Abstract
Large cell neuroendocrine carcinoma (LCNEC) is the rarest type of urinary tract malignancy. Herein, we report a case of LCNEC that arose in the ureter of a 78-year-old Japanese man with a history of ascending colon cancer that had been excised by a right hemicolectomy. Left-sided hydronephrosis associated with the ureteral tumor was discovered during follow-up. A left nephroureterectomy combined with a partial resection of the urinary bladder was performed because atypical cells were detected using voided urine cytology. A histopathological examination revealed that the ureteral tumor contained large atypical epithelial cells of neuroendocrine morphology without a urothelial carcinomatous component. The neoplastic cells were immunohistochemically positive for synaptophysin, chromogranin A, CD56, and cytokeratins, but they were negative for uroplakin III and thyroid transcription factor-1. The Ki-67 labeling index of the neoplastic cells was 50%. Transmission electron microscopy demonstrated the presence of numerous dense granules in the cytoplasm of the neoplastic cells. The ureteral lesion was finally classified as stage III, pT3 cN0 cM0. The patient’s postoperative course was uneventful without chemoradiotherapy, and LCNEC did not recur in the subsequent nine months. This case demonstrates that LCNEC can occur in the ureter, which normally does not contain neuroendocrine cells in the urothelium.
Keywords: Colon cancer, immunohistochemistry, large cell neuroendocrine carcinoma, transmission electron microscopy, ureter, voided urine cytology
Introduction
Primary large cell neuroendocrine carcinoma (LCNEC) of the urinary tract is an extremely rare neoplasm. Given its rarity, the risk factors, biological properties, and effective therapeutic strategies for LCNEC have yet to be determined. LCNECs originate almost exclusively in the urinary bladder [1-17], although they also have been reported, in rare instances, to occur in the kidney [18-20]. To the best of our knowledge, a primary LCNEC of the ureter has not been reported in the literature. Herein, we report the first case of a primary LCNEC of the ureter and focus on the clinicopathological findings.
Materials and methods
Review of clinical data
The patient’s clinical information was obtained from the medical record and was reviewed by the attending urologists.
Pathological examination
The cells collected from voided urine samples provided during the workups were stained with Papanicolaou stain.
The excised left ureteral tumor was fixed in 20% neutral buffered formalin and embedded in paraffin, and tissue sections were subsequently prepared for pathological examination.
The tissue sections were stained with hematoxylin and eosin or were double-stained with Alcian blue and periodic acid-Schiff stain. Immunohistochemistry was performed using an antibody detection kit (Histofine Simple Stain MAX PO (MULTI); Nichirei Bioscience; Tokyo, Japan) according to the manufacturer’s instructions. The following primary antibodies were used in this study: epithelial membrane antigen (E29) (Dako Japan; Tokyo, Japan), pancytokeratin (CK) (CK AE1/AE3; Dako Japan), CK7 (OV-TL 12/30; Dako Japan), CK19 (RCK108; Dako Japan), CK20 (Ks 20.8; Dako Japan), synaptophysin (rabbit polyclonal; Dako Japan), chromogranin A (DAK-A3; Dako Japan), CD56 (1B6; Nichirei Biosciences; Tokyo, Japan), uroplakin III (AU1; Progen Biotechnik; Heidelberg, Germany), thyroid transcription factor-1 (8G7G3/1; Dako Japan), carcinoembryonic antigen (CEA) (rabbit polyclonal; Dako Japan), p53 (DO7; Dako Japan), and Ki-67 (MIB-1; Dako Japan).
We performed transmission electron microscopy on a formalin-fixed, paraffin-embedded specimen of the ureteral tumor. The specimen was deparaffinized using xylene and hydrated using a graded series of ethanol. It was then fixed with 1% osmium tetroxide in 0.1 M phosphate-buffered saline (pH 7.4) for two hours at room temperature and washed with phosphate-buffered saline. Following dehydration using a graded series of ethanol solutions, the tumor sample was infiltrated with propylene oxide and embedded in an epoxy resin (Plain Resin Kit; Nisshin EM; Tokyo, Japan). Ultrathin sections were stained with uranyl acetate for 20 min and then with lead nitrate for 10 min, after which they were examined by transmission electron microscopy (Hitachi H-7500; Hitachi; Tokyo, Japan).
