Abstract
Metabolic impairments in maternal obesity and gestational diabetes mellitus (GDM) induce an abnormal environment in peripheral blood and cause vascular structure alterations which affect the placental development and function. A GDM model was developed using C57BL/6J female mice fed with high fat food (HF) (40% energy from fat) and a control group with control food (CF) (14% energy from fat) for 14 weeks before mating and throughout the gestation period. A subset of dams was sacrificed at gestational day (GD) 18.5 to evaluate the fetal and placental development. HF-fed dams exhibited significant increase in the maternal weight gain and homeostasis model assessment for insulin resistance index (HOMA-IR), impaired insulin secretion of glucose stimulus and glucose clearance of insulin stimulus before pregnancy; in addition, they also had the increase in the fetal and placental weight. HF-fed dams at GD 18.5 showed the high level of circulating maternal inflammation factors and were associated with increased oxidative stress and hypoxia in the labyrinth, abnormal vascular development with a high level of hypoxia inducible factor-1α (HIF-1α) and VEGF-A expression, but without a parallel increase in CD31 level; were induced an exaggerated inflammatory response in placental vascular endothelial cell. Our findings show that GDM induces more maternal weight gain and fetus weight, with abnormal maternal circulating metabolic and inflammation factors, and forms a placental hypoxia environment and impacts the placental vascular development. Our findings indicate that gestational diabetes induce excessive chronic hypoxia stress and inflammatory response in placentas which may contribute mechanisms to the high risks of perinatal complications of obesity and GDM mothers.
Keywords: High fat diet, obesity, GDM, placenta, oxidative stress, hypoxia, inflammation factor
Introduction
With the worldwide epidemic of metabolic syndrome, the proportion of women that are overweight or obese and with GDM during pregnancy has increased [1,2]. The resulting abnormal placenta environment may have deleterious effects on fetal metabolic programming and lead to the increased adverse perinatal outcomes, not only perinatal mortality, macrosomia, shoulder dystocia [3], but also preeclampsia [4,5]. GDM and obesity in pregnancy often increase the risk for the baby to develop metabolic syndrome later in life [4,6].
Placenta plays an important role in this scenario, but basis research is leg behind in this area. Only a few studies with rodent models were designed to address the consequences of maternal obesity to understand the metabolic reprogramming in offspring, but no close attention has been paid to the state changes of placental vascular structure. When capillarization increases rapidly, this tree delivers blood to an extensive and expanding capillary network with a large surface area for exchange and is vital for fetal growth and survival. Nevertheless, the factors that control the normal growth and development of the fetoplacental vasculature in the late gestational are still unknown.
Since murine and human placenta exhibit many same characteristics [7], in the present work, we found that high-fat mouse models are accompanied with increased maternal adiposity, fast glucose and insulin, impaired glucose and insulin tolerance, which is very similar to human GDM symptoms. Because placental function increases dramatically in the last half of gestation to supply enough oxygen and nutrients for exponential fetal growth, maternal hypertension and other complications start and accelerates during the last half of gestation, it is requisite to monitor the pathological events of the placenta in this period. Therefore, based on this GDM animal model, we then took GD 18.5 as a dam time to investigate the changes in placental vascular structure of exchange area and placental environment in this late gestation period.
Our findings show that high fat diet induces more maternal weight gain and fetus weight, with changed maternal circulating metabolic and inflammation factors, and disordered placental vascular environment and development.
Materials and methods
Mice and high fat mouse models
Female C57BL/6J (4 weeks old) mice were purchased from the Shanghai Slac Laboratory Animal Co., Ltd. All of the mice were housed in the animal facility with a 12 h light/dark cycle, 40%-60% humidity and constant temperature (22-24°C), and the mice had free access to water and diet. After 1 week quarantine, these mice were randomly divided into two groups. CF (Control food) group fed a control diet (14% fat, 26% protein, 60% carbohydrate ) [8]. HF (high fat food) [9,10] group fed a research diet (40% fat, 20% protein, 40% carbohydrate), above foods all provided from Slac Laboratory Animal Co., Ltd. After 14 weeks dietary intervention, breeding was conducted overnight in a 1:2 ratio, mating was confirmed by presence of a vaginal mucous plug in the following morning, which represented on gestation day (GD) 0.5 until the next midday of mating. These males were removed once the females were pregnant. All procedures were performed in accordance with national institutes of health guidelines for the care and use of animals and were approved by the institutional animal care and use committee at animal research center of Shanghai 6th People’s Hospital.
