 (B), and MUC5AC (C). No MUC6 expression is present. Immunostaining, x400.
A comparative statistical study of MUC expression between MUC expression of gastric SRCC and that of colorectal SRCC was performed. Significant differences (p<0.05) were found in the expression of MUC1 (stomach 10% vs colorectal 42%), MUC2 (13% vs 92%), MUC5AC (67% vs 33%), and MUC6 (70% vs 0%). Thus, there is a significant tendency that primary gastric SRCC express MUC5AC and MUC6 but not MUC1 and MUC2, while primary colorectal SRCC express MUC1 and MUC2 but not MUC5AC and MUC6.
Discussion
There have been no comprehensive studies of the MUC expression status in the normal human tissues. In the present study, expression pattern of MUC1, MUC2, MUC5AC, and MUC6 were investigated in the normal mucosa of the non-tumorous regions of 44 cases of gastric and colorectal specimens. The results showed that no expression of the transmembranous apomucin MUC1 was seen in any cell types of the normal stomach. Expression of secretory apomucins MUC2, MUC5AC, and MUC6 were consistently recognized in the normal goblet cells (MUC2), foveolar cells (MUC5AC and MUC6) and pyloric glands (MUC6). The normal fundic glands were negative for all MUC apomucins examined. These findings suggest that no MUC1 is present in the normal stomach, while MUC2, MUC5AC and MUC6 are present in the normal stomach with particular localizations. In the colorectum, no expression of MUC1, MUC5AC, and MUC6 was seen in the normal cryptal cells of the normal colorectum, suggesting that MUC1, MUC5AC, and MUC6 were present in the cryptal epithelium of the normal colorectum. In contrast, expression of MUC2 was consistently recognized in the normal cryptal epithelial cells, particularly in the deep parts of the crypts, suggesting consistent presence of MUC2 in the normal cryptal epithelium. MUC1 has been traditionally called “polymorphic epithelial mucin (PEM)”, MUC2 “goblet cell mucin”, MUC5AC “gastric foveolar mucin”, and MUC6 “pyloric gland-type mucin”. The present study confirmed that this designation is almost correct.
The present study revealed that, in primary gastric SRCC, the expression of MUC1 was 10% (3/30), MUC2 13% (4/30), MUC5AC 67% (20/30), and MUC6 70% (21/30). There were significant differences in the positive percentage; the expression percentage of MUC5AC and MUC6 was significantly (p<0.05) higher than that of MUC1 and MUC2. The current study showed that MUC1 was never present in normal gastric mucosal epithelium. The emergence of MUC1 in 10% (3/30) of gastric SRCC case suggests the carcinogenesis of gastric SRCC may involve MUC1 protein expression and MUC1 gene alterations. In the normal stomach, MUC2 was consistently expressed in the gastric foveolar cells and foveolar goblet-like cells in the present study. The expression of MUC2 in primary gastric SRCC was only 13% (4/30) in the present study, entirely different from normal MUC2 expression (100%) in normal stomach. These findings suggest that down-regulation of MUC2 protein and MUC2 gene may be involved in the carcinogenesis, carcinoma progression, malignant potential, and biologic behaviors of primary gastric SRCC. In the present study, MUC5AC and MUC6 were consistently expressed in the normal certain determined epithelial cells. The expression of MUC5AC and MUC6 in the present gastric SRCC was 67% (20/30) in MUC5AC and 70% (21/30) in MUC6. Therefore, the expression of MUC 5AC is down-regulated in 33% (10/30) and that of MUC6 in 30% (9/30). These findings suggest that the down-regulations of MUC5AC and MUC6 proteins and these genes may be associated with the carcinogenesis, malignant potential and biological behaviors of primary gastric SRCC.
