Abstract
There have no comprehensive immunohistochemical studies of primary signet ring cell carcinoma (SRCC) in the stomach and colorectum. The author examined the expression of nine common antigens (EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63) in the non-tumorous normal epithelium of the stomach and colorectum and in 42 cases of primary SRCC of the stomach (30 cases) and colorectum (12 cases). The normal epithelium of the stomach and colon consistently (100%) expressed EMA, CEA, CA19-9, CDX-2, and Ki-67 (labeling <15%). Normal epithelium of these locations never expressed p53, TTF-1, vimentin, and p63. In the primary gastric SRCC, the expression percentage of EMA was 57% (17/30), CEA 100% (30/30), CA19-9 100% (30/30), CDX-2 43% (13/30), p53 83% (25/30), Ki-67 100% (30/30) (labeling index= 36 ± 23 %), TTF-1 0% (0/30), vimentin 0% (0/30), and p63 0% (0/30). In primary colorectal SRCC, the expression percentage of EMA was 25% (3/12), CEA 100% (12/12), CA19-9 100% (12/12), CDX-2 93% (28/30), p53 75% (9/12), Ki-67 100% (30/30) (labeling index= 47% ± 26 %), TTF-1 0% (0/12), vimentin 0% (0/12), and p63 0% (0/12). A comparative statistical analysis showed significant difference in EMA (gastric SRCC 57% vs colorectal SRCC 25%) and CDX-2 (43% vs 93%). There were no significant differences in the other seven antigens’ expression between primary gastric SRCC and primary colorectal SRCC. These findings provide much knowledge of primary SRCC of the stomach and colorectum. The data indicated primary gastric SRCC frequently express EMA but not CDX-2 whereas primary colorectal SRCC frequently express CDX-2 but not EMA. These findings also suggest that EMA and CDX-2 are down-regulated during the gastric SRCC carcinogenesis. This down regulations may be associated with the malignant transformation of gastric SRCC. The data of colorectal SRCC suggest EMA is markedly down-regulated and also suggest that this EMA down-regulation may be associated with the carcinogenesis of colorectal SRCC. The expression pattern of EMA and CDX-2 may be useful in differential diagnosis between primary gastric SRCC and primary colorectal SRCC in the metastatic sites of SRCC.
Keywords: Signet-ring cell carcinoma, common antigens, EMA, CDX-2, stomach, colorectum, histopathology, immunohistochemistry
Introduction
Signet-ring cell carcinoma (SRCC) is characterized by an adenocarcinoma whose carcinoma cells were composed predominantly of SRCC cells [1,2]. SRCC cells are characterized by abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei which are created by the compression by intracytoplasmic mucins [1,2]. SRCC can occur in any organs, but is most prevalent in the stomach, followed in order by colorectum and lung [1,2]. According to the current WHO blue book, SRCC is defined as an adenocarcinoma with the presence of >50% of tumor cells (signet-ring cells) with prominent intracytoplasmic mucins [2]. The author has examined SRCC in the extra-gastric and extra-colorectal SRCC [3-13], and examined many antigenic expressions in primary SRCC of extra-gastric and extra-colorectal organs. There have been no comprehensive immunohistochemical studies on the expression of common antigens in primary gastric SRCC and primary colorectal SRCC. In addition, there have also been no comprehensive immunohistochemical studies on the expression of common antigens in primary gastric ordinary adenocarcinomas and primary colorectal ordinary carcinoma. There have been no comprehensive studies of normal distribution of common antigens in the normal mucosa of the stomach and colorectum.
In the present study, the author reports an immunohistochemical study on the expression of nine common antigens (EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63) in primary gastric SRCC and in primary colorectal SRCC. In addition, the author described the normal distribution of these nine common antigens in non-tumorous normal mucosa of the stomach and colorectum.