Measurements of body weight, blood glucose and insulin levels
Body weight was measured once a week; blood glucose was determined by glucometer (ACCU-CHEK Performa) at indicated time points from cutting tails after overnight fasted (12 h) every 2 weeks; after 14 weeks of dietary intervention, blood samples were collected by retroorbital sinus puncture without anesthetization after overnight fasted (12 h). Following immediate centrifugation at 4000 rpm at 4°C, plasma was separated and stored at -70°C until serum insulin analysis. Homeostasis model assessment of insulin resistance (HOMA-IR) was estimated with the following formula: insulin resistance = fasting plasma insulin (in microunits per ml) × fasting plasma glucose (FPG, in millimoles per litre)/22.5.
Intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IPITT)
After 14 weeks dietary intervention, six mouse of each group were fasted for 12 h and blood was collected from tail for glucose assay by the glucometer at baseline (as 0 min) and 30, 60 and 120 min after glucose (2g/kg) loading through intraperitoneal injection [11]; other six mouse of each group were fasted for 4 h, then the glucose level was measured as the method in IPGTT at the 30, 60 and 120 min after human regular insulin (Novolin R, Novo-Nordisk) through intraperitoneal injection at 0.5 units/kg [12].
Collection of blood and tissue samples
Dams were sacrificed at GD18.5 after fasting 4 h, and maternal blood was collected by cardiac puncture, allowed to clot, and spun at 4000 rpm to collect serum. Maternal serum level of triglyceride and total cholesterol were analysed using triglyceride and total cholesterol test kits (Beijing BHKT Clinical Reagent Co. Ltd) according to the manufacturer’s instructions. Insulin levels were detected by Kit for mouse Insulin Batch (F1093, Shanghai Westang Bio-Tech Inc., Ltd); the levels of TNF-α (12-2720-096, Dakewe Biotech Co., Ltd.) and IL-1β (12-2012-096, Dakewei Biotech Co., Ltd.) were analyzed by ELISA followed manufacturer’s instructions. The marker of insulin resistance was calculated by HOMA-IR as follows: fasting plasma insulin (μU/mL) × fasting glucose (mM)/22.5.
After laparotomy, fetuses and placentas were removed and weighed, and placentas in each litter were pooled, one part of each group after weighing were stored at -70°C for measurement of malondialdehyde (MDA), a common stable marker for OS-induced lipid peroxidation, after placentas homogenized according protocol of MDA (A003-1, Nanjing Jiancheng Bioengineering Institute), the way of tissue homogenate as mentioned elsewhere [13]. One part of the remnant placentas of each group fixed in 4% polymerisatum at once for immunohistochemistry and the other part of placentas were stored at -70°C for real time PCR.
Real time PCR
Real time quantitative PCR to measure mRNA expressions of HIF-1α (NM_010431.2), VEGF-A (NM_001110266.1), CD31(NM_008816.2), TNF-α (NM_013693), IL-6 (NM_031168.1), GAPDH (NM_008084 ). Primer Pairs of above molecular shows in Table 1. Total RNA was extracted from placenta tissues from each group (4 animals) using TRIzol (Invitrogen). Total mRNA (2μg) was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Takaro Biosystems) according to the manufacturer’s instructions. RT-PCR was performed by the Biosystems 7500 Fast Real-Time PCR System using Power SYBR® Green PCR Master Mix kits (Applied Takara BioTechnology). Quantitative Real-time PCR analysis was carried out with the following cycle profile: 1 cycle at 95°C for 30 s, and 40 cycles at 95°C for 5 s, 60°C for 31 s.
Table 1.
