Abstract
Expression of cytokeratin (CK) profiles in primary signet-ring cell carcinoma (SRCC) of the stomach and colorectum have rarely reported; only two such studies are present in the world literature. Herein, an immunohistochemical study on cytokeratin (CK) expression was performed in 42 cases of primary SRCC of the stomach (30 cases) and colorectum (12 cases). SRCC was defined as an adenocarcinoma in which more than 50% adenocarcinoma cells showed SRCC phenotype with prominent intracytoplasmic mucins. In the gastric SRCC, the expression of CK was as follows; CK AE1/3 (30/30, 100%) CK CAM5.2 (30/30, 100%), CK 34BE12 (0/30, 0%), CK5/6 (2/30, 7%), CK7 (26/30, 89%), CK8 (12/30, 40%), CK14 (0/30, 0%), CK18 (30/30, 100%), CK19 (2/30, 7%), and CK20 (3/30, 10%). In the colorectal SRCC, the expression of CK was as follows; CK AE1/3 (12/12, 100%) CK CAM5.2 (12/12, 100%), CK 34BE12 (0/12, 0%), CK5/6 (0/12, 10%), CK7 (2/12, 17%), CK8 (3/12, 25%), CK14 (0/12, 0%), CK18 (12/12, 100%), CK19 (7/12, 58%), and CK20 (8/12, 67%). A statistical analysis showed that significant differences of CK expression between the gastric SRCC and colorectal SRCC were observed in CK7 (stomach 67% vs. colorectum 17%), CK19 (7% vs. 42%) and CK20 (13% vs. 67%); gastric SRCC tended to express CK7, but not CK19 and CK20, while colorectal SRCC tended to express CK19 and CK20, but not CK7. In gastric SRCC, CK7+/CK20- pattern was as follows: CK7+/CK20- (24/30, 81%), CK7+/CK20+ (2/30, 6%), CK7-/CK20+ (1/30, 3%), and CK7-/CK20- (3/30, 10%). CK7/CK19 patterns in gastric SRCC were as follows; CK7+/CK19- (25/30, 83%) CK7+/CK19+ (1/30, 3%), CK7-/CK19+ (1/30, 3%), CK7-/CK19- (3/30, 10%). In colorectal SRCC, the CK7/CK20 patterns were as follows: CK7+/CK20- (2/12, 17%), CK7+/CK20+ (0/12, 0%), CK7-/CK20+ (8/12, 66%), and CK7-/CK20- (2/12, 17%). The CK7/CK19 pattern in colorectal SRCC was as follows; CK7+/CK19- (1/12, 8%), CK7+/CK19+ (1/12, 8%), CK7-/CK19+ (6/12, 50%), and CK7-/CK19- (4/12, 34%). Statistical data indicated that CK7+/CK20- and CK7+/CK19- patterns were significantly prevalent in gastric SRCC, and CK7-/CK20+, CK7-/CK19+ and CK7-/CK20- patterns dominated significantly in colorectal SRCC. CK expression has been studied largely in terms of CD7/CK20 expression pattern in various carcinomas. The present study provided possible usefulness of CK7/19 expression status in various carcinomas including SRCC.
Keywords: Signet-ring cell carcinoma, cytokeratin, stomach, colorectum, histopathology, immunohistochemistry
Introduction
Cytokeratin (CK) or keratin is one of the intermediate filaments predominantly present in epithelial cells [1,2]. At least 20 well-defined subclasses of CK have been identified on the basis of the molecular weight and isoelectric pH value [1,2]. Epithelial cells and epithelial malignancy almost always contains CK, and the presence of CK is a strong evidence for epithelial cells and their malignant counterparts [1,2]. Therefore, in the pathological field, immunohistochemical demonstration of CK almost always indicates that the tumor is not sarcoma, but carcinoma, though CK expression is infrequently seen in certain sarcomas including epithelioid sarcoma [1,2]. Recently, the expression pattern of CK7/CK20 has been well studied in the pathological field [1-3].