EMA (epithelial membrane antigen) is a glycoprotein present in the human milk fat globule membrane, and is an excellent for most normal and neoplastic epithelia but is not restricted to them [14]. EMA is also expressed in mesothelioma, meningioma, a variety of mesenchymal neoplasms, and even some malignant lymphoma. CEA (carcinoembryonic antigen) is a glycoprotein of heterogenous composition (MW 200,000) normally detected in the glycocalix of fetal epithelial cells, particularly those of mucin-secreting glandular nature [15]. It is detectable only in small amounts in normal adult cells and benign tumors but is present in large quantities in carcinomas, particularly in adenocarcinomas of the gastrointestinal tract, lung adenocarcinoma, and thyroid medullary carcinoma. CA19-9 (cancer associated carbohydrate antigen 19-9) is also a member of glycoproteins present on the cancer cell membrane [16]. According to the carbohydrate chain structures and their status of sialylation and fucosylation, there are classified into CA19-9, CA15.3, CA 50, CA-125, CA242, SLEX, B72.3, and CEA. CA19-9 is known to be expressed in most of gastrointestinal cancers, particularly in pancreatico-biliary carcinomas. CDX-2 is a transcriptional factor that plays an important role in the proliferation and differentiation of intestinal epithelial cells [17]. The protein, CDX-2, is highly restricted to intestinal epithelium and therefore promises to become a very useful marker of adenocarcinomas of intestinal origin. p53 is the product of very famous tumor suppresser gene p53 [18]. The product of this gene, p53, is a nuclear protein thought to be involved in the control of the cell cycle, apoptosis, and maintenance of genomic stability. Mutations of the p53 gene represent the most common genetic alteration in human tumors. The altered protein product of mutant has a much extended half-time and can be detected by immunohistochemical techniques. It should be noted, however, that accumulation of the p53 protein can also occur as a result of epigenetic changes, and therefore it is not an obligatory indicator of a gene mutation. However, the immunohistochemical demonstration of p53 highly suggests a p53 gene mutation and malignant potential of tumors. Ki-67 is an antigen that corresponds to a nuclear non-histone protein expressed by cells in the proliferative phases G1, G2, M, and S [19]. Recently a monoclonal antibody was developed that detect formalin-resistant epitope (MIB-1). Ki-67 is a good marker to detect cell proliferative fraction. Other techniques for cell proliferation include AgNOR, PCNA, KiS1, CDK, CdC2, and flow cytometry. TTF-1 (thyroid transcriptional factor-1 is a nuclear transcriptional factor necessary for the development of the thyroid and pulmonary tissue [20]. This DNA-binding protein was first identified in the thymocytes and later in pneumocytes. It is expressed in all types of thyroid carcinoma except anaplastic type. TTF-1 is also present in most cases of lung carcinomas. TT-F-1 is usually not expressed in gastrointestinal carcinomas. Vimentin is one of the five major types of cytoplasmic intermediate filaments (MW 57,000) [21]. It is characteristic of cells of mesenchymal nature, such as endothelial cells and muscle cells. However, vimentin is not restricted to cells of mesodermal origin but is sometimes also expressed in tumors of epithelial or neural nature. Thus, vimentin may be expressed in carcinoma with sarcomatoid differentiation. P63 is a homolog of p53 which is consistently expressed by basal and stem cells of striated epithelium, and myoepithelial cells of breast and salivary glands. Six different isoforms exist, the function of which is largely unknown.
Materials and methods
The author retrieved primary adenocarcinomas with signet-ring cells phenotypes of the stomach and colon in the author’s computer database files of primary SRCC of the digestive organs in the recent 15 years. The computer survey identified 68 cases of primary adenocarcinoma of the stomach and colorectum with signet-ring cell phenotype. The author reviewed these 68 cases under the microscopy. The author confirmed the signet-ring cell phenotype of these adenocarcinomas, and excluded cases of adenocarcinoma with SRCC cells whose percentage was less than 50% of the tumor cells. As the results, 42 cases of SRCC fulfilling the WHO criteria [1,2] remained. The primary nature of these 42 cases of SRCC was confirmed by the clinical and pathological findings. Of the 42 cases, 30 were primary gastric SRCC and the remaining 12 were primary colorectal SRCC. Of the 42 cases, 26 cases were biopsies and the remaining 16 cases were surgically resected cases. In the 30 gastric SRCC cases, 21 were male and 9 were female. The mean age and standard deviation was 74 years ±14 years. In the 12 colorectal SRCC cases, 7 were male and 5 were female. The mean age and standard deviation was 68 years ±12 years.
An immunohistochemical study was performed by the Dako EnVision method (Dako Corp, Glostrup, Denmark), as previously described [22-27]. The antigens examined included a panel of monoclonal and polyclonal antibodies; EMA (clone E29, Dako Corp, dilation=1:100), CEA (clone II-7, Dako Corp, dilution=1:150), CA19-9 (polyclonal, TFB Laboratory, Tokyo, Japan, dilution=1:200), CDX-2 (BioGenex Corp, San Roman, CA, USA; dilution=1:200), p53 (clone DO-7, Dako Corp, dilution=1:200), Ki-67 antigen (clone MIB-1, Dako Corp, dilution=1:100), TTF-1 (clone 8G7G3/1, Dako Corp, dilution=1:200), vimentin (clone V9, Dako Corp, dilution=1:100), and p63 (clone 4A4, Dako Corp, dilution=1:200). Microwave pretreatment was performed in each immunohistochemical run.