Primer Pairs for Real time PCR
| Forward Primer | Reverse Primer | |
|---|---|---|
| HIF-1α | AGCCCTAGATGGCTTTGTGA | TATCGAGGCTGTGTCGACTG |
| CD31 | GCGGTGGTTGTCATTGGAGTG | GTTGGAGTTCAGAAGTGGAGCAG |
| VEGF-A | TGGCTGGCTGGGTCACTAAC | CTGGCTTTGTTCTGTCTTTCTTTGG |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Immunohistochemistry
Placentas preserved were transected perpendicular to the long axis of the disc, processed, and paraffin embedded; and 5-μm sections were stained for morphologic analysis by light microscopy. Placentas from all groups were also observed for overall evidence of HIF-1α (25 ug/ml, MAB1935, R&D Systems), CD31 (PECAM-1) (1:100, sc-31045, Santa Cruz Biotechnology), NFKB P65 (1:100, SC-372, Santa Cruz Biotechnology), pNF-KB P65 (1:50, SC-372, Santa Cruz Biotechnology). Immunohistochemistry was performed by labeled streptavidin/peroxidase method and microwave antigen retrieval technique. The reagents (PV9000 and DAB) were obtained from ZSGB-BIO and staining was performed as protocol. As negative controls, the primary antibody was replaced by anti-mouse IgG isotype.
Microvessel counting was carried out by selecting two center areas in the labyrinth from 4 different samples in each group, that is, average 10 representative microscopic fields of placenta at 400× magnification were enumerated following the methods described previously [14]. The staining of HIF-1α, NFKB and pNF-KB was assessed semiquantitatively by using the Q score method: immunoreactivity was classified according to fractions of positive cells (0, 0-10%; 1, 10-25%; 2, 26-75%; 3, 76-100%) and general intensity of positive cells (0, none; 1, mild; 2, moderate; 3, intense) [14]. Vascular density was measured based on a combination of CD31 staining and morphologic analysis, and was semiquantitatively scored based on the density and area as above described [15], the brown positive staining levels were graded from 0 to 3 as above described, and percentage of staining area were graded from 0 to 4: negative is 0, ≤25% 1, 26-50% 2; 51-75% 3; >75% 4. Each slide was calculated by multiplying staining grade by fractions of positive cells or area. The average score of ten fields was counted by two independent examiners who were blinded as to animal groups.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). The data obtained in the present study were analyzed using an ANOVA. A p value < 0.05 was considered to be statistically significant. Data were analyzed using the statistical package SPSS for Windows version 17.0.
Results
HF increased the biomarkers associated with metabolic impairments
After high fat diet intervention before pregnancy, maternal body weight significantly increased from the second week (P<0.05) (Figure 1A), weight gained 19g compared 7.4g of CF, fasting glucose was increased significantly after 10 weeks (Figure 1A and 1B) (P<0.001). Fast glucose levels were significantly elevated in HF-fed dams prior to mating (P<0.001), although the glucose level was not so stable (Figure 1B), while the highest fast glucose levels of CF was only 131.4mg/dl without changing much during the whole process.
Figure 1.
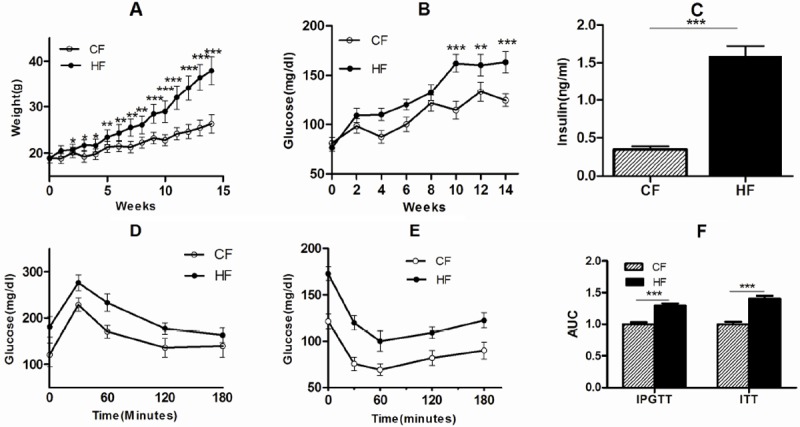
HF led to increase of biomarkers associated with metabolic impairments. Feeding with HF or CF for 14 weeks, body weight (A) was measured every week; fast glucose (B) was detected every two weeks, fast insulin (C), IPGTT (D) and IPTT (E) were detected before mating. AUC of IPGTT and IPTT changed (F). Error bars depicted the standard error of the mean. *P<0.05, **P<0.01 or ***P<0.001 compared to the CF control.
Besides fasting glucose, HF-fed dams also showed significant insulin resistance (Figure 1C) (P<0.001), IPGTT (Figure 1D) and IPTT (Figure 1E) exhibited much worse response to glucose stimulus at all time points (30, 60, 120, and 180 min). AUC levels of both IPGTT and IPTT indicated that HF impaired the response to insulin and glucose stimulus (Figure 1F) (P<0.001).