Signet-ring cell carcinoma (SRCC) is characterized by an adenocarcinoma whose carcinoma cells were composed predominantly of SRCC cells [4,5]. SRCC cells are characterized by abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins [4,5]. SRCC can occur in any organs, but is most prevalent in the stomach, followed in order by colorectum and lung [4,5]. According to the current WHO blue book, SRCC is defined as an adenocarcinoma with the presence of >50% of tumor cells (signet-ring cells) with prominent intracytoplasmic mucins [5].
The author has examined SRCC in the extragastric and extra-colorectal SRCC [6-16]. In the present study, the author reports a study of primary SRCC in the stomach and colorectum.
The CK profiles of ordinary adenocarcinomas of the stomach, colorectum, lung and other organs have been well studied [17-30], although most studies examined only CK7 and CK20. However, the CK profiles of SRCC in the stomach and colorectum have been rarely performed [31,32]; only two studies examined the expression of a few CKs [31,32].
The author herein examined the expression pattern of many CK molecules in primary SRCC of the stomach and colorectum.
Materials and methods
The author retrieved primary adenocarcinoma with signet-ring cells of the stomach and colon in the author’s computer database files of primary SRCC of the digestive organs in the recent 15 years. The computer survey identified 68 cases of primary adenocarcinoma of the stomach and colon with signet-ring phenotype. The author reviewed these 68 cases under the microscopy. The author confirmed the signet ring cell phenotype of these adenocarcinoma, and excluded cases of adenocarcinoma with SRCC cells whose percentage was less than 50% of the tumor cells. As the results, 42 cases of SRCC fulfilling the WHO criteria [4,5] remained. The primary nature of these 42 cases of SRCC was confirmed by clinical and pathological findings. Of the 42 cases, 30 were primary gastric SRCC and the remaining 12 were primary colorectal SRCC. Of the 42 cases, 26 cases were biopsies and the remaining 16 cases were surgically resected cases. In the 30 gastric SRCC cases, 21 were male and 9 were female. The mean age and standard deviation was 74 years ±14 years. In the 12 colorectal SRCC cases, 7 were male and 5 were female. The mean age and standard deviation was 68 years ±12 years.
An immunohistochemical study was performed by the Dako EnVision method (Dako Corp, Glostrup, Denmark), as previously described [33-39]. The antigens examined included CK AE1/3, CK CAM5.2, CK34BE12, CK5/6, CK7, CK8, CK14, CK18, CK19, and CK20. Histochemical investigation was also performed by mucicarmine stain and by combined periodic acid-Schiff after diastase digestion (d-PAS) and Alcian blue (AB) at pH2.5. Statistical analysis was performed by Chi-square test.
Results
The SRCC was composed of medullary proliferation of large clear cells with much intracytoplasmic mucin (neutral mucin and acidic mucin) (Figure 1A-C), which was confirmed by combined d-PAS/AB technique (Figure 1C) and mucicarmine stains. The proportion of signet ring cells in SRCC ranged from 60% to 100%. In most cases, the SRCC contained areas of other histologies such as mucinous adenocarcinoma, and tubular adenocarcinoma.
Figure 1.
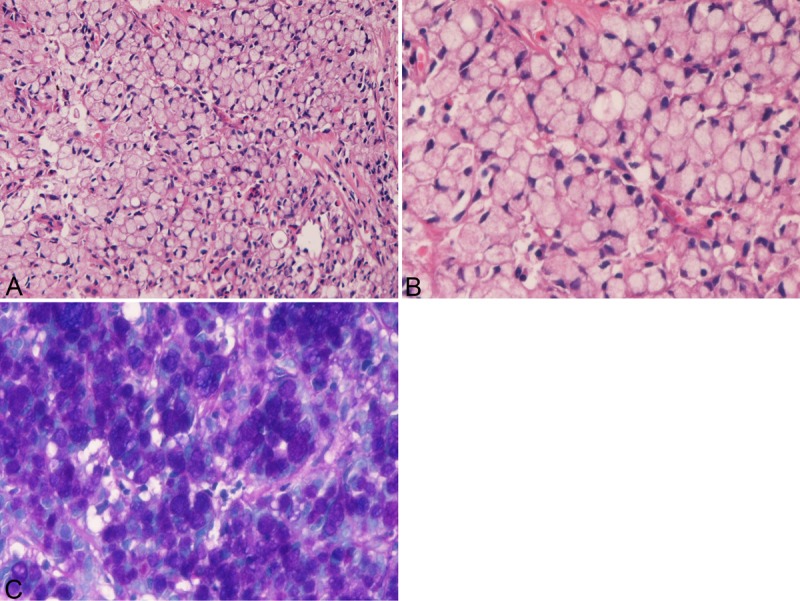
Histology and histochemistry of primary signet-ring cell carcinoma of the stomach. A: Lower power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. The signet-ring cell carcinoma is medullary and the stroma is scant in amount. HE: x100. High power view. The signet-ring features such as abundant intracytoplasmic mucins, ample and clear cytoplasm, and eccentrically located nuclei compressed by intracytoplasmic mucins are apparent. HE: x400. C: Combined d-PAS and AB stains revealed abundant intracytoplasmic mucins composed of neutral (Mazenta color) and acidic (blue color) mucins. Combined d-PAS/AB double staining: x200.