A histochemical investigation was also performed by mucicarmine stain and by combined periodic acid-Schiff after diastase digestion (d-PAS) and alcian blue (AB) at pH2.5. Statistical analysis was performed by Chi-square test.
Results
The SRCC was composed of medullary proliferation of large clear cells with much intracytoplasmic mucin (neutral mucin and acidic mucin) (Figure 1A-C), which was confirmed by the combined d-PAS/AB technique (Figure 1C) and mucicarmine stains. The proportion of signet ring cells in SRCC ranged from 60% to 100%. In most cases, the SRCC contained areas of other histologies such as mucinous adenocarcinoma, and tubular adenocarcinoma.
Figure 1.
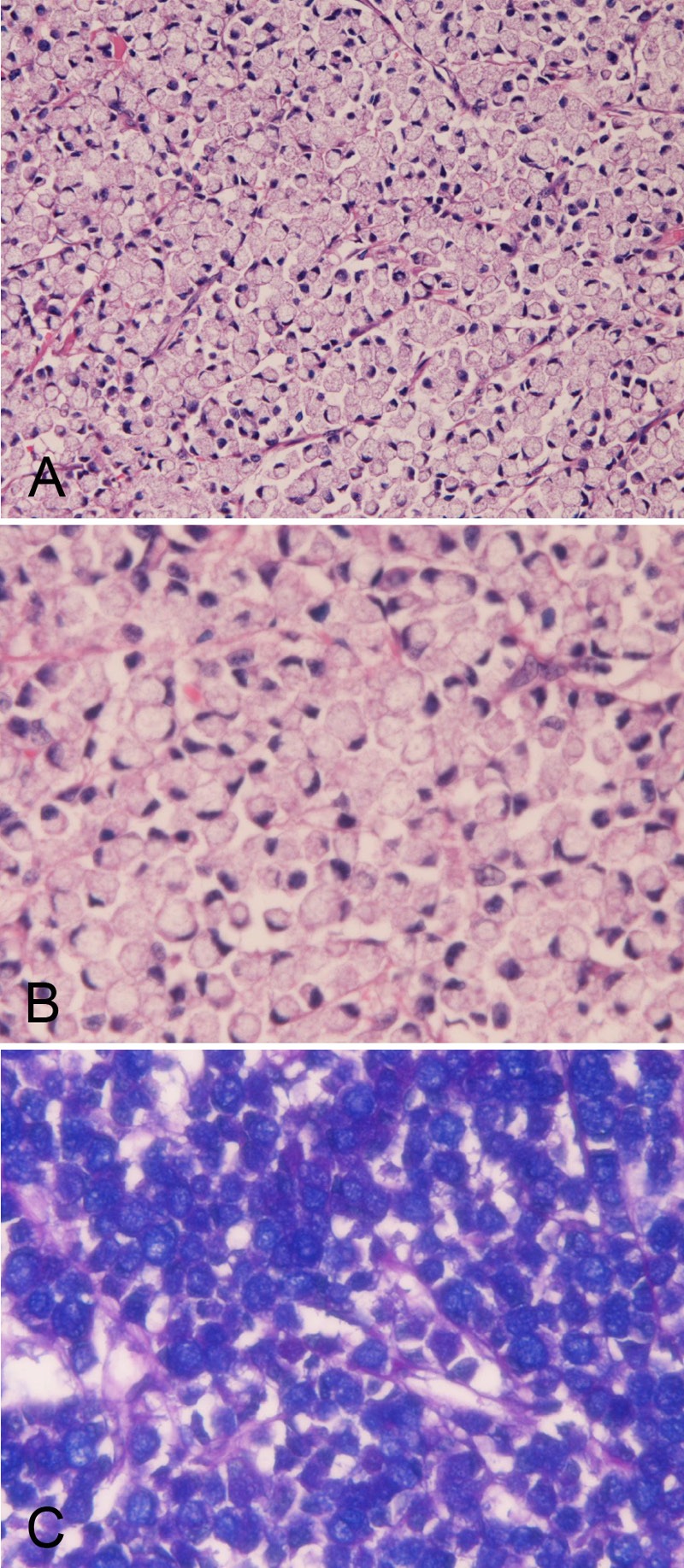
Histology and histochemistry of primary signet-ring cell carcinoma of the stomach. A: Lower power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. The signet-ring cell carcinoma is medullary and the stroma is scant in amount. HE: x100. High power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. HE: x400. C: Combined d-PAS and AB stains revealed abundant intracytoplasmic mucins composed of neutral (Mazenta color) and acidic (blue color) mucins. Combined d-PAS/AB double staining: x200.