The obesity mouse in our study had high levels of fast glucose, insulin and impaired glucose tolerance. And many studies [8,10,12] had showed C57BL/6J mice with a high fat diet is a robust and efficient model for IGT and early type 2 diabetes. , HF mice after pregnancy still had a high serum fast glucose, insulin level (Table 2), and a high triglyceride and total cholesterol level with worse metabolic impairments. HOMA-IR was 7 folds higher than CF (Table 2), Our model of GDM were established and suitable for studying the influence on placental development and function [16]. Body weight of the HF mouse during pregnancy in this study, was increased from 37.9±3.0 to 51.1±5.40g (Table 2) (P<0.001), compared CF from 26.3±2.2 to 39.3±4.1 (P<0.001); Dams were killed to obtain the fetuses and placentas on GD18.5, HF fetuses were significantly heavier than CF fetuses (P<0.001), whereas placental weight did not significantly differ between the two groups (Table 2) (P<0.05), our current results displayed that HF placentas are working in a heavy load.
Table 2.
Biomarkers associated with metabolic impairments
| CF | HF | |
|---|---|---|
| Body weight (g) | 39.3±4.1 | 51.1±5.4*** |
| Fast Glucose (mg/dl) | 122.4±7.2 | 163.8±12.6*** |
| Fast Insulin (ng/ml) | 0.352±0.046 | 1.742±0.140*** |
| HOMA-IR | 2.255±0.017 | 14.9±0.092*** |
| Triglyceride (mg/dl) | 140.4±28.6 | 235.5±27.4*** |
| Total cholesterol (mg/dl) | 235.4±46.9 | 362.3±102.42*** |
| IL-1β ( ng/ml) | 1.87±0.40 | 6.6±0.85*** |
| TNF-α (ng/ml) | 0.59±0.20 | 5.23±0.70*** |
| Placenta weight (g) | 0.1145±0.009 | 0.121±0.017* |
| Fetus weight (g) | 0.76±0.08 | 0.83±0.09** |
| MDA of placenta homogenized (nmol/ml) | 4.12±0.34 | 7.60±0.53*** |
P<0.05;
P<0.01;
P<0.001.
The placentas of GDM are characterized by reduced tissue oxygenation
Obesity and GDM is associated with marked hyperglycaemia, hyperinsulinemia and dyslipidemia, but also with increased oxidative stress [17]. Overproduction of reactive oxygen species may influence arterial branching of exchange region of HF placenta. We assessed the level of systemic oxidative damage in obese pregnant dams by quantifying placenta MDA. MDA level in HF placenta was increased by 84.4% over in whole placental homogenates of GD18.5 (Table 2) (P<0.001), the levels of MDA within the placentas were evaluated as an indicator of tissue hypoxia. HIF-1α, another marker of hypoxia, was also measured in the mouse placenta during late gestation, as shown in Figure 2, HIF-1α mRNA level in HF was increased 4.7 folds, and the protein level of HIF-1α was highly located (P<0.001) (Figure 2A-C). These comprehensive data demonstrated that HF placenta might be in a stronger oxidative stress and deeper hypoxia state.
Figure 2.
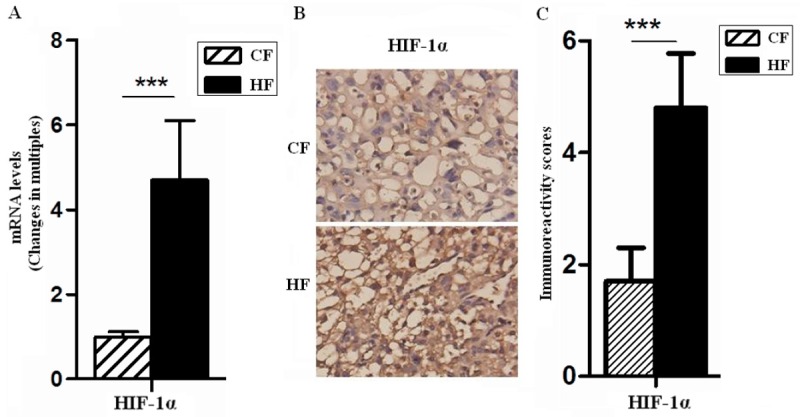
The placentas of GDM were characterized by reduced tissue oxygenation. The mRNA and protein levels of HIF-1α in placentas of GD18.5 were analyzed by quantitative real-time PCR (A) and immunohistochemistry (B), respectively. Moreover, immunoreactivity score showed in photomicrographs (C). The mean fold change of HIF-1α was normalized to the endogenous reference gene (GAPDH). Original magnification: ×400. Error bars depict the standard error of the mean. ***P<0.001 compared to the CF control.
