Abstract
Ferritin L (FTL) and Ferritin H (FTH) subunits are responsible for intercellular iron storage. We previously reported increasing amounts of liver cytoplasmic and nuclear iron content during acute phase response (APR). Aim of the present study is to demonstrate intracellular localization of ferritin subunits in liver compared with extra hepatic organs of rat under physiological and acute phase conditions. Rats were administered turpentine-oil (TO) intramuscularly to induce a sterile abscess (acute-phase-model) and sacrificed at different time points. Immunohistochemistry was performed utilizing horse-reddish-peroxidise conjugated secondary antibody on 4μm thick section. Liver cytoplasmic and nuclear protein were used for Western blot analysis. By means of immunohistology, FTL was detected in cytoplasm while a strong nuclear positivity for FTH was evident in the liver. Similarly, in heart, spleen and brain FTL was detected mainly in the cytoplasm while FTH demonstrated intense nuclear and a weak cytoplasmic expression. Western blot analysis of cytoplasmic and nuclear fractions from liver, heart, spleen and brain further confirmed mainly cytoplasmic expression of FTL in contrast to the nuclear and cytoplasmic expression of FTH. The data presented demonstrate the differential localization of FTL and FTH within hepatic and extra hepatic organs being FTL predominantly in the cytoplasm while FTH predominantly in nucleus.
Keywords: Ferritin, nuclear localization, liver, acute phase, iron regulation
Introduction
Liver is key organ for iron homeostasis and storage under physiological as well as acute phase conditions. Within the cell, iron is stored mainly as ferritin [1]. Ferritin is composed of L and H subunits that are highly conserved [2] nevertheless, genetically separate [3,4] and maintain distinct functions [5]. The storage of iron is considered to take place in the cytoplasm, however iron is required for the nuclear functions as well. L and H subunits spontaneously assemble in a 24-subunit protein “cage” with a flexible H: L ratio. The H: L ratio can vary between different cell types [2,5]. The L gene has very little tissue-specific regulations whereas multiple conditions activate H ferritin gene transcription [6,7] including cell differentiation, changes in the cell proliferation status, oncogenes, cytokines, and heme. Infact, a previous study has showed an association between ferritin expression and cell proliferation [8].
Acute-phase response (APR) is a major physiological defence reaction of the body aimed to eliminate the injuring noxae and to re-establish homeostasis. Clinically, it is characterized by fever, somnolence, weakness, muscular joint pain, adinamia and increased liver activity. Moreover, decrease of serum iron level is also a hallmark of acute-phase reaction [9,10]. This decrease is considered to be due to the sequestration of iron by the reticuloendothelial system [11].
In previous work, we demonstrated that under acute phase conditions the liver takes up serum iron [12,13] and increased hepatic iron level is demonstrable in the nuclear fraction of the liver tissue as well [13]. The increased nuclear iron content was further supported by the nuclear detection of iron import proteins including TfR2 and DMT-1 along with nuclear Fpn-1; the iron export protein under physiological and acute phase conditions [13]. The aim of our prospective study was to determine the intracellular localization of major iron storage proteins; FTH and FTL in hepatic as well as extra hepatic organs.
Methods
Materials
Animals
Rats (170–200 g body weight), were purchased from HarlanWinkelmann (Brochen, Germany). The animals were kept under standard conditions with 12:12-h light dark cycles, and were given ad libitum access to water and food. All animals were cared for in accordance with the guidelines of our institution, the German Convention for the Protection of Animals, and the National Institutes of Health (USA).
Induction of acute phase and harvesting the liver, heart, spleen and brain
APR was induced and organs were removed as described previously [14]. Briefly, tissue damage was induced by injecting 5 ml/kg-TO in both right and left hind limbs of animals. Control animals were treated in the same way for each time point with saline injection. Liver, heart, spleen and brain tissue was harvested, cut into pieces and snapped frozen for further used.