The normal expression pattern of these nine common antigens was investigated in all the sections of SRCC. In both the normal stomach and colorectum, no expressions of p53, p63, and TTF-1 were recognized in all specimens. Vimentin expression was seen in only the stroma. No vimentin expression in the epithelial cells was recognized.
In the normal stomach, moderate expression of EMA was recognized in any epithelial types (100%) of the normal stomach (Figure 2A). CEA was consistently (100%) mildly expressed in the luminal sides of the epithelium (Figure 2B). CA19-9 was consistently (100%) expressed in the cytoplasms of the epithelial cells (Figure 2C). CDX-2 was also consistently (100%) expressed in the epithelial nuclei (Figure 2D). Ki-67 antigen was consistently (100%) expressed in the basal parts of the foveolae (Figure 2E), and Ki-67 labeling index was less than 15%.
Figure 2.
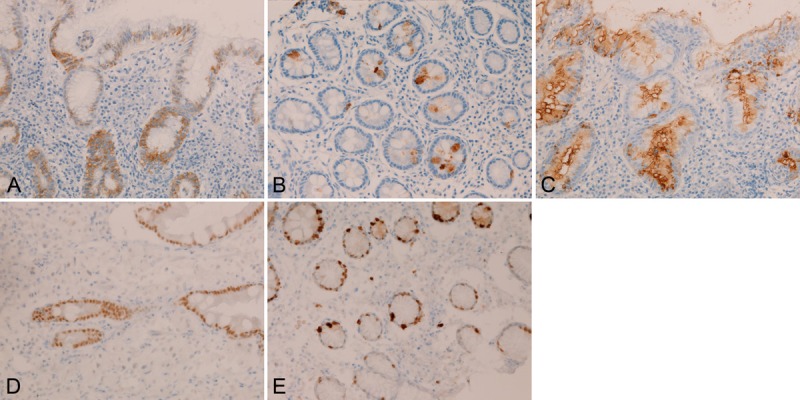
Immunohistochemical findings of the normal stomach. Epithelial cells of the normal stomach are consistently EMA (A), CEA (B), CA19-9 (C), CDX-2 (D), and Ki-67 antigen (labeling <15%) (E). Immunostaining, x300.
In the normal colorectum, mild to moderate expression of EMA was consistently (100%) recognized in the normal cryptal epithelium (Figure 3A). CEA was consistently (100%) mildly expressed in the luminal sides of the normal cryptal epithelium (Figure 3B). CA19-9 was consistently (100%) expressed in the cytoplasms of the normal cryptal epithelial cells (Figure 3C), CDX-2 was also consistently (100%) expressed in the cryptal epithelial nuclei (Figure 3D). Ki-67 antigen was consistently (100%) expressed in the basal parts of the crypts (Figure 3E), and Ki-67 labeling index was less than 10%.
Figure 3.
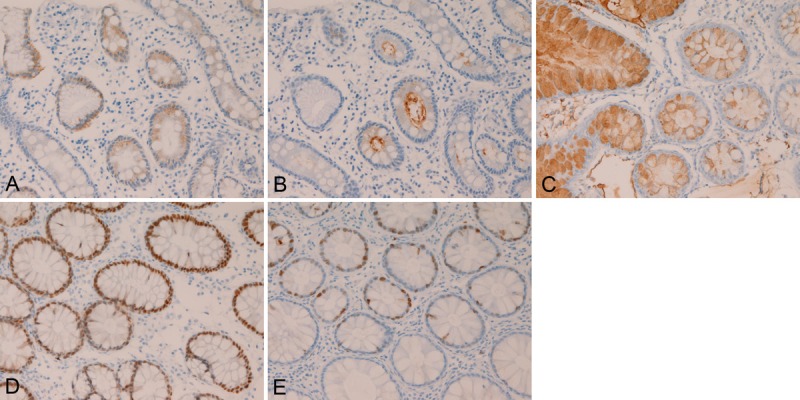
Immunohistochemical findings of the normal colon. The normal colonic epithelium consistently showed EMA (A), CEA (B), CA19-9 (C), CDX-2 (D), and Ki-67 (labeling <10%) (E). Immunostaining, x300.
