Abstract
Angiomatous meningioma (AM) is a rare histological variant of meningioma. Twenty seven patients (14 male and 13 female) with angiomatous meningioma were treated in our institution. Their clinical presentation, neuroimaging studies, treatment and follow-up were investigated. The age of patients ranged from 24 to 72 years with a mean of 51.8 years. The clinical presentation was non-specific and depended on the location of the tumor and was mainly due to the mass effect. On computed tomography (CT) scanning, AMs showed slightly hyperintensity. On magnetic resonance imaging (MRI), AMs demonstrated hypointensity on T1-weighted images (T1WI), hyperintensity on T2-weighted images (T2WI), slight hypointensity on diffusion-weighted images (DWI), enhancement on postcontrast T1WI, peritumoral edema, and rich signal voids of vessels in the tumor. On histology, all tumors exhibited abundant blood vessels with at least focal classic meningothelial differentiation. Thirteen, eight, and six cases were achieved Simpson grade I, II and III-IV resection respectively. Nineteen cases were followed for 8 to 125 months with a mean of 47.9 months. Four patients with residual tumor were treated with postoperative radiation therapy and all of them had stable disease. One patient with Simpson grade II resection was not treated with radiation therapy and developed recurrent tumor in 5 years. In conclusion, angiomatous meningiomas have relative high male to female ratio, more frequent peritumoral edema, and rich blood vessels. Gross total resection is still the treatment of choice. These patients with residual tumor after surgery can benefit from radiation therapy. Overall, the prognosis of AMs are as good as other benign meningiomas.
Keywords: Angiomatous meningioma, computed tomography (CT), magnetic resonance imaging (MRI), surgery, radiation therapy
Introduction
Angiomatous meningioma (AM) is rare World Health Origination (WHO) grade I histological subtype of meningioma, comprising 2.1% of all meningiomas [1]. Histologically, AM has numerous blood vessel channels and at least focal classic meningioma morphology. Since AM has extremely rich blood supply and intraoperative hemorrhage can occur, the operation of AM is more difficult than that of conventional meningiomas. The preoperative neuroimaging studies including computed tomography (CT) and magnetic resonance imaging (MRI) play an important role in the preoperative preparation for a possible AM, which might reduce the chance of intraoperative hemorrhage from the tumor. We performed a retrospective study on 27 patients with AM between January 1997 and February 2011 at Qilu Hospital. To our knowledge, this is one of largest case series for angiomatous meningiomas [2-8].
Materials and methods
In total, there were 27 angiomatous meningioma cases at Qilu Hospital between January 1997 and February 2011. Clinical information was obtained by reviewing electronic medical records according to the regulation of the National Research Ethics Committee for Neurology and Neurosurgery.
CT and/or MRI were performed for all patients. We adopted Elster AD et al’s score criteria to determine the signal of angiomatous meningioma on T1WI and T2WI as follows [9]. T1WI: Signal similar to cerebrospinal fluid was scored as 1; slightly lower than the gray matter scored as 2; equal to the gray matter scored as 3; slightly higher than the gray matter scored as 4; close to the fat scored as 5. T2WI: Signal significantly lower than the gray matter was scored as 1; slightly lower than the gray matter was scored 2; equal to the gray matter scored as 3; slightly higher than the gray matter scored as 4; close to the cerebrospinal fluid scored as 5. According to the edema bandwidth measured on the plane of the largest diameter of the tumors and most obvious peritumoral edema, we graded the peritumoral edema. No edema: No visible edema in MRI; slight edema: the edema bandwidth ≤ 2 cm; moderate edema: the edema bandwidth ≥ 2cm, but ≤ 1/2 hemisphere; severe edema: the edema bandwidth ≥ 1/2 hemisphere.
All patients were treated with surgical resection. In these cases with large feeding artery or large draining vein, the silver clips were used to block the blood flow to avoid catastrophic hemorrhage. Bipolar coagulation was performed to stop bleeding. The goal of surgery was gross total resection if it was possible without damage the surrounding crucial anatomical structures. Four patients with residual tumor were received radiation therapy.