In the 30 gastric SRCC, the expression of CK was as follows; CK AE1/3 (30/30, 100%) (Figure 2A), CK CAM5.2 (30/30, 100%) (Figure 2B), CK 34BE12 (0/30, 0%), CK5/6 (2/30, 7%) (Figure 2C), CK7 (26/30, 89%) (Figure 2D), CK8 (12/30, 40%) (Figure 2E), CK14 (0/30, 0%), CK18 (30/30, 100%) (Figure 2F), CK19 (2/30, 7%) (Figure 2G), and CK20 (3/30, 10%) (Figure 2H). There was a tendency that the CK immunoreactivity was strong and diffuse in cases with high expression percentage of a given CK, and that the CK immunoreactivity was weak and focal in cases with low expression percentage of a given CK. The expression percentage was significantly (p<0.05) higher in CK AE1/3, CK CAM5.2, CK7, and CK18 than in CK34BE12, CK5/6, CK14, CK19 and CK20. That is, there was a tendency that gastric SRCC tends to express CK7 and tend not express CK19 and CK20.
Figure 2.
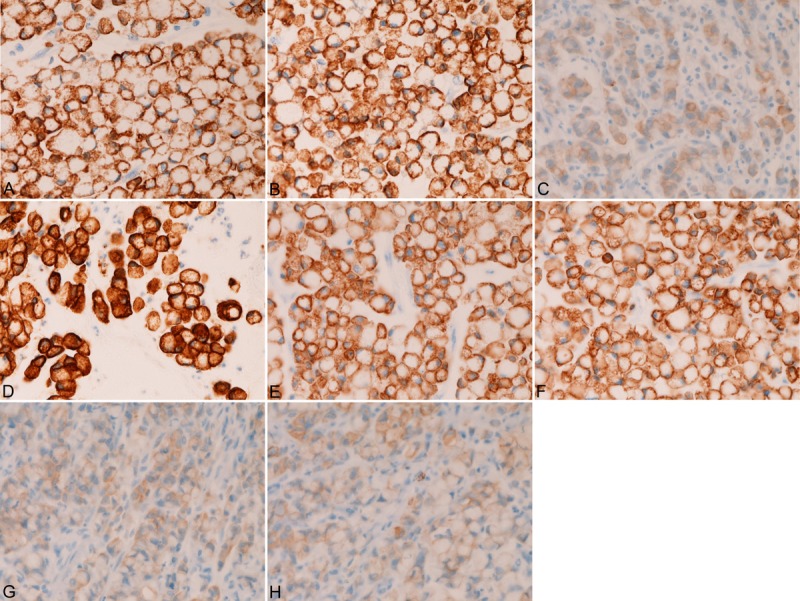
Immunohistochemical findings of primary gastric signet-ring cell carcinoma. The signet-ring cell carcinoma cells are positive for CK AE1/3 (A), CK CAM5.2 (B), CK5/6 (C), CK7 (D), CK8 (E), CK18 (F), CK19 (G), and CK20 (H). The CK expression is strong in CK AE1/3 (A), CK CAM5.2 (B), CK7 (D), CK8 (E), and CK18 (F), while it is relatively weak in CK5/6 (C), CK19 (G), and CK20 (H).