In the primary gastric SRCC, EMA was expressed in the cytoplasm of the SRCC cells (Figure 4A). CEA was expressed in the cytoplasms of the SRCC cells (Figure 4B). The expression of CA19-9 was very strong and was located in the cytoplasms of SRCC cells (Figure 4C). CDX-2 was strongly expressed in the nuclei of the SRCC cells (Figure 4D). p53 was expressed in the nuclei (Figure 4E). Ki-67 was expressed in the nuclei (Figure 4F), and Ki67 labeling index was 36 ± 23 %. There were no expressions of TTF-1, vimentin, and p63 in the SRCC cells. The expression percentage (positive cases/total cases) of EMA was 57% (17/30), CEA 100% (30/30), CA19-9 100% (30/30), CDX-2 43% (13/30), p53 83% (25/30), Ki-67 100% (30/30) (labeling index= 36 ± 23 %), TTF-1 0% (0/30), vimentin 0% (0/30), and p63 0% (0/30).
Figure 4.
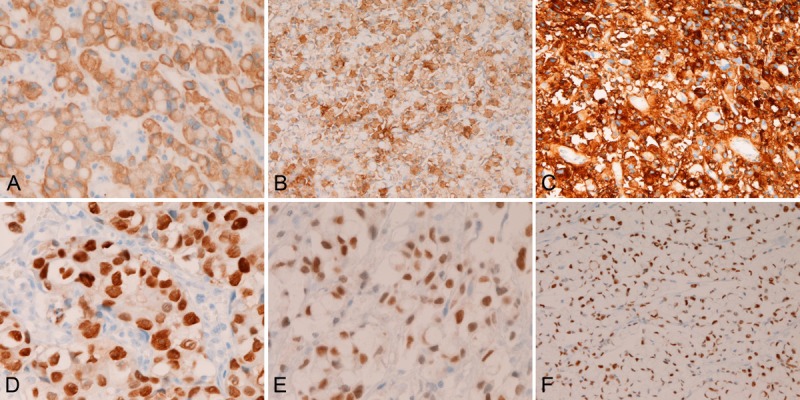
Immunohistochemical findings of primary gastric signet-ring cell carcinoma. The gastric SRCC cells express EMA (A), CEA (B), CA19-9 (C), CDX-2 (D), p53 (E), and Ki-67 antigen (labeling=36 ± 23 %) (F). Immunostaining, x300.
In the primary colorectal SRCC, EMA was expressed in the cytoplasm of the SRCC cells (Figure 5A). CEA was expressed in the cytoplasms of the SRCC cells (Figure 5B). The expression of CA19-9 was very strong and was located in the cytoplasms of SRCC cells (Figure 5C). CDX-2 was strongly expressed in the nuclei of the SRCC cells (Figure 5D). p53 was expressed in the nuclei (Figure 5E). Ki-67 was expressed in the nuclei (Figure 5F), and Ki67 labeling index was 47% ± 26 %. There were no expressions of TTF-1, vimentin, and p63 in the colorectal SRCC cells. The expression percentage (positive cases/ total cases) of EMA was 25% (3/12), CEA 100% (12/12), CA19-9 100% (12/12), CDX-2 93% (28/30), p53 75% (9/12), Ki-67 100% (30/30) (labeling index= 47% ± 26 %), TTF-1 0% (0/12), vimentin 0% (0/12), and p63 0% (0/12).
Figure 5.
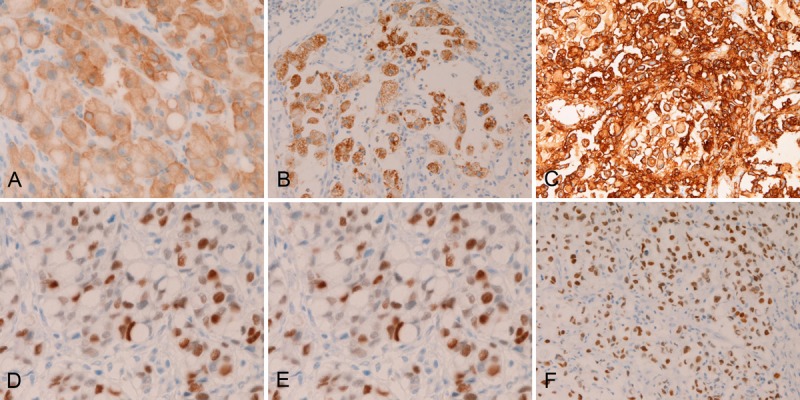
Immunohistochemical findings of primary colorectal signet ring cell carcinoma. The colorectal SRCC cells express EMA (A), CEA (B), CA19-9 (C), CDX-2 (D), p53 (E), and Ki-67 antigen (labeling=47% ± 26 %) (F). Immunostaining, x300.