Immunohistochemistry was also performed on samples of the patient’s previous ascending colon cancer to enable a comparison with the current ureteral tumor.
Results
Clinical findings
A 78-year-old Japanese man with a history of ascending colon cancer that had been treated by a right hemicolectomy was hospitalized due to left hydronephrosis. Prior to this hospital admission, he had been followed-up for 14 months without a remarkable episode. His family and occupational histories were unremarkable. He did not drink alcohol but had been a smoker for approximately 50 years (Brinkman index 500). At the time of admission to the hospital, the results of his general physical examination, complete blood count, and biochemistry were within normal ranges. His voided urine was slightly turbid but not bloody. Light microscopic observation of the urine sediment revealed one to four red blood cells per high power field (HPF), more than 50 white blood cells per HPF, one to four squamous cells per HPF, no urothelial or tubular epithelial cells per HPF, and only a few bacteria per HPF. A midstream urine sample contained more than 105 colony-forming units/mL of Streptococcus sp.. Computed tomography of the pelvis revealed a left ureteral tumor (Figure 1A), and retrograde urography demonstrated an obstruction of the lower portion of the left ureter (Figure 1B). A systematic radiographic examination revealed no additional neoplastic lesions other than the left ureteral tumor. A left nephroureterectomy and a partial resection of the urinary bladder were performed, and the ureteral tumor was submitted for pathology. The patient was not given chemoradiotherapy. His postoperative course was uneventful, and the ureteral tumor did not recur in the subsequent nine months.
Figure 1.
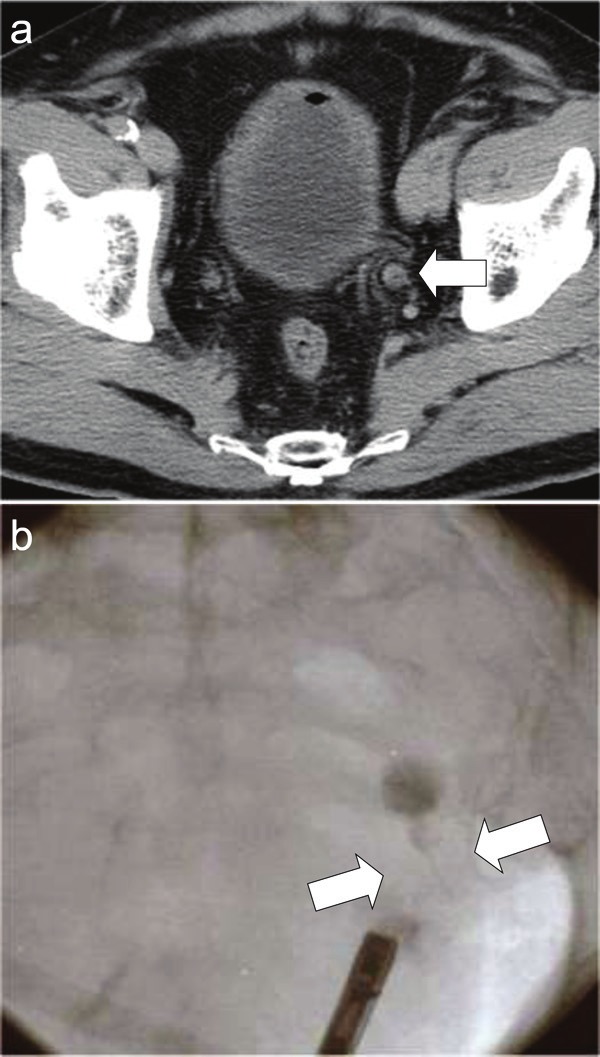
Radiological images of the ureteral tumor. A: Computed tomographic image of the pelvis demonstrating the tumor in the lower portion of the left ureter (arrow). B: Retrograde urographic image revealing an obstruction of the lower portion of the left ureter (arrows).