In the colorectal SRCC, the expression of CK was as follows; CK AE1/3 (12/12, 100%) CK CAM5.2 (12/12, 100%), CK 34BE12 (0/12, 0%), CK5/6 (0/12, 10%), CK7 (2/12, 17%) (Figure 3A), CK8 (3/12, 25%), CK14 (0/12, 0%), CK18 (12/12, 100%), CK19 (7/12, 58%) (Figure 3B), and CK20 (8/12, 67%) (Figure 3C). There was a tendency that the CK immunoreactivity was strong and diffuse in cases with high expression percentage of a given CK, and that the CK immunoreactivity was weak and focal in cases with low expression percentage of a given CK. The expression percentage was significantly (p<0.05) higher in CK AE1/3, CK CAM5.2, CK18, CK19 and CK20 than in CK34BE12, CK5/6, CK7, CK8, and CK14. That is, there was a significant tendency that colorectal SRCC tends to express CK19 and CK29 and tend not express CK7.
Figure 3.
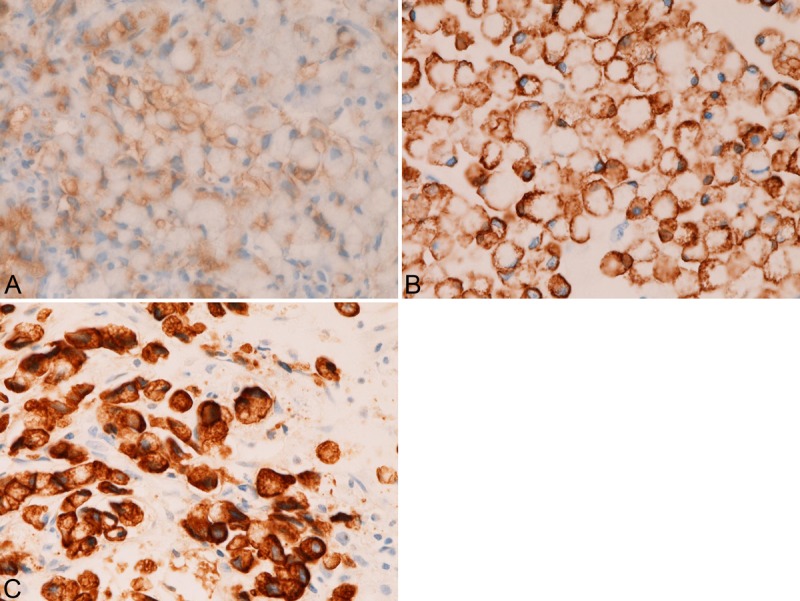
Some examples of immunohistochemical findings of primary colorectal signet-ring cell carcinoma. The signet-ring cell carcinoma cells are positive for CK AE1/3 (A), CK CAM5.2 (B), CK5/6 (C), CK7 (D), CK8 (E), CK18 (F), CK19 (G), and CK20 (H). The CK expression is strong in CK AE1/3 (A), CK CAM5.2 (B), CK7 (D), CK8 (E), and CK18 (F), while it is relatively weak in CK5/6 (C), CK19 (G), and CK20 (H).
A comparative statistical analysis showed that significant differences (p<0.05) of CK expression between the primary gastric SRCC and primary colorectal SRCC were observed in CK7 (67% vs 17%), CK19 (7% vs 42%) and CK20 (13% vs 67%); gastric SRCC tended to express CK7, but not CK19 and CK20, while colorectal SRCC tended to express CK19 and CK20, but not CK7.
In primary gastric SRCC, CK7+/CK20- pattern was as follows: CK7+/CK20- (24/30, 81%), CK7+/CK20+ (2/30, 6%), CK7-/CK20+ in (1/30, 3%), and CK7-/CK20- (3/30, 10%). CK7/CK19 patterns in gastric SRCC were as follows; CK7+/CK19- (25/30, 83%) CK7+/CK19+ (1/30, 3%), CK7-/CK19+ (1/30, 3%), CK7-/CK19- (3/30, 10%). The CK7+/CK20- and CK7+/CK19- patterns were significantly predominated over other patterns.