A comparative statistical analysis showed significant difference in EMA (gastric SRCC 57% vs colorectal SRCC 25%) and CDX-2 (43% vs 93%). There were no significant differences in the other seven antigens’ expression between primary gastric SRCC and primary colorectal SRCC.
Discussion
There have been no comprehensive immunohistochemical studies of the expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in the normal mucosa of the normal stomach and normal colorectum. The present study revealed the expression and localization of these nine common antigens. In the normal stomach, EMA, CEA, and CA19-9, were consistently (100%) expressed in the cytoplasms or cell membranes facing the lumens in the epithelial cells of the normal stomach and normal colon. CDX-2 and Ki-67 were also consistently (100%) expressed in the epithelial nuclei in the normal epithelial cells of the normal stomach and normal colorectum. Ki-67 labeling index was less than 15% in the normal stomach and less than 15% in the normal colorectum. No expression of p53, p63, TTF-1 and vimentin were seen in the normal epithelial cells of the normal stomach and normal colorectum. These findings provide the basic knowledge of the expression status of these nine antigens in normal condition, and may be useful in investigations of the expressions of these nine antigens in abnormal conditions.
There has been only one comprehensive study of primary SRCC of the stomach and colon [28]. Goldstein et al [28], using 27 gastric SRCC and 14 colonic SRCC, studies the immunohistochemical features of SRCC. They used eight antibodies (cytokeratin (CK) 7, CK17, CK19, CK20, CA19-9, CA125, estrogen receptor, and gross-cystic disease fluid protein 15). They found frequent expression of CA19-9 in both gastric and colonic SRCC. They focused the CK pattern of CK7/CK20, and stressed that gastric SRCC tended to express CK7+/CK20- pattern, while colonic SRCC tended to show CK7-/20+ pattern [28]. This tendency of SRCC was similar to that of ordinary adenocarcinomas of the stomach and colon. The expression status of CA 19-9 of the present study is very similar to that of Goldstein et al [28]. However, they did not investigate the expression of EMA, CEA, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63.
In the present study, EMA, CEA, CA19-9 and CDX-2 were consistently expressed in normal epithelial cells in the stomach and colorectum. However, the expressions of these four molecules were variable in primary SRCC of the stomach and colorectum. In stomach SRCC, the expression percentage of EMA was 57% (17/30), CEA 100% (30/30), CA19-9 100% (30/30), and CDX-2 43% (13/30). Thus, the expressions of CEA and CA19-9 in gastric SRCC were the same as those of normal gastric epithelium. However, the expressions of EMA and CDX-2 in gastric SRCC was low than those of normal gastric epithelium. This finding suggests that EMA and CDX-2 are down-regulated during the gastric SRCC carcinogenesis. This down regulations may be associated with the malignant transformation of gastric SRCC. In the colorectal SRCC, the expression of EMA was 25% (3/12), CEA 100% (12/12), CA19-9 100% (12/12), and CDX-2 93% (28/30). Thus, the expressions of CEA and CA19-9 maintained during the carcinogenesis of colorectal SRCC.
However, the expression of EMA is markedly down-regulated, suggesting that this EMA down-regulation may be associated with the carcinogenesis of colorectal SRCC. The expression of CDX-2 in SRCC (93%) was not statistically different from the normal counterpart (100%), suggesting that CDX-2 is not involved in the pathogenesis of primary colorectal SRCC. The p53 status of primary SRCC was different from the normal epithelium. While normal epithelium never expressed p53, the gastric SRCC expressed p53 in 83% (25/30) and primary colorectal SRCC in p53 75% (9/12). These findings suggest that the development of most of the primary SRCC of the stomach and colorectum involves the p53 tumor suppresser gene mutations. Molecular studies of p53 mutations are required in primary SRCC of the digestive organs. In the present study of normal epithelium, the Ki-67 labeling was relatively low (<15%), while Ki-67 labeling of primary gastric and colorectal SRCC were high; 36 ± 23% in gastric SRCC and 47% ± 26% in colorectal SRCC. Thus, it seems that the SRCC of these locations have high cell proliferative activity. The proliferative fraction was higher in colorectal SRCC than in gastric SRCC. In general, SRCC have poor outcome than ordinary adenocarcinoma. This may al least because of the high p53 mutations and high proliferative activity in primary SRCC.