Pathological findings
The voided urine cytology performed before the nephroureterectomy revealed the presence of small numbers of atypical cells among numerous necrotic cells and inflammatory cells (Figure 2). Although highly degenerated, the atypical cells exhibited hyperchromatic patterns in nuclei of various sizes. A subset of the atypical cells possessed a relatively rich cytoplasm and marginally situated nuclei, whereas other cells presented as naked nuclei. These naked nuclei were observed to be round, oval, or polygonal. The relatively well-preserved neoplastic cells displayed a fine or coarse granular pattern of nuclear chromatin and occasionally had an enlarged nucleolus. In contrast, the poorly preserved neoplastic cells exhibited a dark blue diffuse pattern of chromatin, inconspicuous nucleoli, and a relatively high nucleus to cytoplasm ratio with karyopyknotic changes. Nuclear molding and rosette arrangements were seldom observed, and no mitotic figures were detected among the few atypical cells. These cytological findings were characteristic of the malignant cells of adenocarcinomas or urothelial carcinomas, but they were insufficient to render a diagnosis of neuroendocrine carcinoma.
Figure 2.
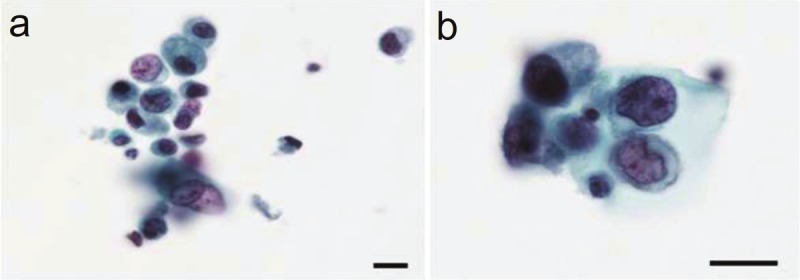
Photomicrographs of cells from the voided urine. A: Degenerating, incohesive atypical cells of various sizes containing hyperchromatic nuclei of various sizes are present (Papanicolaou stain; the scale bar indicates 10 μm). B: Neoplastic cells exhibit a relatively rich cytoplasm and a marginally situated nucleus containing fine or coarse granular hyperchromatic material and an enlarged nucleolus (Papanicolaou stain; the scale bar indicates 10 μm).
A gross examination of the ureteral tumor excised by nephroureterectomy revealed a sessile mass, which measured approximately 23 x 20 x 6 mm, attached to a thickened region of the ureteral wall situated in the lower portion of the left ureter (Figure 3).
Figure 3.
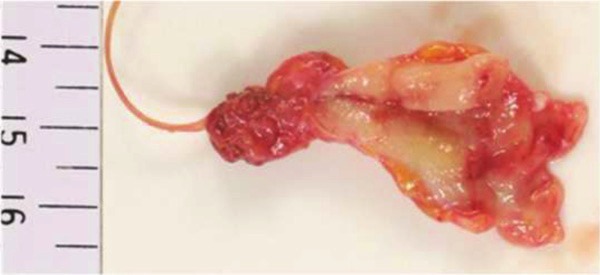
A photograph of the lower portion of the ureter reveals a sessile tumorous lesion (approximately 23 x 20 x 6 mm) projecting from a thickened portion of the ureteral wall.
Histologically, the ureteral tumor contained abundant atypical epithelial cells, some of which had penetrated the muscular layer (Figure 4A). The neoplastic cells occurred in rosette-like, palisading, trabecular, or organoid patterns (Figure 4B). The neoplastic cells, which were characterized by a rich cytoplasm and enlarged nuclei with fine or coarse granular patterns of nuclear chromatin and enlarged nucleoli, were accompanied by mitotic cells and apoptotic bodies (Figure 4C). The average mitotic count was 42 per 10 HPF, and small necrotic foci were observed within the nests of tumor cells. The neoplastic cells were observed to spread in a small region of the ureter in situ, but dysplasia was not evident. Neither urothelial carcinoma nor squamous cell carcinoma was observed in the neoplastic lesion. Alcian blue and periodic acid-Schiff double-staining detected glandular structures that accounted for less than 2% of the neoplasm. Minimal lymphovascular invasion was observed, and the surgical margins were entirely negative.
Figure 4.