Abnormal vascular development in the placentas of HF
Hypoxia, an additional mechanism whereby hypoxia could influence VEGF action of the factors known to induce VEGF gene expression, is by far the most potent stimulus. Low oxygen tension significantly increased VEGF mRNA levels in a reversible manner [18]. Since VEGF plays a role not only in angiogenesis and vascular permeability, but also in maintenance of the endothelium. Thus, the hypoxic induction of VEGF expression was most likely achieved by transcriptional activation. It was found that VEGF-A mRNA level of placentas in HF group increased 7.4 folds (P<0.001) (Figure 3A), therefore, we speculated that maternal obesity and GDM may affected blood vessel density and function.
Figure 3.
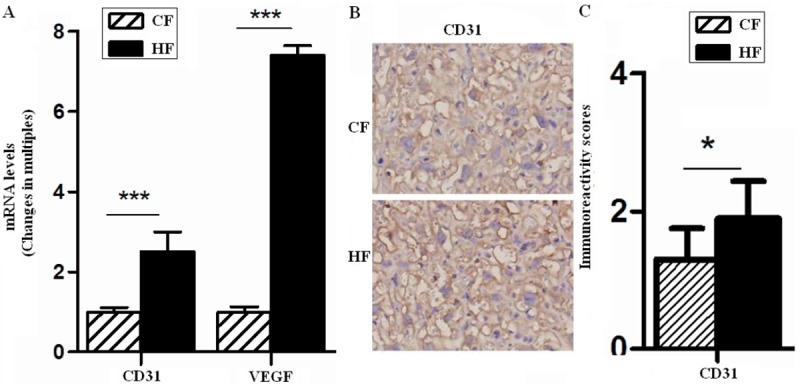
Vascular development is altered in the placentas of GDM. The mRNA levels of VEGF and CD31 and protein level of CD31 in placentas of GD18.5 were analyzed by quantitative real-time PCR (A) and immunohistochemistry (B and C), respectively. The mean fold change of target genes was normalized to GAPDH. Original magnification: ×400. Error bars depict the standard error of the mean. *P<0.05 or ***P<0.001 compared to the CF control.
Subsequently, we evaluated fetoplacental vasculature changes. As a common endothelial cell specific marker, the transcriptional level of CD31 in HF placentas was significantly higher than that of the CF group (P<0.001) (Figure 3A), but the result of immunohistochemistry showed that fetal microvessels with positive CD31 only increased 1.3 folds (P<0.05) (Figure 3B and 3C) than CF, increased CD31-postived areas suggested that there was a growing exchange area. However, these data also showed that HF vasculature changes could not catch up with its load, and as shown in figures, vascular structure was confused and disordered in HF group.
HF stimulates inflammatory response in placentas
Finally, we found that the placenta in HF developed an exaggerated inflammatory response to GDM and obesity with a high level of circulation inflammation factors (IL-1β and TNF-α) (Table 2) (P<0.001). As shown, pro-inflammatory factors TNF-α and IL-6 of placentas were markedly elevated in HF group. TNF-α levels in HF was increased 1.3 folds (P<0.05) (Figure 4A) than CF along with a significant increase in IL-6 levels (9.79 folds, P<0.001) (Figure 4A). IL-6 also could stimulate angiogenesis, so HF was possibly involved in modulating vascular development through promoting IL-6 expression and other factors in placentas.
Figure 4.