Immunohistochemistry and immunocytology
Four to five micrometer thick cryostat sections (Reichert Jung, Wetzlar, Germany) from rat liver, heart, spleen and brain were used for immunodetection of FTL and FTH. The slides were air-dried, fixed with ice cold methanol (-20°C, 10 min) and ice cold acetone (-20°C, 10 sec) and stored at -20°C. After inhibition of endogenous peroxidase by incubating the slides with phosphate-buffered saline (PBS) containing glucose/glucose oxidase/sodium azide, the sections were treated with FCS for 30 min to minimize nonspecific staining. Peroxidase immunostaining was performed utilizing two different commercially available antibodies for FTL (abcam; UK and Santa Cruz; USA) and FTH (LS Bio and Santa Cruz from USA). The primary antibodies were diluted FTH (1:10), FTL (1:50). Negative controls were incubated with isotype-specific IgGs, instead of the specific primary antibody. After washing, the slides were covered with peroxidase-conjugated anti-rabbit/anti-mouse immunoglobulins pre-absorbed with normal rat serum to avoid cross-reactivity. Slides were washed and incubated with PBS containing 3,3-diaminobenzidine (0.5 mg/ml) and H2O2 (0.01%) for 10 min to visualize immune complexes. Nuclei were counterstained with Meyer’s hemalaun solution before the slides were mounted with cover slips.
Cellular fractionation for protein isolation
Liver, heart, spleen and brain cytoplasmic and nuclear proteins were isolated using NucBuster Protein Extraction kit (Novagen USA) as described by manufacturer, with some modifications. Briefly, 100 mg of tissue sample was homogenized in 300 μl of NucBuster reagent 1 followed by collection of supernatant as cytoplasmic fraction. Pellet was washed thrice with sterile ice-cold PBS and dissolved in 50 μl of NucBuster reagent 2.1 μl of 100mMDTT and Protease Inhibitor Cocktail Set I was added to each sample to inhibit proteases activity. Samples were stored at -20°C for further use.
Western blot analysis
30 μg of protein from tissue fraction was applied per well and were subjected to electrophoresis using NuPAGEÒ (4-12% Bis-Tris Gel; Invitrogen) under reducing conditions [15]. After electrophoresis the proteins were transferred to Hybond-ECL nitrocellulose membranes [16]. Immunodetection was performed according to the ECL Western blotting protocol. The anti-Ferritin L (abcam and Santa cruz) and anti-Ferritin H (LSBio, Santa cruz) were used in the study.
Results
Hepatic expression and localization of FTL and FTH
Immunohistochemical analysis of normal liver showed FTL granular positivity mainly in the cytoplasm of hepatic cells, which kept on increasing in TO treated rats. Intensity of FTL immunoexpression was found to be stronger after TO-injection with an intense expression in hepatocytes (distinguished on visual morphology; Figure 1 enlarged insets). The number of cells positive for FTL cytoplasmic expression was highest at 24h after TO-injection. The reaction was negative when the primary antibody was omitted (negative control; Figure 1; upper right panel).
Figure 1.
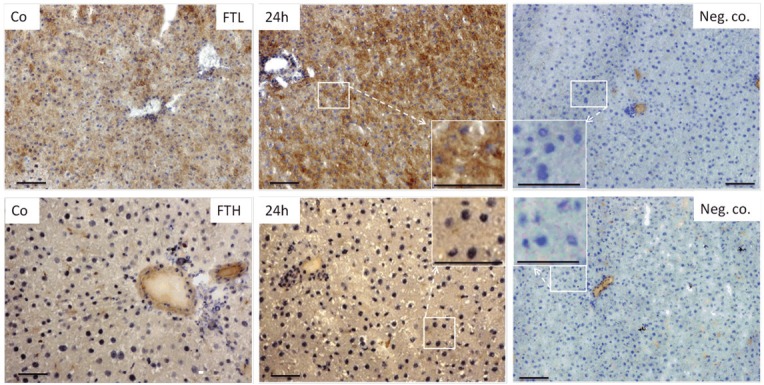
Immunodetection of FTL (upper panel) and FTH (lower panel) on cryostat sections of rat liver from control and TO-injected animals utilizing horse reddish peroxidase conjugated secondary antibody. Negative control represents immunostaining when primary antibody was omitted. Insets show the enlarged magnification of selected box. Original magnification 200x bar 50μm.
In contrast to FTL immunolocalization, peroxidase staining of FTH showed intense granular positivity in the nucleus of hepatic cells of control and TO-injected rats. Compared to negative controls (primary antibody omission) where nuclei were stained clear blue, liver tissue from control and TO-injected rats showed FTH blotching (brown dots) mainly in the large nuclei of hepatocytes (Figure 1; lower panel).