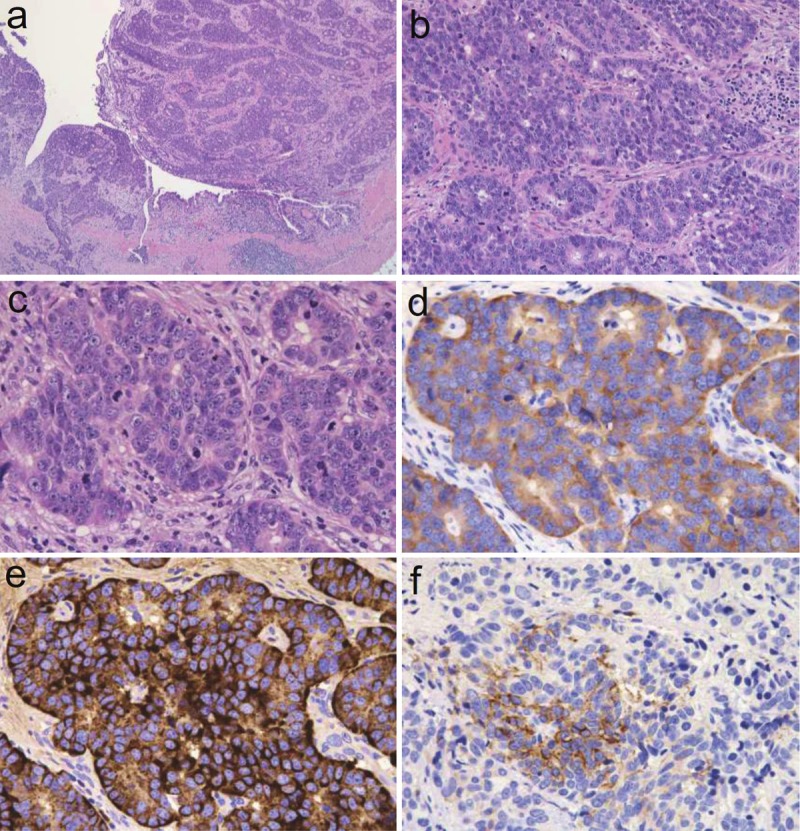
Photomicrographs of the ureteral tumor. A: Low-power view of neoplastic cells in irregularly shaped nests and neoplastic cells that penetrated the muscular layer (hematoxylin and eosin stain, objective 20x). B: Medium-power view of neoplastic cells arranged in rosette-like, palisading, or organoid patterns (hematoxylin and eosin stain, objective 20x). C: High-power view of neoplastic cells harboring a rich cytoplasm with large nuclei containing fine or coarse granular hyperchromatic material and nuclei and moderately enlarged nucleoli. These cells are accompanied by large numbers of mitotic cells and apoptotic bodies (hematoxylin and eosin stain, objective 20x). D: Neoplastic cells positive for the expression of synaptophysin (immunohistochemistry, objective 40x). E: Neoplastic cells positive for the expression of chromogranin A (immunohistochemistry, objective 40x). F: Neoplastic cells positive for the expression of CD56 (immunohistochemistry, objective 40x).
Immunohistochemistry showed that the ureteral neoplastic cells were positive for synaptophysin (Figure 4D), chromogranin A (Figure 4E), CD56 (Figure 4F), epithelial membrane antigen, pan-CK, CK7, CK19, CK20, and CEA, but these cells were negative for uroplakin III and thyroid transcription factor-1. The p53-positive rate was approximately 90%, and Ki-67 labeling index was approximately 50%.
Transmission electron microscopy of the tumor revealed numerous electron-dense granules with diameters of 200 to 400 nm in the cytoplasm of neoplastic cells, which resembled neuroendocrine granules (Figure 5).
Figure 5.
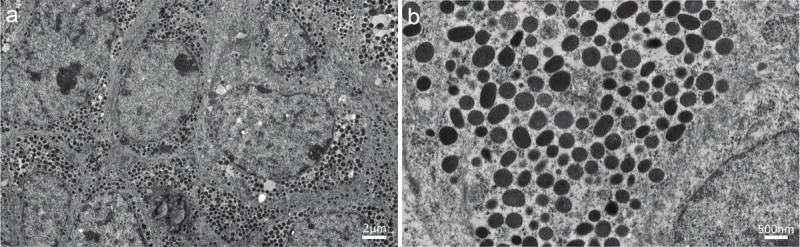
Transmission electron micrographs of the ureteral tumor. A: Neoplastic cells harboring abundant dense granules in a relatively rich cytoplasm (the scale bar indicates 2 μm). B: The cytoplasm of a neoplastic cell containing numerous dense granules ranging in size from 200 to 400 nm in diameter (the scale bar indicates 500 nm).