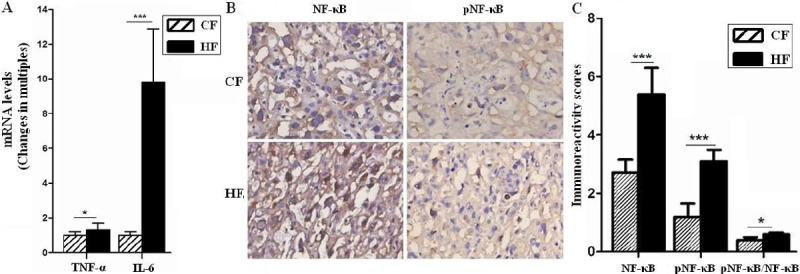
HF stimulated inflammatory response in placentas. The mRNA levels of TNF-α and IL-6 were analyzed by quantitative real-time PCR (A), and the expression of NF-κB and pNF-κB in placentas of GD18.5 were analyzed by immunohistochemistry (B and C), respectively. The mean fold change of target genes was normalized to GAPDH. Original magnification: ×400. Error bars depict the standard error of the mean. *P<0.05 or ***P<0.001 compared to the CF control.
As we known, NF-κB signaling pathways play an important role in activating inflammation response. Our data showed in Figure 4, both NF-κB and phosphorylation of NF-κB (pNF-κB) in vascular structure of HF group were higher than that in CF group (P<0.001) (Figure 4B and 4C), moreover, the ratio of pNF-κB to total NF-κB was also up-regulated in HF group. These data showed an exaggerated inflammation response in HF placenta, the effect was achieved partly through activating NF-κB signals.
Discussion
In GDM, there are strong gene-environment interactions that trigger the pathogenetic process. Thus, despite their different genetic predisposition, environmental factors, mainly lifestyle-related factors such as high-saturated-fat diet, may enhance glucose intolerance and promote insulin resistance and obesity during pregnancy. In this study, we have developed an attractive model to further study the physiologic and molecular abnormalities in GDM. Mice model was fed with a moderately high fat diet for a long time before pregnancy [10], as increased maternal adiposity, fast glucose and insulin, impaired glucose and insulin tolerance, which were very similar to the common metabolic abnormalities of human GDM, and Liang et [16] fed high fat food to female C57BL/6J mouse for 8 weeks before mating and considered them as GDM model, changes in fetus and placentas with these metabolic impairments also may be applied to GDM woman. In our study, we found that maternal and litter’s weight gain in HF group was more than that in CF group, placental weight also increased, which promoted an increase level in exchange nutrient function in HF group, but placenta weight did not show a parallel growth in litter weight.
A physiological state of insulin resistance is required to preferentially direct the maternal nutrients toward the fetoplacental unit, allowing adequate growth of the fetus. When women develop GDM, insulin resistance is more severe and disrupts the intrauterine milieu, which resulting in accelerated fetal development with increased risk of macrosomia. Jones et [9] found high fat diet before and during pregnancy could cause marked up-regulation of placental nutrient transport and fetal overgrowth in mice, in which glucose transporter 1 (GLUT1) and sodium-coupled neutral amino acid transporter (SNAT) 2 were elevated. They suggested that up regulation of specific placental nutrient transporter isoforms constituted a mechanism linking maternal high fat diet and obesity to fetal overgrowth. It coincided with our results on this point in placenta.
To further elucidate the mechanisms that a heavy function burden in HF group leads to preeclampsia, fetal growth restriction, infant death, stillbirth and other complications, the key finding in these studies was that GDM results in increased placental oxidative stress, as the most frequently used indicators of lipid peroxidation and biomarker of oxidative stress [19], MDA level in HF placenta was increased by 84.4 % over in whole placental homogenates of GD18.5. At the first trimester, excessive production of reactive oxygen species may break the balance of hypoxia-reoxygenation on trophoblast development such as invading shallowly; and in late gestation, hypoxia has been shown to cause apoptosis of trophoblast and vascular endothelium cells [20,21], which changes were all observed in preeclampsia, These may explains why GDM has a high incidence of preeclampsia [22,23].