These specimens were formalin fixed and paraffin embedded. Four-micrometer-thick sections were prepared for hematoxylin and eosin (H&E) stain. The pathological diagnosis of angiomatous meningioma was rendered by pathologists.
Result
Clinical presentation
Twenty seven angiomatous meningioma cases were diagnosed at Qilu Hospital between January 1997 and February 2011. They were 14 males and 13 females with a male to female ratio of 1.08:1.0. Their age ranged from 24 to 72 years with a mean of 51.8 years. Fifteen of 27 (55.6%) patients presented with Headache and dizziness, nine (33.3%) patients with temporary loss of consciousness, seven (25.9%) patients with epileptic attack, and five patients with nausea and vomiting. The rest of patients presented with stool incontinence, visual impairment, facial hypoesthesia, tinnitus and deafness, or hoarseness and drinking bucking (Table 1). Papilledema and unilateral Limb muscle strength declined were seen in 5 and 3 patients respectively. Visual field defect, corneal reflex sluggish, nasolabial fold shallow, unilateral limb muscle tone increased, ataxia, positive Babinski sign, or chewing muscle weakness was noted in these patients (Table 1).
Table 1.
The symptoms and signs of 27 patients with angiomatous meningioma
| Symptoms | No. of cases (%) | Signs | No. of cases (%) |
|---|---|---|---|
| Headache, dizziness | 15 (55.6) | Papilledema | 5 (18.5) |
| Transient loss of consciousness | 9 (33.3) | Unilateral Limb muscle strength declined | 3 (11.1) |
| Epileptic attack | 7 (25.9) | Visual field defect | 2 (7.4) |
| Nausea, vomiting | 5 (18.5) | Corneal reflex sluggish | 2 (7.4) |
| Stool incontinence | 3 (11.1) | Nasolabial fold shallow | 2 (7.4) |
| Visual impairment | 2 (7.4) | Unilateral Limb muscle tone increased | 2 (7.4) |
| Facial hypoesthesia | 2 (7.4) | Ataxia | 2 (7.4) |
| Tinnitus and deafness | 1 (3.7) | Positive babinski sign | 2 (7.4) |
| Hoarseness, drinking bucking | 1 (3.7) | Chewing muscle weakness | 1 (3.7) |
Neuroimaging findings
MRI was performed in all patients. CT scanning was performed in 11 patients and MRA was done in 2 patients. All patients had a solitary mass and the maximal diameter of the tumor ranged from 1.5 cm to 8 cm with a mean of 4.5 cm. Convexity and sphenoidal crest were the most common locations with 18 and 4 cases respectively. These less common locations were saddle area, petroclival region, cerebellopontine angle, and the tentorium of cerebellum (Table 2). On CT scan, the tumor from all 11 patients show slight hyperintensity compared to normal brain parenchyma. On MRI, all tumors are hypointense on T1-weighted images (T1WI) and hyperintense on T2-weighted images (T2WI), slightly hypointense on DWI, signal voids of vessels and obvious peritumoral edema (Figures 1A, 1B, 2A-C and 2E). The signal score on TIWI were 1 for 9 cases and 2 for 18 cases. The signal score on T2WI were 4 for 13 cases and 5 for 14 cases. Three, seventeen and seven cases showed mild, moderate and severe peritumoral edema respectively. Twenty three of 27 cases showed homogeneous enhancement (Figures 1C, 2D) while 4 cases demonstrated cystic changes and heterogeneous enhancement. Obvious dural tail sign was seen in 18 cases (66.7%). No calcification, necrosis, or hemorrhage was noted in any case. In 2 cases with MRA, hypervascularity in the tumor and the feeding artery were noted (Figure 1D).
Table 2.