In primary colorectal SRCC, CK7+/CK20- patterns were as follows: CK7+/CK20- (2/12, 17%), CK7+/CK20+ (0/12, 0%), CK7-/CK20+ (8/12, 66%), and CK7-/CK20- (2/12, 17%). The CK7/CK19 pattern in colorectal SRCC was as follows; CK7+/CK19- (1/12, 8%) CK7+/CK19+ (1/12, 8%), CK7-/CK19+ (6/12, 50%), and CK7-/CK19- (4/12, 34%). The CK7-/CK20+, CK7-/CK19+ and CK7-/CK19- patterns dominated significantly (p<0.05) in colorectal SRCC.
Discussion
Our previous studies of SRCC of non-gastric and non-colorectal organs have indicated that the expression of CKs including CK AE1/3, CK CAM5.2, CK5/6, CK34BE12, CK7, CK8, CK14, CK17, CK18, CK19 and CK20 is not restricted, but showed diverse expression patterns in SRCC of the non-gastric and non-colorectal organs [6-16].
Most of studies of CK expression of the stomach and colorectum employed only CK7 and CK20 [17-30]. There are only two studies of CK expression of SRCC in the stomach and colorectum [31,32]. In the ordinary gastric adenocarcinomas excluding SRCC, Kim et al [26] showed that CK expression in 329 gastric carcinomas was as follows: CK4 84.7%, CK5 3.2%, CK6 28.7%, CK7 71.1%, CK8 96.6%, CK10 0%, CK13 81.6%, CK14 0.3%, CK16 4.1%, CK17 0%, CK18 99.4 %, CK19 89.7%, and CK20 30.0%. In the present study using 30 cases of gastric SRCC, the CK expression was as follows: CK AE1/3 (30/30, 100%) CK CAM5.2 (30/30, 100%), CK 34BE12 (0/30, 0%), CK5/6 (2/30, 7%), CK7 (26/30, 89%), CK8 (12/30, 40%), CK14 (0/30, 0%), CK18 (30/30, 100%), CK19 (2/30, 7%), and CK20 (3/30, 10%). The results of these two studies are very similar except for CK19 and CK20. The positive percentage of CK 19 and CK20 of gastric non-SRCC adenocarcinoma was 89.7% and 30.0% according to Kim et al [26], while the percentage of CK19 and CK20 expression in the current study of gastric SRCC was 19 7% (CK19) and 10% CK20), respectively. This difference may suggest that gastric SRCC lose CK19 and CK20 during the carcinogenesis. However, the CK expression pattern seems similar between gastric ordinary adenocarcinomas and gastric SRCC except for CK19 and CK20. Goldstein et al [31] who used 27 cases of gastric SRCC, also stated that there was no difference of CK expression of CK7, CK17, CK19, and CK20 between gastric ordinary adenocarcinoma and gastric SRCC. Chu et al [32] demonstrated that gastric SRCC expressed CK7 in 67% (20/ 30 cases) and CK 20 in 57% (17/30 cases). The present study was the first one demonstrating the expression of wide ranges of CKs in primary gastric SRCC. The present study strongly suggests that the expression of CKAE1/3, CK CAM5.2 and CK18 is always expressed in gastric SRCC, that expression of CK34BE12, CK5/6, and CK14 is none or very low in gastric SRCC, that expression of CK19 and CK20 is very low (less than 10%), that expression of expression of CK7 is very high (89%), and that expression of CK8 is intermediate. The author wants to stress the very high expression of CK7 and very low expression in CK19 and CK20 in primary gastric SRCC.
Most studies of CK expression in colorectal carcinoma employed only CK7 and CK20, because of well known specificity of CK7/20 pattern [2,3,17-32].