The MUC profile in gastric ordinary adenocarcinoma has rarely been described [19-21]. Koseki et al [19] immunohistochemically examined human gastric mucin, MUC2 and CD10 in early gastric carcinomas, and stated that mucins of gastric adenocarcinoma were classified into gastric type, intestinal type, and mixed gastric and intestinal type. In any way, Koseki et al [19] founded the expression of MUC2 in gastric ordinary adenocarcinoma. This is similar to the present study of primary gastric SRCC, which showed positive MUC2 in 13% (4/30) of gastric SRCC. Ilhan et al [20] also demonstrated very high expressions of MUC1 (90%) and MUC2 (98%) and a low expression MUC5AC (10%) in gastric ordinary carcinoma, the results of which is entirely different from the present results of primary gastric SRCC, in which the MUC1 expression was 10% (3/30), MUC2 13% (4/30), MUC5AC 67% (20/30), and MUC6 70% (21/30). These findings show that the MUC profile of primary gastric SRCC is entirely different from that of primary gastric ordinary adenocarcinoma. This also suggests the different pathogenesis, carcinogenesis, malignant potential and biological behaviors between gastric SRCC and gastric ordinary adenocarcinoma; the malignant potential, aggressiveness, infiltrative features and poor prognosis are more recognized in SRCC than in ordinary adenocarcinoma.
The expression patterns of MUC apomucins (MUC2, MUC5AC and MUC6), similar to the data of Ilhan [20], have been reported by Tsukashita et al [21], further supporting the current concept that the pathogenesis, carcinogenesis, malignant potential, and biological behaviors are different between gastric SRCC and gastric ordinary adenocarcinoma. The present study revealed that, in primary colorectal SRCC, the expression of MUC1 was 42% (5/12), MUC2 92% (11/12), MUC5AC 33% (4/12), and MUC6 0% (0/12). There were significant differences in the positive percentage; the expression percentage of MUC5AC and MUC6 was significantly (p<0.05) higher than that of MUC1 and MUC2. In the current study, only MUC2 was expressed in the normal colorectal epithelium, while no expressions of MUC1, MUC5AC, and MUC6 were seen in any cell types of the normal colorectum. These findings strongly suggest the up-regulation of MUC1 and MUC5AC frequently occurs in primary colorectal SRCC, and that those up-regulations of the proteins and genes of MUC1 and MUC5AC are associated with the pathogenesis, carcinogenesis, malignant potential, and biological behaviors of he colorectal SRCC. The expression of MUC2 and MUC6 is not different between normal colorectal epithelium and colorectal SRCC. This finding suggests that the protein expression and gene status of MUC2 and MUC6 are not involved in the pathogenesis, carcinogenesis, malignant potential, and biological behaviors of the colorectal SRCC.
The MUC profile in colorectal ordinary adenocarcinoma has rarely been described [22,23]. Byrd et al [22] showed high MUC1 expression, low MUC2 expression, and high MUC5AC expression in colorectal ordinary adenocarcinoma. The expression of MUC1 of Byrd et al [22] is similar to that of the present colorectal SRCC which showed 42% (5/12) of cases were positive for MUC1. However, the expressions of MUC2 and MUC5AC of Byrd are entirely different from the present colorectal SRCC, in which the expression of MUC2 was high (92%, 11/12) and that of MUC5AC was low (33%, 4/12). These findings suggest that the molecular mechanisms of carcinogenesis are entirely different between primary colorectal SRCC and ordinal colorectal adenocarcinoma. However, the MUC1 status may be similar between colorectal SRCC and colorectal ordinary carcinoma, suggesting that MUC1 protein and gene may be involved similarly in the pathogenesis of both colorectal SRCC and colorectal ordinary adenocarcinoma. Bu [23] et al. demonstrated high MUC2 expression (100%, 15/15) and high MUC5AC expression (100%, 15/15), and speculated that the high expression of MUC2 and MUC5AC may play a role of progression of colorectal ordinary adenocarcinoma. In the current study, the expression of MUC2 was high (92%, 11/12), but expression of MUC5AC is relatively low (33%, 4/12), further suggesting the different role in cancer carcinogenesis and progression between colorectal SRCC and ordinary adenocarcinomas.