A pathological review of the patient’s prior ascending colon cancer confirmed the diagnosis of a moderately differentiated tubular adenocarcinoma with negative surgical margins that was classified as Stage IIA, pT3 pN0 cM0, R0 according to the TNM classification system for malignant tumors (7th edition of the International Union against Cancer). Characteristically, true glandular structures with intracytoplasmic mucin were prevalent in the ascending colon cancer, which was negative for synaptophysin, chromogranin A, CD56, uroplakin III, and CK7, as shown in Table 1.
Table 1.
Immunohistochemical results of the ureteral cancer and colon canter
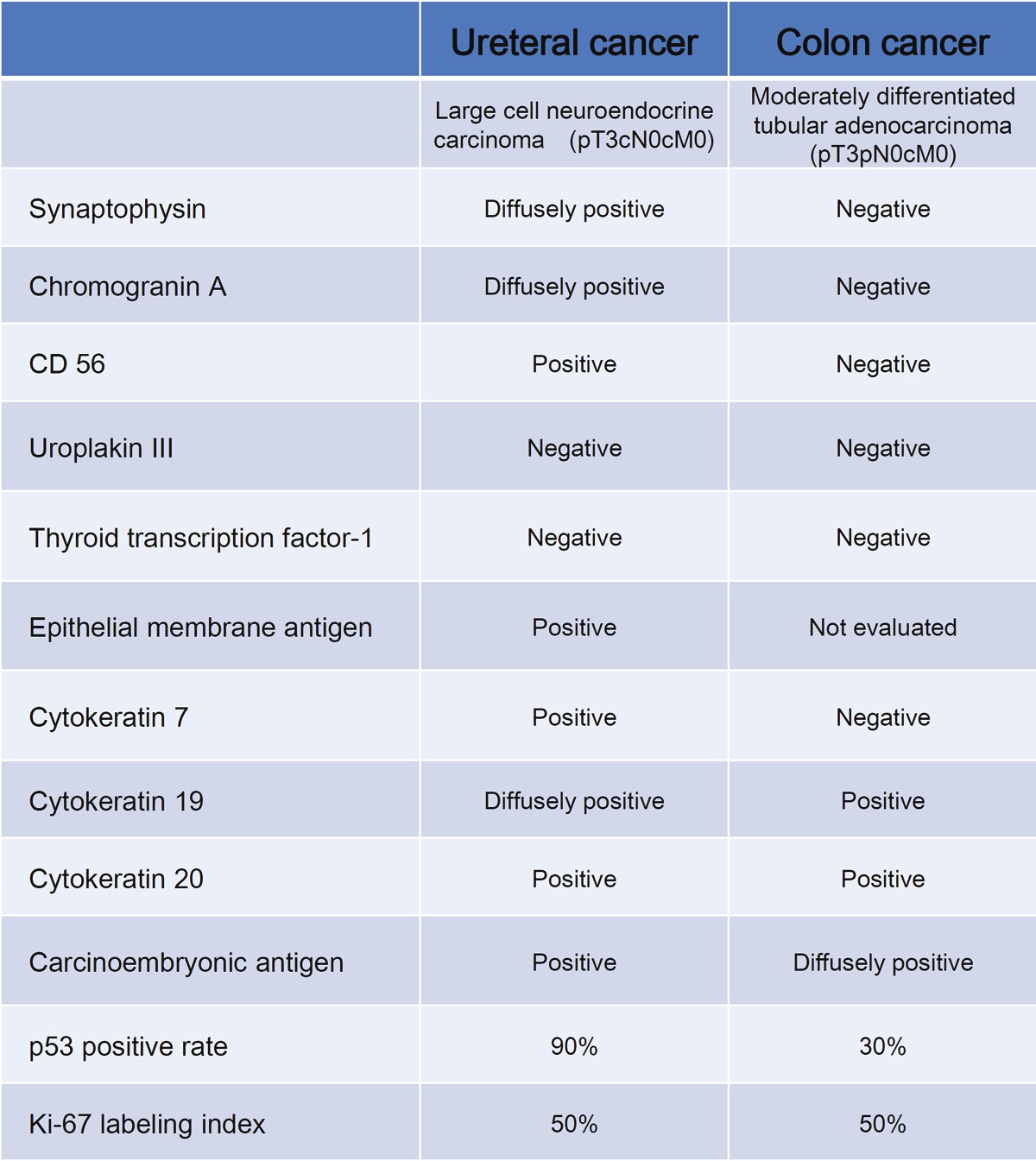
|
Diffusely positive: reactive for more than 80% of neoplastic cells. Negative: not reactive or reactive for less than 1% of neoplastic cells.