One report of ordinary colorectal carcinomas done by Lee et al [29] showed expression of CKs; CK7 (10% 7/70), CK8 (95.6%, 65/68), CK13 (95.6%, 65/68), CK14 (2.7%, 2/72), CK18 (92.4%, 61/66), CK19 (95.6%, 65/68), and CK20 (76.5%, 52/68). In the current study of primary colorectal SRCC, the expression of CK was as follows; CK AE1/3 (12/12, 100%) CK CAM5.2 (12/12, 100%), CK 34BE12 (0/12, 0%), CK5/6 (0/12, 10%), CK7 (2/12, 17%), CK8 (3/12, 25%), CK14 (0/12, 0%), CK18 (12/12, 100%), CK19 (7/12, 58%), and CK20 (8/12, 67%). Compared to the ordinary adenocarcinoma of colorectum reported by Lee et al [29], primary colorectal SRCC in the present study showed basically similar CK profiles. However, expression of CK8, CK19, and CK20 is low in colorectal SRCC, compared to that of colorectal ordinary adenocarcinoma [29]. The CK expression of SRCC in the colorectal region has been reported by only two studies [31,32]. Goldstein et al [31] who examined CK expression in 14 colonic SRCCs, showed low expression of CK7 (37%) and high expression of CK20 (72%). Chu et al [32] demonstrated low CK7 expression (44%, 4/9) and high CK20 expression (78%, 7/9). Taken together, it is strongly suggested that the expression of CK7 is low while the expression of CK19 and CK20 is high in primary colorectal SRCC.
No comparative studies have been performed between gastric SRCC and colorectal SRCC. In the present study, the statistical analysis showed that significant differences (p<0.05) of CK expression between the gastric SRCC and colorectal SRCC were observed in CK7 (67% vs 17%), CK19 (7% vs 42%) and CK20 (13% vs 67%) among the many CKs examined. Primary gastric SRCC tended to express CK7, but not CK19 and CK20, while primary colorectal SRCC tended to express CK19 and CK20, but not CK7. This tendency is similar to the previous studies of gastric ordinary adenocarcinoma and colorectal ordinary adenocarcinoma [17-32], except for CK19. In the present study, low expression of CK19 was seen in gastric SRCC and high expression of CK19 in colorectal SRCC. The expression pattern of CK 19 was similar to CK20. The author wants to stress that the expression pattern of CK19 resembles that of CK20 in gastric and colorectal malignancies. These are new findings.
Most studies of CK expression in gastric and colorectal ordinary adenocarcinomas employed only CK7 and CK20, because CK7/CK20 expression pattern is relatively specific in carcinomas in various organs [2,3,17-32] and also because CK7/20 pattern is useful in the differential diagnosis of carcinoma of various organs [2,3,17-32]. The current study confirmed that there is also a significant importance of the CK7/CK20 pattern in primary gastric and colorectal SRCC, similar to the CK7/20 pattern of the previous reported cases of ordinary adenocarcinoma of the stomach and colorectum. In the present primary gastric SRCC, 81% of gastric SRCC showed CK7+/CK20-, 6% CK7+/CK20+, and 10% CK7-/CK20-. Goldstein et al [31] showed the expression status of CK7/20 very similar to that of the present study in their 27 cases of gastric SRCC. These findings suggest that the CK7/20 pattern is useful in determining the primary and metastatic origin of SRCC. Of particular importance in the present study is that the CK7/CK19 pattern in primary gastric SRCC showed the expression pattern similar to CK7/CK20. Namely, 83% was CK7+/CK19-, 3% CK7+/CK19+, 3% CK7-/CK19+, and 10% CK7-/CK19-. The statistical analysis showed that the CK7+/CK20- and CK7+/CK19- patterns were significantly (p<0.05) predominated in gastric SRCC. The author wants to stress that the CK7/CK19 pattern is also useful in primary gastric SRCC, in addition to the CK7/CK20 pattern.
Similar tendency was observed in the primary colorectal SRCC in the current study. In contrast to primary gastric SRCC, in the primary colorectal SRCC, CK7+/CK20- was 17%, CK7+/CK20+ 0%, CK7-/CK20+ 66%, and CK7-/CK20- 17%. Similar expression pattern of CK7/20 was reported by Goldstein [31] in colorectal SRCC. In the present study, the difference was statistically significant. Therefore, the CK7-/CK20+ pattern predominates over other patterns in colorectal SRCC. The similar tendency was observed in the CK7/CK19 pattern in colorectal SRCC; CK7+/CK19- (8%), CK7+/CK19+ (8%), CK7-/CK19+ (50%), and CK7-/CK19- (34%). Therefore, the CK7-/CK20+, CK7-/CK19+ and CK7-/CK19- patterns dominated significantly (p<0.05) over other combination pattens of CK in colorectal SRCC. The study of CK expression pattern of CKs combination has been performed in only once [31] in gastric and colorectal SRCC. The only study of Goldstein [31] used only CK7, CK17, CK19 and CK20. In the present study, the expression pattern of much more CK molecules was investigated, and found the significance of CK7/20 and CK7/19 patterns.