Localization of FTL and FTH in extra-hepatic organs
In control heart tissue, the immunodetection of FTL indicated a very weak expression as compared to liver tissue. However, it was localized exclusively within the cytoplasm. The protein expression of FTL showed more intense granular expression in TO-injected animals at 24h Figure 2; upper panel). In contrast to FTL expression within the heart tissue, FTH immunodetection was more intense and was spread all over the tissue structure including the cytoplasm and the nuclei of tissue (Figure 2; lower panel).
Figure 2.
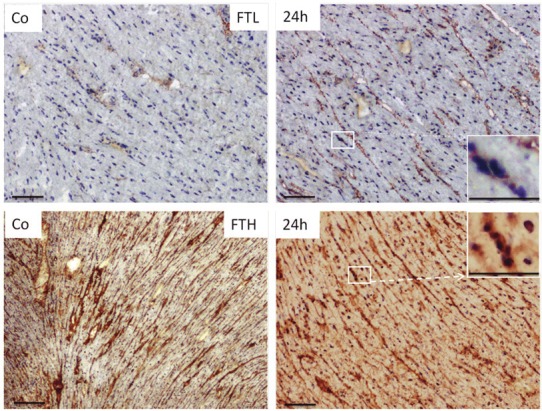
Immunodetection of FTL (upper panel) and FTH (lower panel) on cryostat sections of rat heart from control and TO-injected animals utilizing horse reddish peroxidase conjugated secondary antibody. Insets show the enlarged magnification of selected box. Original magnification 100x bar 25μm.
In control spleen tissue, FTL was detected within the cytoplasm of red pulp and white pulp cell population. This expression was more copious in spleen tissue of TO-injected rats (Figure 3; upper panel). However, within the spleen tissue, FTH protein showed a dual localization. We could detect a strong nuclear and comparatively weak cytoplasmic expression of FTH within spleen tissue of control and TO-injected rats (Figure 3; lower panel).
Figure 3.

Immunodetection of FTL (upper panel) and FTH (lower panel) on cryostat sections of Rat spleen from control and TO-injected animals utilizing horse reddish peroxidase conjugated secondary antibody. Insets show the enlarged magnification of selected box. Original magnification 100x bar 25μm.
However, within the brain tissue of both control and TO-injected rats, FTL was plentiful in the cytoplasm and a slight nuclear expression was also detected (Figure 4; upper panel). However, in both control and TO-injected animals, FTH was localized strongly within the brain nuclei and weakly in the cytoplasm (Figure 4; lower panel).
Figure 4.

Immunodetection of FTL (upper panel) and FTH (lower panel) on cryostat sections of Rat brain from control and TO-injected animals utilizing horse reddish peroxidase conjugated secondary antibody. Insets show the enlarged magnification of selected box. Original magnification 100x bar 25μm.
Western blot analysis of hepatic and extra hepatic protein fractions for FTL and FTH
The immunohistochemical data was further confirmed by means of Western blot analysis of cytoplasmic and nuclear fractions proteins from hepatic and extra hepatic organs of control animals. Western blot analysis demonstrated mainly cytoplasmic expression of FTL in liver, heart, spleen and brain. FTL was found to be more abundant in liver followed by spleen and then heart and brain. While, only a very slight FTL nuclear expression was found in spleen. FTH was detected mainly in the nuclear fraction of liver, heart, spleen and brain. However, it was also detectable in the liver and heart cytoplasmic fraction (Figure 5).
Figure 5.

Western blot analysis of FTL and FTH in protein extracted from nuclear and cytoplasmic fractions of different organs of control animals.
Discussion
To our best knowledge, this is the first attempt to determine the predominantly nuclear localization of FTH in contrast to cytoplasmic expression of FTL under physiological and acute phase conditions. Immunodetection protocols and Western blot analysis showed a strong cytoplasmic and very weak nuclear expression of FTL as compared to the strong nuclear and weak cytoplasmic localization of FTH in hepatic and extra hepatic organs of rat including heart, spleen and brain. Moreover, protein expression was found to be elevated for both FTL and FTH by Immunohistology with the onset of APR.