The MRI findings of 27 patients with angiomatous meningioma
| MRI findings | No. | % |
|---|---|---|
| Tumor diameter (cm) | ||
| ≤3 | 6 | 22.2% |
| 3-5 | 13 | 48.1% |
| 5-7 | 7 | 25.9% |
| ≥7 | 1 | 3.7% |
| Tumor location | ||
| Convexity | 18 | 66.7% |
| Sphenoidal crest | 4 | 14.8% |
| Saddle area | 2 | 7.4% |
| Petroclival | 1 | 3.7% |
| Cerebellopontine angle area | 1 | 3.7% |
| Tentorium of cerebellum | 1 | 3.7% |
| Tumor shape | ||
| Oval | 22 | 81.5% |
| Nodular lobulated | 1 | 3.7% |
| Irregular | 4 | 14.8% |
| Signal voids of vessel | 27 | 100% |
| Tumor enhancement | ||
| Homogeneous | 23 | 85.2% |
| Heterogeneous | 4 | 14.8% |
| Obvious meningeal tail sign | 18 | 66.7% |
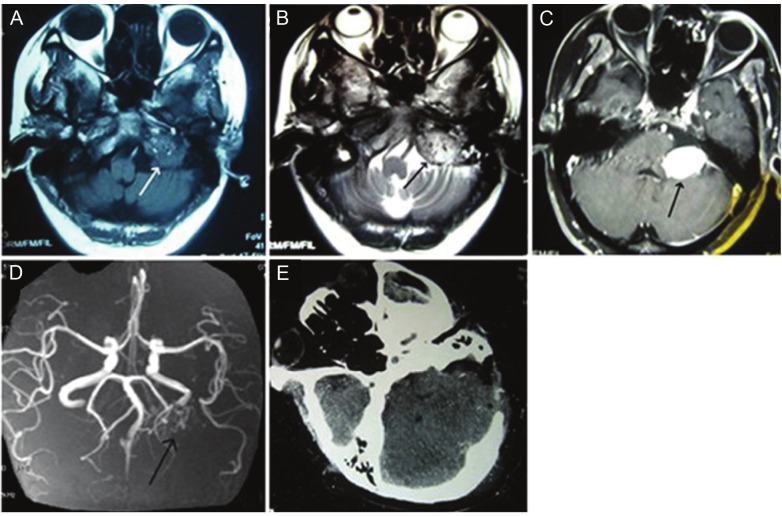
Figure 1.
Neuroimaging for an angiomatous meningioma at cerebellopontine angle. A. Axial T1WI: Slightly hypointense mass with the voids of blood vessels. B. Axial T2WI: Hyperintense mass with peritumoral edema and the voids of blood vessels. C. Axial postcontrast T1WI: Tumor with homogeneously enhancement. D. MRA: Hypervascularity was noted in the tumor and the feeding artery was seen. E. Follow-up CT scan: Gross total resection of tumor without evidence of recurrence.
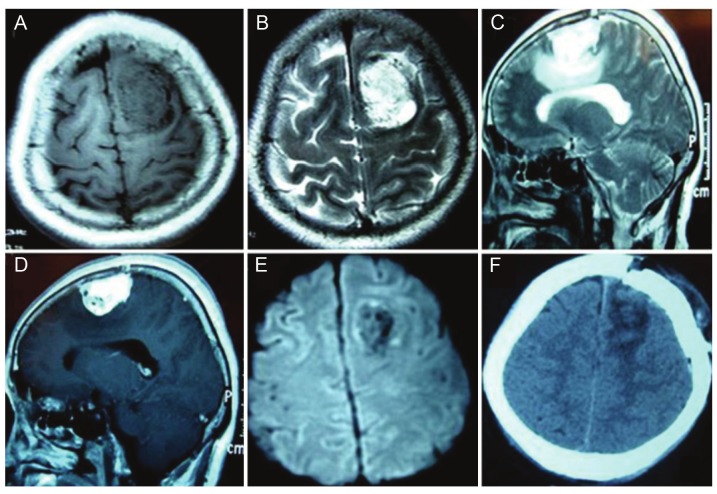
Figure 2.