In conclusion, the authors demonstrated the expression of 10 types of CK in 42 cases of primary gastric and colorectal SRCC, and described the frequency of positive expression of these 10 CK antigens. In addition, the author demonstrated the significant importance of CK7/20 and CK 7/19 patterns in primary gastric and colorectal SRCC. The present study is the third one for CK expression of primary gastric and colorectal SRCC, and the second one examining the CK7/20 pattern, and the first one for CK expression using many CK types, and the first one demonstrating the significance of CK7/19 expression in primary gastric and colorectal SRCC. The present study indicated that CK7+/CK20- and CK7+/CK19- patterns were significantly prevalent in primary gastric SRCC, and CK7-/CK20+, CK7-/CK19+ and CK7-/CK20- patterns dominated significantly in primary colorectal SRCC. The author wants to stress the possible importance of CK7/CK19 pattern in various carcinomas of carious organs, which await further studies.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Rosai J. Keratins. In: Rosai J, editor. Rosai and Ackermans Surgical Pathology. New York: V Mosby; 2004. pp. 55–56. [Google Scholar]
- 2.Moll R, Divo M, Langbein L. The human keratin: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002;38:758–763. doi: 10.1016/s0959-8049(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 4.Lauwers GY, Franceschi S, Carneiro F, Montgomery E, Graham DY, Tatematsu M, Curado MP, Hattori . Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 48–58. [Google Scholar]
- 5.Hamilton SR, Nakamura S, Bosman FT, Quirke P, Boffetta P, Riboli E, IIyas M, Sobin LH, Morreau H. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the the digestive organs. Lyon: IARC; 2010. pp. 134–146. [Google Scholar]
- 6.Terada T. Primary signet-ring cell carcinoma of the lung: a case report with an immunohistochemical study. Int J Clin Exp Pathol. 2012;5:171–174. [PMC free article] [PubMed] [Google Scholar]
- 7.Terada T. Primary signet-ring cell carcinoma of the ampulla of Vater: a case report with an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2012;20:427–428. doi: 10.1097/PAI.0b013e31823b7052. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report. Endoscopy. 2012;44(Suppl 2):E141–142. doi: 10.1055/s-0030-1257045. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Primary pure signet ring cell adenocarcinoma of the non-Barrett’s esophagus: a case report with immunohistochemical study. Endoscopy. 2011;43(Suppl 2 UCTN):E397–8. doi: 10.1055/s-0030-1256944. [DOI] [PubMed] [Google Scholar]
- 10.Terada T. Primary pure signet-ring cell adenocarcinoma of the urinary bladder: a report of three cases with an immunohistochemical study. Med Oncol. 2012;29:2866–9. doi: 10.1007/s12032-011-0122-7. [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Signet-ring cell carcinoma of the non-ampullary duodenum and proximal jejunum: a case report with an immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291528. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Terada T. Primary pure signet-ring cell carcinoma of the anus: a case report with immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291516. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Terada T. An immunohistochemical study of a primary signet-ring cell carcinoma of the ampulla of Vater: A case report. J Gastrointest Cancer. 2012 Dec 19; doi: 10.1007/s12029-012-9469-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Terada T. Signet-ring cell carcinoma of the esophagus in dermatomyositis: a case report with immunohistochemical study. J Gastrointest Cancer. 2013 Jan 6; doi: 10.1007/s12029-012-9473-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Terada T. Ovarian malignant Mullerian mixed tumor (heterologous) whose epithelial component is composed predominantly of signet ring cell carcinoma. Arch Gynecol Obstet. 2011;283:1403–1406. doi: 10.1007/s00404-010-1591-1. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Small Cell Carcinoma of the Ileum That Developed 10 Years After Total Gastrectomy for Gastric Signet-ring Cell Carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:618–619. doi: 10.1097/PAI.0b013e31823eb34f. [DOI] [PubMed] [Google Scholar]