The primary molecular sensor used by trophoblasts and the developing embryo to sense and respond to changes in O2 tension is HIF-1α [24]. Through transcriptional activation the HIF-1α regulates many different cellular processes in response to hypoxia, including angiogenesis. In human placenta, Myatt et al [25] suggested that oxidative stress may cause vascular dysfunction in the placenta that compromises fetal growth. The higher level of HIF-1α expression showed a lower O2 tension. Our results proved that HF induced HIF-1α not only in mRNA level but also in protein level. HIF-1α and low O2 tension are important in early pregnant, including regulation of angiogenesis, migration/invasion and cell metabolism, but in late placenta, it seems not so good for pregnancy. Our findings suggested that HF had a confused and disordered vascular structure. Pietro et al [26] indicated that the change in the placental VEGF/VEGFR expression ratio in mild hyperglycemia may favor angiogenesis in placental tissue. In our studies, the increase of VEGF level might be also induced by HIF-1α in HF group. However, we also found that CD31 staining intensity in placenta of HF group is not inconsistent with the increase degree of HIF-1α and VEGF, which suggested that angiogenesis in HF placenta may be not enough, although the level of angiogenesis related factors, such as VEGF, IL-6, was significantly increased. These results are partly echoed with Liang et al [16]. VEGF levels highly increased in human late gestational placenta, but vascular structure was not growing because one of VEGF receptor FLT-1 in placenta was decreased, but soluble FLT-1 (sFLT-1) expression in a free serum could combine with VEGF, which could not ensure the neoformation of placental vessels and their organization to meet the needs of the fetus and placentas [27]. Our results also showed that GDM and obesity placentas could not have an adaptive capacity, besides impaired angiogenesis.
Radaelli et al [28] showed placental transcriptome emerged as a primary target of the altered environment of diabetic pregnancy. The genes identified provide the basis to elucidate links between inflammatory pathways and GDM-associated insulin resistance. Desoye et [29] showed a similar results with Radaelli’s that GDM induced a significantly increase in IL-6 mRNA levels in GDM placenta, there were no major differences in immune cell populations within the placental villi and only a greater degree of muscularity in the vessel walls [30,31]. Zhou et al [32] showed that increased TNF-α/IL-6 signaling in mice was a key mechanism underlying increased preeclampsia, and IL-6 blockade inhibited preeclamptic features in autoantibody-injected pregnant mice. In our studies, it could be observed that an exaggerated inflammation response in HF placentas. Because these factors and biological behaviors were closely related with each other, when the balance in placenta disturbed, such as misbalance of endothelial hypoxia, proliferation and apoptosis behaviors, which may contribute to a high risk of complications.
Qian et al [33] had demonstrated that HIF-1α activation by IL-1β and TNF-α resulted in increased secretion of VEGF, the process of HIF-1α activation was driven by both IL-1β and TNF-α, up-regulating expression of the extracellular signal-regulated kinase (ERK) phosphorylation pathway. NF-kB is the key link that drives cytokine cellular signal. There is a close relationship between HIF-1α and NF-kB during hypoxia, which leads to the up-regulation of inflammatory modulators such as cycloxygenase-2 (COX-2), TNF-a, IL-6. Therefore, it can be speculated that the secretion of inflammatory factors in placenta induced by HF may further up-regulate the expression of HIF-1α and activate NF-kB signals, which in turn increases the production of inflammatory factors, and forms a positive feedback. However, the mechanism of these factors expression changes need to be further investigated.
In summary, our findings showed that GDM mouse models developed by moderate high fat diet had a maternal nutritional imbalance and metabolic disturbance, an elevated circulating and placental inflammatory response, an aggravated placental hypoxia environment and an altered placental vascular development. Our data supported the hypothesis that the altered placental vascular structure and function contributed to the mechanisms of the high risks of perinatal complications in obesity and GDM mothers.
Acknowledgments
This study was supported by National Basic Research Program of Shanghai (12ZR1422200) to Hua-Ping Li.
We would like to thank Pro, Da-Jin Li, Ming-Qing Li, PhD, Dr, Kai-Kai Chan, Yu-Han Men, Hui Li, from the Institute of Obstetrics and Gynecology of Fudan University of Shanghai for their excellent technical support.