Neuroimaging for an angiomatous meningioma at left frontal region a. A. Axial T1WI: Hypointense masswith the voids of blood vessels. B. Axial T2WI: Hyperintense mass with the voids of blood vessels. C. Sagittal T2WI:Hyperintense mass with peritumoral edema. D. Sagittal postcontrast T1WI: Tumor with homogeneously enhancementwith the voids of blood vessels. E. DWI: Hypointense and no restriction with the voids of blood vessels. F.Follow-up CT scan: Gross total resection without evidence of recurrence.
Treatment and follow-up
Intraoperatively, angiomatous meningioma had abundant blood supply with a lot of perforating branches of the feeding artery from the surrounding tissue and exhibited soft texture, flesh-red color in most cases, thin capsule and clear boundaries without brain invasion. Tumor tissue contained large amount of immature artery-like blood vessels, and many blood vessels were distributed on the surface of tumor. The blood flow within these thin wall vessels could be clearly seen. All patients were treated surgically. According to Simpson grade criteria [10], grade I resection was achieved in 13 cases; grade II resection in 8 cases; grade III-IV resection in 6 cases. Postoperative oculomotor nerve palsy occurred in two cases, abducens nerve palsy in one case and trigeminal nerve damage in one case. No serious post-operative complications or death occurred during the perioperative period in this series.
Four patients (3 had Simpson grade II resection and 1 had grade III resection) underwent gamma-knife radiotherapy after surgery. Clinical follow-up with neuroimaging studies was done for 19 cases (Figures 1E, 2F). The duration of follow-up was from 8 to 125 months with a mean of 47.9 months. One case with Simpson grade II resection did not receive radiotherapy after surgery and developed recurrent tumor in 5 years. The 4 patients treated with postoperative radiotherapy had stable disease.
Pathological findings
Hematoxylin and eosin (H&E) stained slides from all tumors show these tumors contained numerous blood vessels exceeding 50% of the area of the tumor. At least focal meningothelial differentiation was noted in all tumors especially in the hypercellular areas at the periphery of the tumor mass. The mitotic activity was inconspicuous. The mean MIB-1(Ki-67) proliferation index was 1.9 %. No brain invasion was identified. The histological characteristics was diagnostic of angiomatous meningioma, WHO grade I (Figure 3A, 3B) [1,7,11].
Figure 3.
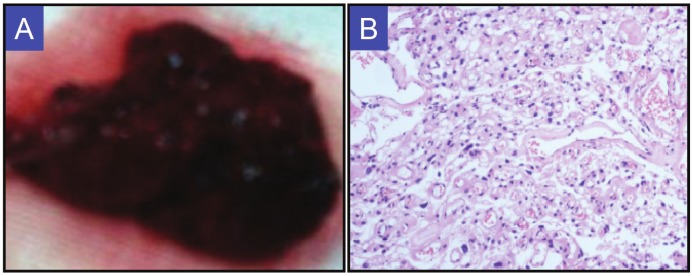
Pathological features for angiomatous meningioma. A. Gross specimen: Soft, red to brown in color. B. Hematoxylin and Eosin stain (200X). Rich thin-walled and thick-walled blood vessels and scattered meningothelial cells in the tumor.
Discussion
In the present study, we use the same criteria for angiomatous meningioma as Hasselblatt M et al [1]. A meningioma with more than 50% vascular component is designated as an angiomatous meningioma. Although all our angiomatous meningiomas are WHO grade I meningioma and share similar features of benign meningiomas such as the mean age of onset (angiomatous meningiomas: 51.8 years old, meningioma in general: 49.7 years old), five-year recurrence rate (angiomatous meningiomas: 5.3%, meningioma in general: 7%), and gross total surgical resection rate (angiomatous meningiomas: 77.8%, meningioma in general: 50-83%) (Table 3) [12-14].
Table 3.