The final diagnosis of the ureteral tumor was primary LCNEC of the ureter, which was classified as stage III, pT3 cN0 cM0.
Discussion
LCNEC is a malignant neoplasm consisting of large epithelial cells with neuroendocrine features [21]. Originally established as a distinct clinicopathologic entity of lung tumors [22], primary LCNECs have since been reported in various organs. However, LCNEC is the rarest type of urinary tract malignancy. Indeed, only 25 cases have been reported to date, of which 21 were in the bladder [1-17] and four were in the kidney [18-20]. To the best of our knowledge, there have been no published reports of LCNEC in the ureter.
The clinical presentations of LCNEC in the urinary tract have varied from case to case, but hematuria has been the most commonly reported symptom of LCNEC in the bladder [1-17]. For cases of LCNEC in the kidney, the symptoms have been shown to depend on the extent of spread or dissemination of the neoplasm [18-20]. This type neoplasm tends to show aggressive behavior, and distant metastases have been discovered at the time of diagnosis in the majority of LCNEC cases in the bladder and kidney. Although the data are limited, organ-confined cases are expected to have good prognoses from surgical resection alone or in combination with chemotherapy or radiotherapy.
Diagnoses of primary urinary tract LCNEC are based on the criteria proposed for pulmonary LCNECs, which include the following: (1) a neuroendocrine morphology (organoid nesting, palisading, rosettes, or trabeculae); (2) a high mitotic rate: 11 or more mitotic cells per 2 mm2 (10 HPF), with a median of 70 mitotic cells per 2 mm2 (10 HPF); (3) necrosis (often a large necrotic zone); (4) the cytologic features of a non-small cell carcinoma, including large cell size; a low ratio of nucleus to cytoplasm, and nuclei with vesicular, coarse or fine chromatin, and/or frequent nucleoli. Some of these tumors contain cells with diffuse nuclear chromatin and no nucleolus, but these tumors still qualify as non-small cell carcinomas because of the presence of large cells with abundant cytoplasm; and (5) positive immunohistochemical staining for one or more neuroendocrine markers (other than neuron-specific enolase) and/or the presence of neuroendocrine granules identified by electron microscopy. The present case fulfills all of these diagnostic criteria.
The differential diagnoses for primary LCNECs of the urinary tract included carcinoid tumor, small cell carcinoma, high-grade urothelial carcinoma, and poorly differentiated adenocarcinoma.
Carcinoid tumors generally exhibit small cells, low-grade nuclear atypia, a small number of mitotic cells (generally fewer than two mitotic cells per 10 HPF), and a low Ki-67 labeling index (generally less than 2%), all of which are characteristics that differentiate LCNECs from carcinoid tumors [23].
Small cell carcinoma is characterized by (1) small cell size (generally less than the diameter of three small quiescent lymphocytes), (2) scant cytoplasm, (3) finely granular nuclear chromatin and absent or faintly observed nucleoli, and (4) a high mitotic rate (11 or more mitotic cells per 2 mm2 (10 HPF), with a median of 80 mitotic cells per 2 mm2, and (5) frequent necrosis, often in large zones [21]. Using these criteria, trained pathologists can distinguish small cell carcinoma from LCNEC.
In general, neoplastic cells in high-grade urothelial carcinomas exhibit a high degree of variation in size, shape, and cytoplasmic morphology. The nuclei are usually centrally located and have thick, rough nuclear membranes, easily identifiable nucleoli, and irregularly distributed chromatin. Immunohistochemically, urothelial carcinomas are generally positive for uroplakin but not for neuroendocrine markers. The ureteral tumor in the current case did not exhibit the characteristic features of high-grade urothelial carcinoma.