Conflict of interest statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Working group on gestational diabetes. National guidelines for gestational diabetes. Duodecim. 2008;124:1556–1569. [Google Scholar]
- 2.Kaaja R, Rönnemaa T. Gestational Diabetes: Pathogenesis and Consequences to Mother and Offspring. Rev Diabet Stud. 2008;5:194–202. doi: 10.1900/RDS.2008.5.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker T, Vermeulen MJ, Wyatt PR, Meier C, Ray JG. Prepregnancy diabetes and risk ofplacental vascular disease. Diabetes Care. 2007;30:2496–2498. doi: 10.2337/dc07-0364. [DOI] [PubMed] [Google Scholar]
- 4.Leddy MA, Power ML, Schulkin J. The Impact of Maternal Obesity on Maternal and Fetal Health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 5.Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, Mansikkamäki K, Lamberg S, Vasankari T, Komulainen T, Tulokas S. Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial. PLoS Med. 2011;8:e1001036. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity: what is the link? Curr Opin Obstet Gynecol. 2012;24:376–81. doi: 10.1097/GCO.0b013e328359f0f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maltepe E, Bakardjiev AI, Fisher J. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winzell MS, Magnusson C, Ahrén B. Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metabolism. 2007;56:122–128. doi: 10.1016/j.metabol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buettner R, Schölmerich J, Bollheimer LC. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 11.Serre-Beinier V, Toso C, Morel P, Gonelle-Gispert C, Veyrat-Durebex C, Rohner-Jeanrenaud F, Calandra T, Roger T, James RW, Montet X, Bühler L, Bosco D, Berney T. Macrophage migration inhibitory factor deficiency leads to age-dependent impairment of glucose homeostasis in mice. J Endocrinol. 2010;206:297–306. doi: 10.1677/JOE-09-0342. [DOI] [PubMed] [Google Scholar]
- 12.Gallou-Kabani C, Vigé A, Gross MS, Rabès JP, Boileau C, Larue-Achagiotis C, Tomé D, Jais JP, Junien C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity. 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MY, Abdul AB, Wahab SIA, Elhassan MM, Alzubairi AS, Syamet MM. Attenuation of cisplatin induced hepatotoxicity in rats using zerumbone. Res J Biol Sci. 2009;4:777–784. [Google Scholar]
- 14.Noguera R, Fredlund E, Piqueras M, Pietras A, Beckman S, Navarro S, Påhlman S. HIF-1alpha and HIF-2alpha are differentially regulated in vivo in neuroblastoma: high HIF-1alpha correlates negatively to advanced clinical stage and tumor vascularization. Clin Cancer Res. 2009;15:7130–7136. doi: 10.1158/1078-0432.CCR-09-0223. [DOI] [PubMed] [Google Scholar]
- 15.Tang L, Yi R, Yang B, Li H, Chen H, Liu Z. Valsartan inhibited HIF-1α pathway and attenuated renal interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res Clin Pract. 2012;97:125–131. doi: 10.1016/j.diabres.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 16.Liang CY, DeCourcy K, Prater MR. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism. 2010;59:943–950. doi: 10.1016/j.metabol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura L. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol. 2009;296:C1162–1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 19.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Myatt L, Cui XL. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed A. New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb Res. 2011;127:S72–75. doi: 10.1016/S0049-3848(11)70020-2. [DOI] [PubMed] [Google Scholar]
- 22.Rani N, Dhingra R, Arya DS, Kalaivani M, Bhatla N, Kumar R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J Obstet Gynaecol Res. 2010;36:1189–1194. doi: 10.1111/j.1447-0756.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- 23.Scholl TO, Leskiw M, Chen XH, Sims M, Stein TP. Oxidative stress, diet, and the etiology of preeclampsia. Am J Clin Nutr. 2005;81:1390–1396. doi: 10.1093/ajcn/81.6.1390. [DOI] [PubMed] [Google Scholar]
- 24.Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31:951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Myatt L, Kossenjans W, Sahay R, Eis A, Brockman D. Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med. 2000;9:79–82. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<79::AID-MFM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Pietro L, Daher S, Rudge MV, Calderon IM, Damasceno DC, Sinzato YK, Bandeira C, Bevilacqua E. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta. 2010;31:770–80. doi: 10.1016/j.placenta.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, Liebermann DA, Tront JS, Holtzman EJ, Huang Y, Hoffman BO, Geifman-Holtzman O. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol. 2009;220:632–639. doi: 10.1002/jcp.21800. [DOI] [PubMed] [Google Scholar]
- 28.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 29.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30:S120–126. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]
- 30.Jawerbaum A, González E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13:2127–2138. doi: 10.2174/092986706777935302. [DOI] [PubMed] [Google Scholar]
- 31.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186:6024–6034. doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian D, Lin HY, Wang HM, Zhang X, Liu DL, Li QL, Zhu C. Normoxic induction of the hypoxic-inducible factor-1 alpha by interleukin-1 beta involves the extracellular signal-regulated kinase 1/2 pathway in normal human cyto-trophoblast cells. Biol Reprod. 2004;70:1822–1827. doi: 10.1095/biolreprod.103.025031. [DOI] [PubMed] [Google Scholar]