The comparison of our angiomatous meningiomas and meningiomas in general
| Angiomatous meningiomas (present cases) | Meningiomas in general | Comparison | |
|---|---|---|---|
| Mean age of onset (years) | 51.8 | 49.7 [13] | No significantly different |
| Male and female ratio | 14:13 | 1:2 [19] | Significantly different |
| Incidence rate at brain convexity (Predilection site) | 66.7% | 15% [26] | Significantly different |
| Calcification rate | 0% | 15% [9,25] | Significantly different |
| Cystic change rate | 14.8 % | 0.9%~14% [25] | No significantly different |
| Total surgical resection rate | 77.8% | 50%~83% [24] | No significantly different |
| Dural tail sign rate | 66.7% | 52%~72% [25] | No significantly different |
| Moderate to severe peritumoral edema | 88.9% | 40% [9,25] | Significantly different |
Angiomatous meningioma demonstrates some distinct features compared to other benign meningiomas. The male to female ratio for meningioma in general is 1:2 [15]. It seems that the male to female ratio for angiomatous meningioma is much higher than that for meningioma in general. In Hasselblatt M et al’s study, the male to female ratio is 16:22 (1:1.4) while the male to female ratio in the present study is 14:13 (1.08:1.0) [1]. In Asian population, convexity meningiomas only count 15% of meningiomas [16]. All our angiomatous meningiomas are dura based and most commonly (66.7%) located over the convexity while 42% of angiomatous meningiomas in Hasselblatt M et al’s study are located over the convexity [1]. Fifteen percent of meningiomas have calcification while none of our angiomatous meningiomas has calcification [17,18]. Forty percent of meningiomas including all WHO grades show peritumoral brain edema [17,18]. In our study, 88.9% of angiomatous meningiomas and 74% of cases in Hasselblatt M et al’s study demonstrate moderate to severe brain edema, which is much more common than meningiomas in general [1]. Angiomatous meningioma seems to have more obvious signal voids of vessels in the tumor on MRI. On MRA, there is increased vascullarity in angiomatous meningioma compared to conventional meningioma. Venous obstruction, hypervascularity, pial-meningeal anastomoses, increased capillary permeability, sex hormones and their receptors in meningiomas, and the secretion of vascular endothelial growth factor (VEGF) contribute to peritumoral edema in meningioma [18-21]. Obvious peritumoral edema for angiomatous meningioma is probably due to hypervascularity, increased capillary permeability and VEGF secretion.
On histology, the main differential diagnosis for angiomatous meningioma is hemangioblastoma, which usually shows a cystic lesion with a mural enhancing nodule on MRI. During intraoperative consultation, the hemangioblastoma can closely mimic angiomatous meningioma on frozen section. However, the cellularity for hemangioblastoma on the cytological preparation is very low and tumor cells have obvious intracellular vacuoles. On the permanent tissue sections, it is not a problem for us to separate angiomatous meningioma from hemangioblastoma since all of our angiomatous meningiomas at least focally exhibit classic meningothelial morphology.
On MRI, if a dura based mass shows homogeneous enhancement, rich blood flow void shadow and obvious peritumoral edema while there is clear CSF signal between the mass and brain tissue, the possibility of angiomatous meningioma should be considered. A cerebral angiography can be performed in order to identify the feeding artery. Especially for the skull base tumor, it is better to perform the preoperative embolization to reduce tumor blood supply. Sufficient compatible blood for possible transfusion or autologous blood should be prepared before the surgery. Multiple intravenous lines for rehydration or possible blood transfusion should be established. Clear exposure of the tumor is the key for successful operation. It is preferable to find and block the feeding artery, and then cut off the perforating branches of the feeding artery. Bipolar forceps can be used to fulgurate the surface of the tumor repeatedly to shrink the tumor. Gross total resection is the goal if it is possible.