One might have misinterpreted the present case of ureteral LCNEC as a metastatic or primary adenocarcinoma because of the presence of glandular or pseudoglandular structures. However, the morphological and immunohistochemical findings of the patient’s prior ascending colon cancer were distinct from those of the ureteral tumor, and thus it is highly unlikely that the ureteral LCNEC was derived from a metastasis of the ascending colon cancer. In the ureteral tumor, true glandular structures were observed only in foci, whereas cells positive for neuroendocrine markers were widely observed throughout the neoplastic population, including in the glandular structures. These findings suggest that the neoplastic neuroendocrine cells in the tumor exhibit some glandular alterations, not that the tumor is a poorly differentiated adenocarcinoma.
Because most urinary LCNEC cases present at advanced stages, developing methods for early detection remains a high priority issue. Voided urine cytology can be one of the diagnostic modalities for early detection. However, only one case report has successfully demonstrated the cytological characteristics of a primary LCNEC of the bladder using cells obtained from a voided urine sample [14]. Diagnosing LCNEC by conventional urine cytology alone is difficult because neoplastic cells are highly susceptible to degeneration in urine, as was observed in the present case.
The histogenesis of LCNEC in the urinary tract remains to be elucidated. However, at least two hypotheses have been proposed regarding the origin of LCNEC of the urinary tract. First, it has been suggested that these tumors derive from neuroendocrine cells that are susceptible to oncogenesis and are situated in the normal urothelial mucosa. Alternatively, it has been proposed that LCNECs derive from genetically unstable progenitor cells or pluripotent stem cells [24]. Because neuroendocrine cells are not found in the urothelium of the normal ureter [25], the latter concept appears more feasible for the current case.
In summary, the present case study demonstrates that LCNEC can occur in the ureter, which does not normally contain neuroendocrine cells in the urothelium. Further investigations are required to clarify the biological properties of these tumors and to develop effective therapeutic strategies.
Conflict of interest statement
We declare that we have no conflict of interest regarding this study.
Financial/nonfinancial disclosures
This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 24590242 to H. Oshiro and No. 24700501 to T. Nagai).
References
- 1.Abenoza P, Manivel C, Sibley RK. Adenocarcinoma with neuroendocrine differentiation of the urinary bladder. Clinicopathologic, immunohistochemical, and ultrastructural study. Arch Pathol Lab Med. 1986;110:1062–1066. [PubMed] [Google Scholar]
- 2.Hailemariam S, Gaspert A, Komminoth P, Tamboli P, Amin M. Primary, pure, large-cell neuroendocrine carcinoma of the urinary bladder. Mod Pathol. 1998;11:1016–1020. [PubMed] [Google Scholar]
- 3.Evans AJ, Al-Maghrabi J, Tsihlias J, Lajoie G, Sweet JM, Chapman WB. Primary large cell neuroendocrine carcinoma of the urinary bladder. Arch Pathol Lab Med. 2002;126:1229–1232. doi: 10.5858/2002-126-1229-PLCNCO. [DOI] [PubMed] [Google Scholar]
- 4.Dundr P, Pesl M, Povysil C, Vitkova I, Dvoracek J. Large cell neuroendocrine carcinoma of the urinary bladder with lymphoepithelioma-like features. Pathol Res Pract. 2003;199:559–563. doi: 10.1078/0344-0338-00462. [DOI] [PubMed] [Google Scholar]
- 5.Vincendeau S, de Lajarte-Thirouard AS, Bensalah K, Guille F, Lobel B, Patard JJ. [Neuroendocrine differentiation of bladder tumors] . Prog Urol. 2003;13:375–384. [PubMed] [Google Scholar]