- 17.Gürbüz Y, Köse N. Cytokeratin expression patterns of gastric carcinomas according to Lauren and Goseki classification. Appl Immunohistochem Mol Morphol. 2006;14:303–308. doi: 10.1097/00129039-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Tsuta K, Ishii G, Nitadori J, Murata Y, Kodama T, Nagai K, Ochiai A. Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol. 2006;209:78–87. doi: 10.1002/path.1947. [DOI] [PubMed] [Google Scholar]
- 19.Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884–92. doi: 10.1309/A09E-RYMF-R64N-ERDW. [DOI] [PubMed] [Google Scholar]
- 20.Merchant SH, Amin MB, Tamboli P, Ro J, Ordóñez NG, Ayala AG, Czerniak BA, Ro JY. Primary signet-ring cell carcinoma of lung: immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol. 2001;25:1515–1519. doi: 10.1097/00000478-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Castro CY, Moran CA, Flieder DG, Suster S. Primary signet ring cell adenocarcinomas of the lung: a clinicopathological study of 15 cases. Histopathology. 2001;39:397–401. doi: 10.1046/j.1365-2559.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 22.Tot T. The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. APMIS. 2000;108:467–472. doi: 10.1034/j.1600-0463.2000.d01-84.x. [DOI] [PubMed] [Google Scholar]
- 23.Osborn M, Mazzoleni G, Santini D, Marrano D, Martinelli G, Weber K. Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas. Virchows Arch A Pathol Anat Histopathol. 1988;413:303–312. doi: 10.1007/BF00783022. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi H, Kitamura H, Nakatani Y, Inayama Y, Ito T, Kitamura H. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol. 1999 Apr;30:378–83. doi: 10.1016/s0046-8177(99)90111-9. [DOI] [PubMed] [Google Scholar]
- 25.Thomas AA, Stephenson AJ, Campbell SC, Jones JS, Hansel DE. Clinicopathologic features and utility of immunohistochemical markers in signet-ring cell adenocarcinoma of the bladder. Hum Pathol. 2009;40:108–116. doi: 10.1016/j.humpath.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Kim MA, Lee HS, Yang HK, Kim WH. Cytokeratin expression profile in gastric carcinomas. Hum Pathol. 2004;35:576–581. doi: 10.1016/j.humpath.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86:1884–1887. doi: 10.1038/sj.bjc.6600326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SY, Kim HS, Hong EK, Kim WH. Expression of cytokeratin 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol. 2002;33:1078–1085. doi: 10.1053/hupa.2002.129422. [DOI] [PubMed] [Google Scholar]
- 29.Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in parimary carcinomas of the digestive system. Mod Pathol. 2003;16:403–410. doi: 10.1097/01.MP.0000067683.84284.66. [DOI] [PubMed] [Google Scholar]
- 30.Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX-2, cytokeratins 7 and 20 immunoreactivity of rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196–201. doi: 10.1097/PAI.0b013e31819268f2. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein NS, Long A, Kuan SF, Hart J. Colon signet ring cell adenocarcinoma; immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol. 2000;8:183–188. doi: 10.1097/00129039-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884–892. doi: 10.1309/A09E-RYMF-R64N-ERDW. [DOI] [PubMed] [Google Scholar]
- 33.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 34.Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type) Int J Clin Exp Pathol. 2011;4:797–798. [PMC free article] [PubMed] [Google Scholar]
- 35.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens: I. Benign lesions. Int J Clin Exp Pathol. 2012;5:46–51. [PMC free article] [PubMed] [Google Scholar]
- 36.Terada T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens in a single Japanese hospital: II. Malignant lesions. Int J Clin Exp Pathol. 2012;5:52–57. [PMC free article] [PubMed] [Google Scholar]
- 37.Terada T. Malignant tumors of the small intestine: A histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203–209. [PMC free article] [PubMed] [Google Scholar]
- 38.Terada T. Gastrointestinal malignant lymphoma: a pathologic study of 37 cases in a single Japanese institution. Am J Blood Res. 2012;2:194–200. [PMC free article] [PubMed] [Google Scholar]
- 39.Terada T. A clinical-histopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–198. [PMC free article] [PubMed] [Google Scholar]