For these cases with residual tumor after surgery, radiation therapy can be used for treating these patients [22,23]. Moreover, Rowe J et al have shown that gamma-knife stereotactic radiosurgery does not increase the probability of malignant transformation of meningiomas [24]. The molecular basis of the effectiveness of radiation therapy is that radiotherapy can inhibit the expression of vascular endothelial growth factor (VEGF) and somatostatin receptor, which causes blood vessel contraction to reduce blood supply and then shrink the tumor [25]. Nicolato A et al reported that, in the 122 cases of cavernous sinus meningiomas who received gamma-knife stereotactic radiosurgery, 118 (97%) cases had stable clinical symptoms or greatly improved [26]. Subach et al think that patients with residual tumor, recurrent tumors, or tumor progression after subtotal resection, and inoperable patients, can receive the radiosurgery [27]. In our 27 cases, four received postoperative gamma-knife radiotherapy; none of them had progression of the tumor. One case with grade II resection did not receive radiotherapy after surgery and developed recurrent tumor in 5 years. It is very clear that radiotherapy, as postoperative adjuvant therapy, can reduce the recurrence rate of angiomatous meningioma in these cases with residual tumor.
Conclusion
In summary, although angiomatous meningiomas share similar clinical features and prognosis with benign meningiomas, they have some unique characteristics such as, relative high male to female ratio compared with meningiomas in general, more frequent peritumoral edema, and rich blood vessels in the tumor. Gross total resection is still the treatment of choice. These patients with residual tumor after surgery can benefit from radiation therapy.
Acknowledgments
We thank Ms. Yan Song, Mr. Tao Li, Mr. Guodong Yang and Mr. Xinguo Sun for administrative and operational support; Dr. Shugan Zhu, Dr. Xiangshui Meng, and Dr. Donghai Wang for their helpful discussions and critical reading of the manuscript.
Conflict of interest statement
None.
References
- 1.Hasselblatt M, Nolte KW, Paulus W. Angiomatous meningioma: a clinicopathologic study of 38 cases. Am J Surg Pathol. 2004;28:390–393. doi: 10.1097/00000478-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bodla AA, Mehta P, Mushtaq F, Durrani OM. Angiomatous meningioma of orbit mimicking as malignant neoplasm: a case report and literature review. Orbit. 2011;30:183–185. doi: 10.3109/01676830.2011.582980. [DOI] [PubMed] [Google Scholar]
- 3.Deb P, Sahni H, Bhatoe HS. Cystic angiomatous meningioma in the cerebellopontine angle mimicking hemangioblastoma. J Cancer Res Ther. 2010;6:560–563. doi: 10.4103/0973-1482.77074. [DOI] [PubMed] [Google Scholar]
- 4.Dietzmann K, von BP, Warich-Kirches M, Kirches E, Synowitz HJ, Firsching R. Immunohistochemical detection of vascular growth factors in angiomatous and atypical meningiomas, as well as hemangiopericytomas. Pathol Res Pract. 1997;193:503–510. doi: 10.1016/s0344-0338(97)80104-5. [DOI] [PubMed] [Google Scholar]
- 5.Elahi E, Meltzer MA, Friedman AH, Som PM. Primary orbital angiomatous meningioma. Arch Ophthalmol. 2003;121:124–127. doi: 10.1001/archopht.121.1.124. [DOI] [PubMed] [Google Scholar]
- 6.Marsh C, Vogel PJ, Hurley C, Deeb PH. Suprasellar angiomatous meningioma simulating a large carotid aneurysm; report of case. Bull Los Angel Neuro Soc. 1957;22:40–44. [PubMed] [Google Scholar]
- 7.Rao S, Rajkumar A, Kuruvilla S. Angiomatous meningioma: a diagnostic dilemma. Indian J Pathol Microbiol. 2008;51:53–55. doi: 10.4103/0377-4929.40397. [DOI] [PubMed] [Google Scholar]