In the present study, no expressions of TTF-1, vimentin, and p63 were observed in normal epithelium and SRCC cells. TTF-1 expression is seen exclusively in thyroid and pulmonary tumors. Thus, the present SRCCs are not metastatic lesions of very rare pulmonary SRCC [29,30]. Vimentin may be positive in certain carcinomas, especially those with sarcomatoid features [21]. The present 42 cases were all negative for vimentin, implying that primary SRCCs of the stomach and colon do not express vimentin. P63 is a marker of myoepithelial cells and squamous and urothelial differentiations. This fact suggests that the SRCC cells of the present study do not shows myoepithelial, basal cellular, squamous cellular, and urothelial differentiations.
A comparative study between primary gastric and colorectal SRCC showed significant difference were present in EMA expression (gastric SRCC 57% vs colorectal SRCC 25%) and CDX-2 expression (43% vs 93%). There were no significant differences in the other seven antigens’ expression between primary gastric SRCC and primary colorectal SRCC. These findings suggest that the expressions of EMA and CDX-2 are useful in determining the primary site in metastatic SRCC. Namely, the EMA+/CDX-2- pattern may suggest gastric SRCC and the EMA-/CDX-2+ pattern may suggest colorectal SRCC.
There have been sporadic reports of the expression of the nine antigens examined in the present study of SRCC in gastric and colorectal ordinary adenocarcinomas [31-37]. The results of the present primary gastric and colorectal SRCC are essentially very similar to the results of ordinary adenocarcinomas of the stomach and colorectum [31-37].
Although Nguyen et al [29] reported 21 cases of gastric SRCC, 11 cases of colorectal SRCC, and 6 cases of breast SRCC, their study focused on only MUC apomucin expressions, and did not examined the antigens of the present study. Hayashi et al [30], who studies MUC profile of five cases of SRCC of the lung described that the MUC profile of the SRCC of the lung was different from SRCC of the gastrointestinal tract. However, they did not perform immunohistochemical study of other molecules. Bu et al [37] mentioned that MUC2 expression was 88% (7/8) and MUC5AC 100% (8/8) in colorectal SRCC. These figures are also different from the studies of Nguyen et al [29]. However, they studied other molecules. In the author’s literature of SRCC of extra-gastric and extra-colorectal organs showed frequent expression of p53, CEA, CA19-9, and MUC apomucins [3-13]. Please refer to these references [3-13,29,30] of SRCC in non-gastric and non-colorectal origin.
In conclusion, the author immunohistochemically examined the expression of nine common antigens (EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63) in the normal epithelial cells of the stomach and colorectum in 30 cases of primary gastric SRCC, and in 12 cases of primary colorectal SRCC. It was found that the normal epithelium of these locations consistently (100%) express (100%) EMA, CEA, CA19-9, CDX-2, and Ki-67 (labeling <15%). Normal epithelium never expressed p53, TTF-1, vimentin, and p63. In primary gastric SRCC, In the primary gastric SRCC, the expression percentage of EMA was 57% (17/30), CEA 100% (30/30), CA19-9 100% (30/30), CDX-2 43% (13/30), p53 83% (25/30), Ki-67 100% (30/30) (labeling index= 36 ± 23%), TTF-1 0% (0/30), vimentin 0% (0/30), and p63 0% (0/30). In primary colorectal SRCC, the expression percentage of EMA was 25% (3/12), CEA 100% (12/12), CA19-9 100% (12/12), CDX-2 93% (28/30), p53 75% (9/12), Ki-67 100% (30/30) (labeling index= 47% ± 26%), TTF-1 0% (0/12), vimentin 0% (0/12), and p63 0% (0/12). A comparative statistical analysis showed significant difference in EMA (gastric SRCC 57% vs colorectal SRCC 25%) and CDX-2 (43% vs 93%). There were no significant differences in the other seven antigens’s expression between primary gastric SRCC and primary colorectal SRCC. These findings provide much knowledge of primary SRCC of the stomach and colorectum. The data indicated primary gastric SRCC frequently express EMA but not CDX-2 and primary colorectal SRCC frequently express CDX-2 but not EMA. The expression pattern of EMA and CDX-2 may be useful in differential diagnosis between primary gastric SRCC and primary colorectal SRCC in the metastatic sites of SRCC.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Lauwers GY, Franceschi S, Carneiro F, Montgomery E, Graham DY, Tatematsu M, Curado MP, Hattori . Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 48–58. [Google Scholar]
- 2.Hamilton SR, Nakamura S, Bosman FT, Quirke P, Boffetta P, Riboli E, IIyas M, Sobin LH, Morreau H. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 134–146. [Google Scholar]
- 3.Terada T. Primary signet-ring cell carcinoma of the lung: a case report with an immunohistochemical study. Int J Clin Exp Pathol. 2012;5:171–174. [PMC free article] [PubMed] [Google Scholar]