Ferritin has been investigated as a cytosolic iron storage protein [17]. Its localization within the cell however, is controversially debated. So far, nuclear localization of FTH is reported in-vitro in human astrocytoma cell line [18], in corneal epithelial cells [19] and in-vitro in mice hepatocytes under iron overload conditions [20]. Indeed, we showed constitutive nuclear FTH detection not only in the hepatic but also in extra-hepatic organs of rat under physiological and acute phase conditions. As increasing amounts of iron seem to be temporarily needed in the nucleus during the APR, therefore, it is not surprising to detect iron storage proteins not only in cytoplasm, but also in the nucleus of the cells.
Nuclear FTH of our current study suggests iron sequestration not only in cytoplasm but also in nucleus of liver cells indicating an important role of iron for nuclear metabolism. It could also suggest that iron may be necessary for the activity of nuclear enzymes for DNA synthesis and repair and/or to regulate the initiation of transcript [21]. Another possibility could be that under acute-phase conditions, extra iron may be needed to satisfy the increased metabolic work of the liver [10].
We previously reported elevated liver iron stores in the same model [13]. Likewise, our previous and current study showed an increased mRNA and protein expression of FTL and FTH in the liver, in parallel to the increased hepatic uptake of iron during APR [13]. Most of the initial observations reported that the amount of intracellular ferritin could be modified by changes in iron status [17] and accumulation of H-chain [22,23]. However, our previous study also showed that gene expression of FTL, FTH and of other iron regulatory genes is modulated also by acute phase cytokines [13,24]. In other words, the increase in hepatic FTL and FTH expression is not only due to the increase in hepatic iron concentration but it is also due to the direct effect of acute-phase cytokines produced during TO-induced APR.
A previous study [25] reported that FTH is the main iron storage protein in the liver. Depletion of FTH in hepatocytes makes these cells more susceptible to the toxic effect of iron [25]. Moreover, it has been shown that in non-hepatic cell lines (K562 cells) the over-expression of FTH resulted in reduced free iron pool [26]. This may indicate that not only ferritin L but also ferritin H subunit could be required to reduce free available iron level in the “stressed” hepatocytes during APR.
FTL shares “the iron storage” function in liver tissue and extra hepatic organs (heart, spleen and brain), however the secretory function of liver makes it unique to extra hepatic organs. We reported FTL as a secretory protein (manuscript submitted) so liver should have more FTL as compared to extra hepatic organs.
An earlier study reported evidence of stainable iron within the nuclei of hepatocytes and Kupffer cells in mouse liver under conditions of iron overload [27]. Our current finding supports not only the presence of iron storage protein within the nuclei of liver cells under non-physiological conditions but suggests that nuclear iron is important in the initiation of defense mechanism.
In summary, FTH is localized not only in the cytoplasm but also in the nucleus of liver, heart, spleen and brain cells. This suggests that iron is not only stored in the nucleus but also that nuclei need to be defended from possible dangerous effects of iron overload on DNA as has been hypothesized previously [25]. This might become more important when the metabolic challenges increase during APR.
References
- 1.Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012;2:a011668. doi: 10.1101/cshperspect.a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–99. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Caskey JH, Jones C, Miller YE, Seligman PA. Human ferritin gene is assigned to chromosome 19. Proc Natl Acad Sci U S A. 1983;80:482–6. doi: 10.1073/pnas.80.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worwood M, Brook JD, Cragg SJ, Hellkuhl B, Jones BM, Perera P, Roberts SH, Shaw DJ. Assignment of human ferritin genes to chromosomes 11 and 19q13.3----19qter. Hum Genet. 1985;69:371–4. doi: 10.1007/BF00291657. [DOI] [PubMed] [Google Scholar]