- 8.Taraszewska A, Bogucki J. A case of cystic form of angiomatous meningioma with prominent microvascular pattern mimicking haemangioblastoma. Folia Neuropathol. 2001;39:119–123. [PubMed] [Google Scholar]
- 9.Elster AD, Challa VR, Gilbert TH, Richardson DN, Contento JC. Meningiomas: MR and histopathologic features. Radiology. 1989;170:857–862. doi: 10.1148/radiology.170.3.2916043. [DOI] [PubMed] [Google Scholar]
- 10.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg MS. Handbook of Neurosurgery. 1990. [Google Scholar]
- 13.Ayerbe J, Lobato RD, de la CJ, Alday R, Rivas JJ, Gomez PA, Cabrera A. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien) 1999;141:921–932. doi: 10.1007/s007010050398. [DOI] [PubMed] [Google Scholar]
- 14.Tokumaru A, O’uchi T, Eguchi T, Kawamoto S, Kokubo T, Suzuki M, Kameda T. Prominent meningeal enhancement adjacent to meningioma on Gd-DTPA-enhanced MR images: histopathologic correlation. Radiology. 1990;175:431–433. doi: 10.1148/radiology.175.2.2326470. [DOI] [PubMed] [Google Scholar]
- 15.Roser F, Nakamura M, Ritz R, Bellinzona M, Dietz K, Samii M, Tatagiba MS. Proliferation and progesterone receptor status in benign meningiomas are not age dependent. Cancer. 2005;104:598–601. doi: 10.1002/cncr.21192. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita J, Handa H, Iwaki K, Abe M. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14:33–40. [PubMed] [Google Scholar]
- 17.Kim BW, Kim MS, Kim SW, Chang CH, Kim OL. Peritumoral brain edema in meningiomas: correlation of radiologic and pathologic features. J Korean Neurosurg Soc. 2011;49:26–30. doi: 10.3340/jkns.2011.49.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363:1535–1543. doi: 10.1016/S0140-6736(04)16153-9. [DOI] [PubMed] [Google Scholar]
- 19.Pistolesi S, Fontanini G, Camacci T, De IK, Boldrini L, Lupi G, Padolecchia R, Pingitore R, Parenti G. Meningioma-associated brain oedema: the role of angiogenic factors and pial blood supply. J Neurooncol. 2002;60:159–164. doi: 10.1023/a:1020624119944. [DOI] [PubMed] [Google Scholar]
- 20.Domingo Z, Rowe G, Blamire AM, Cadoux-Hudson TA. Role of ischaemia in the genesis of oedema surrounding meningiomas assessed using magnetic resonance imaging and spectroscopy. Br J Neurosurg. 1998;12:414–418. doi: 10.1080/02688699844600. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85:936–944. doi: 10.1002/(sici)1097-0142(19990215)85:4<936::aid-cncr23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Malik I, Rowe JG, Walton L, Radatz MW, Kemeny AA. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13–20. doi: 10.1080/02688690500080885. [DOI] [PubMed] [Google Scholar]
- 23.Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56:546–559. doi: 10.1227/01.neu.0000153906.12640.62. [DOI] [PubMed] [Google Scholar]
- 24.Rowe J, Grainger A, Walton L, Silcocks P, Radatz M, Kemeny A. Risk of malignancy after gamma knife stereotactic radiosurgery. Neurosurgery. 2007;60:60–65. doi: 10.1227/01.NEU.0000255492.34063.32. [DOI] [PubMed] [Google Scholar]
- 25.Nicolato A, Giorgetti P, Foroni R, Grigolato D, Pasquin IP, Zuffante M, Soda C, Tomassini A, Gerosa M. Gamma knife radiosurgery in skull base meningiomas: a possible relationship between somatostatin receptor decrease and early neurological improvement without tumour shrinkage at short-term imaging follow-up. Acta Neurochir (Wien) 2005;147:367–374. doi: 10.1007/s00701-005-0483-9. [DOI] [PubMed] [Google Scholar]
- 26.Nicolato A, Foroni R, Alessandrini F, Bricolo A, Gerosa M. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153–1159. doi: 10.1097/00006123-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Subach BR, Lunsford LD, Kondziolka D, Maitz AH, Flickinger JC. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437–443. doi: 10.1097/00006123-199803000-00001. [DOI] [PubMed] [Google Scholar]