- 4.Terada T. Primary signet-ring cell carcinoma of the ampulla of Vater: a case report with an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2012;20:427–428. doi: 10.1097/PAI.0b013e31823b7052. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report. Endoscopy. 2012;44(Suppl 2):E141–142. doi: 10.1055/s-0030-1257045. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Primary pure signet ring cell adenocarcinoma of the non-Barrett’s esophagus: a case report with immunohistochemical study. Endoscopy. 2011;43:E397–8. doi: 10.1055/s-0030-1256944. [DOI] [PubMed] [Google Scholar]
- 7.Terada T. Primary pure signet-ring cell adenocarcinoma of the urinary bladder: a report of three cases with an immunohistochemical study. Med Oncol. 2012;29:2866–9. doi: 10.1007/s12032-011-0122-7. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Signet-ring cell carcinoma of the non-ampullary duodenum and proximal jejunum: a case report with an immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291528. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Primary pure signet-ring cell carcinoma of the anus: a case report with immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291516. (in press) [DOI] [PubMed] [Google Scholar]
- 10.Terada T. An immunohistochemical study of a primary signet-ring cell carcinoma of the ampulla of Vater: A case report. J Gastrointest Cancer. 2012 Dec 19; doi: 10.1007/s12029-012-9469-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Signet-ring cell carcinoma of the esophagus in dermatomyositis: a case report with immunohistochemical study. J Gastrointest Cancer. doi: 10.1007/s12029-012-9473-3. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Terada T. Ovarian malignant Mullerian mixed tumor (heterologous) whose epithelial component is composed predominantly of signet ring cell carcinoma. Arch Gynecol Obstet. 2011;283:1403–1406. doi: 10.1007/s00404-010-1591-1. [DOI] [PubMed] [Google Scholar]
- 13.Terada T. Small Cell Carcinoma of the Ileum that developed 10 Years after total gastrectomy for gastric signet-ring cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:618–619. doi: 10.1097/PAI.0b013e31823eb34f. [DOI] [PubMed] [Google Scholar]
- 14.Rosai J. Epithelial membrane antigen (EMA) In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 52. [Google Scholar]
- 15.Rosai J. Carcinoembryonic antigen (CEA) In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 50. [Google Scholar]
- 16.Rosai J. Cancer-associated carbohydrates. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 50. [Google Scholar]
- 17.Rosai J. CDX2. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 50. [Google Scholar]
- 18.Rosai J. P53. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 59. [Google Scholar]
- 19.Rosai J. Other methods for analysis of cell proliferation. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. pp. 65–66. [Google Scholar]
- 20.Rosai J. hyroid transcription factor-1 (TTF-1) In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 61. [Google Scholar]
- 21.Rosai J. Vimentin. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 62. [Google Scholar]
- 22.Rosai J. p63. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. Ninth Edition. New York: Mosby; 2004. p. 59. [Google Scholar]
- 23.Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type) Int J Clin Exp Pathol. 2011;4:797–798. [PMC free article] [PubMed] [Google Scholar]
- 24.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens: I. Benign lesions. Int J Clin Exp Pathol. 2012;5:46–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens in a single Japanese hospital: II. Malignant lesions. Int J Clin Exp Pathol. 2012;5:52–57. [PMC free article] [PubMed] [Google Scholar]
- 26.Terada T. Malignant tumors of the small intestine: A histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203–209. [PMC free article] [PubMed] [Google Scholar]
- 27.Terada T. A clinical-histopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–198. [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein NS, Long A, Kuan SF, Hart J. Colon signet ring cell adenocarcinoma: immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol. 2000;8:183–1886. doi: 10.1097/00129039-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen MD, Plasil B, Wen P, Frankle WL. Mucin profiles in signet-ring cell carcinoma. Arch Pathol Lab Med. 2006;130:799–804. doi: 10.5858/2006-130-799-MPISCC. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi H, Kitamura H, Nakatani Y, Inayama Y, Ito T, Kiutamura H. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol. 1999;30:378–383. doi: 10.1016/s0046-8177(99)90111-9. [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 32.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 34.Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sigihara K. Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer. 2000;89:724–732. [PubMed] [Google Scholar]
- 35.Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166–170. doi: 10.1002/ijc.1460. [DOI] [PubMed] [Google Scholar]
- 36.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 37.Bu XD, Li N, Tian XG, Li L, Wang JS, Yu XJ, Huang PL. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J Gastroenterol. 2010;16:4089–4094. doi: 10.3748/wjg.v16.i32.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]