- 5.Sammarco MC, Ditch S, Banerjee A, Grabczyk E. Ferritin L and H subunits are differentially regulated on a post-transcriptional level. J Biol Chem. 2008;283:4578–87. doi: 10.1074/jbc.M703456200. [DOI] [PubMed] [Google Scholar]
- 6.Briat JF, Ravet K, Arnaud N, Duc C, Boucherez J, Touraine B, Cellier F, Gaymard F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811–22. doi: 10.1093/aob/mcp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 8.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103:2377–83. doi: 10.1182/blood-2003-06-1842. [DOI] [PubMed] [Google Scholar]
- 9.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, Sabharwal V, Algül H, Akira S, Schmid RM, Pelton SI, Spira A, Mizgerd JP. Hepatocyte-specific mutation of both NF-kappaB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest. 2012;122:1758–63. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141–55. doi: 10.1055/s-2007-1007106. [DOI] [PubMed] [Google Scholar]
- 11.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–7. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Malik IA, Naz N, Sheikh N, Khan S, Moriconi F, Blaschke M, Ramadori G. Comparison of changes in gene expression of transferrin receptor-1 and other iron-regulatory proteins in rat liver and brain during acute-phase response. Cell Tissue Res. 2011;344:299–312. doi: 10.1007/s00441-011-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naz N, Malik IA, Sheikh N, Ahmad S, Khan S, Blaschke M, Schultze F, Ramadori G. Ferroportin-1 is a ‘nuclear’-negative acute-phase protein in rat liver: a comparison with other iron-transport proteins. Lab Invest. 2012;92:842–56. doi: 10.1038/labinvest.2012.52. [DOI] [PubMed] [Google Scholar]
- 14.Ramadori P, Ahmad G, Ramadori G. Cellular and molecular mechanisms regulating the hepatic erythropoietin expression during acute-phase response: a role for IL-6. Lab Invest. 2010;90:1306–24. doi: 10.1038/labinvest.2010.85. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm. 2011;118:337–47. doi: 10.1007/s00702-011-0582-0. [DOI] [PubMed] [Google Scholar]
- 18.Surguladze N, Patton S, Cozzi A, Fried MG, Connor JR. Characterization of nuclear ferritin and mechanism of translocation. Biochem J. 2005;388:731–40. doi: 10.1042/BJ20041853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai CX, Linsenmayer TF. Nuclear translocation of ferritin in corneal epithelial cells. J Cell Sci. 2001;114:2327–34. doi: 10.1242/jcs.114.12.2327. [DOI] [PubMed] [Google Scholar]
- 20.Smith AG, Carthew P, Francis JE, Edwards RE, Dinsdale D. Characterization and accumulation of ferritin in hepatocyte nuclei of mice with iron overload. Hepatology. 1990;12:1399–405. doi: 10.1002/hep.1840120622. [DOI] [PubMed] [Google Scholar]
- 21.Thompson KJ, Fried MG, Ye Z, Boyer P, Connor JR. Regulation, mechanisms and proposed function of ferritin translocation to cell nuclei. J Cell Sci. 2002;115:2165–77. doi: 10.1242/jcs.115.10.2165. [DOI] [PubMed] [Google Scholar]
- 22.Hentze MW, Caughman SW, Rouault TA, Barriocanal JG, Dancis A, Harford JB, Klausner RD. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238:1570–3. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- 23.Goralska M, Holley BL, McGahan MC. Overexpression of H- and L-Ferritin Subunits in Lens Epithelial Cells: Fe Metabolism and Cellular Response to UVB Irradiation. Invest Ophthalmol Vis Sci. 2001;42:1721–7. [PubMed] [Google Scholar]
- 24.Sheikh N, Batusic DS, Dudas J, Tron K, Neubauer K, Saile B, Ramadori G. Hepcidin and hemojuvelin gene expression in rat liver damage: in vivo and in vitro studies. Am J Physiol Gastrointest Liver Physiol. 2006;291:G482–G490. doi: 10.1152/ajpgi.00586.2005. [DOI] [PubMed] [Google Scholar]
- 25.Darshan D, Vanoaica L, Richman L, Beermann F, Kuhn LC. Conditional deletion of ferritin H in mice induces loss of iron storage and liver damage. Hepatology. 2009;50:852–60. doi: 10.1002/hep.23058. [DOI] [PubMed] [Google Scholar]
- 26.Picard V, Epsztejn S, Santambrogio P, Cabantchik ZI, Beaumont C. Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J Biol Chem. 1998;273:15382–6. doi: 10.1074/jbc.273.25.15382. [DOI] [PubMed] [Google Scholar]
- 27.Magens B, Dullmann J, Schumann K, Wulfhekel U, Nielsen P. Nuclear iron deposits in hepatocytes of iron-loaded HFE-knock-out mice: a morphometric and immunocytochemical analysis. Acta Histochem. 2005;107:57–65. doi: 10.1016/j.acthis.2004.08.006. [DOI] [PubMed] [Google Scholar]