There has been only one comprehensive study of MUC apomucins expression in SRCC of the stomach, colorectum and breast, performed by Nguyen et al [24]. They showed that the expression of MUC1 was 24% (5/21), MUC2 48% (10/21), MUC5AC 38% (8/21), and MUC6 28% (6/21) in primary gastric SRCC. In the current study of gastric SRCC, expression of MUC1 was 10% (3/30), MUC2 13% (4/30) (Figure 4B), MUC5AC 67% (20/30), and MUC6 70% (21/30). The results of the present SRCC are different from those of Nguyen et al [24]. The expression of MUC 1 and MUC2 is significantly (p<0.05) lower in the present SRCC than SRCC of Nguyen et al, and the expression of MUC5AC and MUC6 is significantly (p<0.05) higher in the current SRCC than SRCC of Nguyen et al [24]. This may be due to race difference or may imply that the MUC profile of gastric SRCC is not restricted but shows diverse patterns. Otherwise, the interpretation of the immunostaining was different between the two. In colorectal SRCC, Nguyen et al [24] reported that the expression of MUC1 was 0% (0/11), MUC2 100% (11/11), MUC5AC 9% (1/11), and MUC6 0% (0/11). In the colorectal SRCC of the current cases, the expression of MUC1 was 42% (5/12), MUC2 92% (11/12), MUC5AC 33% (4/12), and MUC6 0% (0/12). A statistical analysis shows that no significant differences are seen in the expression of MUC2 and MUC6, but significant differences are seen in the expression MUC1 and MUC5A, between the colorectal SRCC of Nguyen et al [24] and the colorectal SRCC of the present study. These further suggest that these differences in colorectal SRCC may be due to race difference or different interpretation of the immunostaining, or may imply that the MUC profile of colorectal SRCC is not restricted but shows diverse patterns. Bu et al [23] mentioned that MUC2 expression was 88% (7/8) and MUC5AC 100% (8/8) in colorectal SRCC. These figures are also different from the studies of Nguyen et al [24] and the present cases. Much more studies using large number of cases are required in determining the MUC status in SRCC. Hayashi et al [25], who studies MUC profile of five cases of SRCC of the lung described that the MUC profile of the SRCC of the lung was different from SRCC of the gastrointestinal tract. They mentioned that lung SRCC were positive for MUC1 but negative for MUC2 whereas colon SRCC were negative for MUC1 but positive for MUC2. They stressed that this difference in the expression of MUC1 and MUC2 can differentiate the origins of SRCC. However, their study is not enough because the number of SRCC is too small (five cases). In breast SRCC, Nguyen et al [24] showed expression of MUC1 was 100% (6/6), MUC2 33% (2/6), MUC5AC 16% (1/6), and MUC6 33% (2/6), thus being different from the data of SRCC of the stomach, colorectum, and lung.
In conclusion, the author demonstrated the normal distribution of MUC1, MUC2, MUC5AC, and MUC6 in the normal stomach and colorectum. The author investigated the expression pattern of these MUC apomucins in SRCC of the stomach and colorectum. The expression pattern of gastric SRCC was as follows: MUC1, 3/30 (10%); MUC2, 4/30 (13%); MUC5AC, 20/30 (67%), and MUC6 21/30 (70%). The expression pattern of colorectal SRCC was as follows: MUC1, 5/12 (42%); MUC2, 11/12 (92%); MUC5AC, 4/12 (33%); and MUC6, 0/12 (0%). A comparative study of the present SRCC and presently reported ordinary adenocarcinoma and SRCC cases of the stomach and colorectum was performed.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 2.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 4.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 5.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 6.Lauwers GY, Franceschi S, Carneiro F, Montgomery E, Graham DY, Tatematsu M, Curado MP, Hattori T. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 48–58. [Google Scholar]
- 7.Hamilton SR, Nakamura S, Bosman FT, Quirke P, Boffetta P, Riboli E, IIyas M, Sobin LH, Morreau H. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 134–146. [Google Scholar]
- 8.Terada T. Primary signet-ring cell carcinoma of the lung: a case report with an immunohistochemical study. Int J Clin Exp Pathol. 2012;5:171–174. [PMC free article] [PubMed] [Google Scholar]
- 9.Terada T. Primary signet-ring cell carcinoma of the ampulla of Vater: a case report with an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2012;20:427–428. doi: 10.1097/PAI.0b013e31823b7052. [DOI] [PubMed] [Google Scholar]
- 10.Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report. Endoscopy. 2012;44(Suppl 2):E141–142. doi: 10.1055/s-0030-1257045. [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Primary pure signet ring cell adenocarcinoma of the non-Barrett’s esophagus: a case report with immunohistochemical study. Endoscopy. 2011;43:E397–8. doi: 10.1055/s-0030-1256944. [DOI] [PubMed] [Google Scholar]
- 12.Terada T. Primary pure signet-ring cell adenocarcinoma of the urinary bladder: a report of three cases with an immunohistochemical study. Med Oncol. 2012;29:2866–9. doi: 10.1007/s12032-011-0122-7. [DOI] [PubMed] [Google Scholar]
- 13.Terada T. Signet-ring cell carcinoma of the non-ampullary duodenum and proximal jejunum: a case report with an immunohistochemical study. Endoscopy. 2013 doi: 10.1055/s-0031-1291528. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Terada T. Primary pure signet-ring cell carcinoma of the anus: a case report with immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291516. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Terada T. An immunohistochemical study of a primary signet-ring cell carcinoma of the ampulla of Vater: A case report. J Gastrointest Cancer. 2012 doi: 10.1007/s12029-012-9469-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Signet-ring cell carcinoma of the esophagus in dermatomyositis: a case report with immunohistochemical study. J Gastrointest Cancer. doi: 10.1007/s12029-012-9473-3. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Terada T. Ovarian malignant Mullerian mixed tumor (heterologous) whose epithelial component is composed predominantly of signet ring cell carcinoma. Arch Gynecol Obstet. 2011;283:1403–1406. doi: 10.1007/s00404-010-1591-1. [DOI] [PubMed] [Google Scholar]
- 18.Terada T. Small Cell Carcinoma of the Ileum That Developed 10 Years After Total Gastrectomy for Gastric Signet-ring Cell Carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:618–619. doi: 10.1097/PAI.0b013e31823eb34f. [DOI] [PubMed] [Google Scholar]
- 19.Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sigihara K. Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer. 2000;89:724–732. [PubMed] [Google Scholar]
- 20.Ilhan O, Han U, Onal B, Celik SY. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol. 2010;21:345–352. doi: 10.4318/tjg.2010.0119. [DOI] [PubMed] [Google Scholar]
- 21.Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166–170. doi: 10.1002/ijc.1460. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 23.Bu XD, Li N, Tian XG, Li L, Wang JS, Yu XJ, Huang PL. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J Gastroenterol. 2010;16:4089–4094. doi: 10.3748/wjg.v16.i32.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen MD, Plasil B, Wen P, Frankle WL. Mucin profiles in signet-ring cell carcinoma. Arch Pathol Lab Med. 2006;130:799–804. doi: 10.5858/2006-130-799-MPISCC. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi H, Kitamura H, Nakatani Y, Inayama Y, Ito T, Kiutamura H. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol. 1999;30:378–383. doi: 10.1016/s0046-8177(99)90111-9. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type) Int J Clin Exp Pathol. 2011;4:797–798. [PMC free article] [PubMed] [Google Scholar]
- 27.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens: I. Benign lesions. Int J Clin Exp Pathol. 2012;5:46–51. [PMC free article] [PubMed] [Google Scholar]
- 28.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens in a single Japanese hospital: II. Malignant lesions. Int J Clin Exp Pathol. 2012;5:52–57. [PMC free article] [PubMed] [Google Scholar]
- 29.Terada T. Malignant tumors of the small intestine: A histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203–209. [PMC free article] [PubMed] [Google Scholar]
- 30.Terada T. A clinical-histopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–198. [PMC free article] [PubMed] [Google Scholar]