- 6.Li Y, Outman JE, Mathur SC. Carcinosarcoma with a large cell neuroendocrine epithelial component: first report of an unusual biphasic tumour of the urinary bladder. J Clin Pathol. 2004;57:318–320. doi: 10.1136/jcp.2003.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quek ML, Nichols PW, Yamzon J, Daneshmand S, Miranda G, Cai J, Groshen S, Stein JP, Skinner DG. Radical cystectomy for primary neuroendocrine tumors of the bladder: the university of southern california experience. J Urol. 2005;174:93–96. doi: 10.1097/01.ju.0000162085.20043.1f. [DOI] [PubMed] [Google Scholar]
- 8.Lee KH, Ryu SB, Lee MC, Park CS, Juhng SW, Choi C. Primary large cell neuroendocrine carcinoma of the urinary bladder. Pathol Int. 2006;56:688–693. doi: 10.1111/j.1440-1827.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 9.Trimeche M, Mutijima E, Ziadi S, Mestiri S, Sorba NB, Sriha B, Mosbah AT, Korbi S. [Primary large-cell neuro-endocrine carcinoma of the bladder] . Prog Urol. 2006;16:610–612. [PubMed] [Google Scholar]
- 10.Alijo Serrano F, Sanchez-Mora N, Angel Arranz J, Hernandez C, Alvarez-Fernandez E. Large cell and small cell neuroendocrine bladder carcinoma: immunohistochemical and outcome study in a single institution. Am J Clin Pathol. 2007;128:733–739. doi: 10.1309/HTREM6QYQDYGNWYA. [DOI] [PubMed] [Google Scholar]
- 11.Akamatsu S, Kanamaru S, Ishihara M, Sano T, Soeda A, Hashimoto K. Primary large cell neuroendocrine carcinoma of the urinary bladder. Int J Urol. 2008;15:1080–1083. doi: 10.1111/j.1442-2042.2008.02168.x. [DOI] [PubMed] [Google Scholar]
- 12.Bertaccini A, Marchiori D, Cricca A, Garofalo M, Giovannini C, Manferrari F, Gerace TG, Pernetti R, Martorana G. Neuroendocrine carcinoma of the urinary bladder: case report and review of the literature. Anticancer Res. 2008;28:1369–1372. [PubMed] [Google Scholar]
- 13.Lee WJ, Kim CH, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol. 2009;31:166–169. doi: 10.1097/DAD.0b013e31818eba4c. [DOI] [PubMed] [Google Scholar]
- 14.Oshiro H, Gomi K, Nagahama K, Nagashima Y, Kanazawa M, Kato J, Hatano T, Inayama Y. Urinary cytologic features of primary large cell neuroendocrine carcinoma of the urinary bladder: a case report. Acta Cytol. 2010;54:303–310. doi: 10.1159/000325039. [DOI] [PubMed] [Google Scholar]
- 15.Tsugu A, Yoshiyama M, Matsumae M. Brain metastasis from large cell neuroendocrine carcinoma of the urinary bladder. Surg Neurol Int. 2011;2:84. doi: 10.4103/2152-7806.82250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin IJ, Vilar DG, Aguado JM, Perello CG, Aliaga MR, Argente VG, Ferreres LA, Gomez JG. Large cell neuroendocrine carcinoma of the urinary bladder. Bibliographic review. Arch Esp Urol. 2011;64:105–113. [PubMed] [Google Scholar]
- 17.Engles CD, Slobodov G, Buethe DD, Lightfoot S, Culkin DJ. Primary mixed neuroendocrine carcinoma of the bladder with large cell component: a case report and review of the literature. Int Urol Nephrol. 2012;44:1021–1025. doi: 10.1007/s11255-012-0148-6. [DOI] [PubMed] [Google Scholar]
- 18.Moukassa D, Leroy X, Bouchind’homme B, Saint F, Lemaitre L, Gosselin B. [Primary large cell neuroendocrine carcinoma of the kidney: morphologic and immunohistochemical features of two cases] . Ann Pathol. 2000;20:357–360. [PubMed] [Google Scholar]
- 19.Lane BR, Chery F, Jour G, Sercia L, Magi-Galluzzi C, Novick AC, Zhou M. Renal neuroendocrine tumours: a clinicopathological study. BJU Int. 2007;100:1030–1035. doi: 10.1111/j.1464-410X.2007.07116.x. [DOI] [PubMed] [Google Scholar]
- 20.Ratnagiri R, Singh SS, Majhi U Kathiresan, Sateeshan. Large-cell neuroendocrine carcinoma of the kidney: Clinicopathologic features. Indian J Urol. 2009;25:274–275. doi: 10.4103/0970-1591.52928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis W. The concept of pulmonary neuroendocrine tumours. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 19–20. [Google Scholar]
- 22.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB Jr, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Rindi GAR, Bosman FT, Capella C, Klimstra DS, Kloppel G, Komminoth P, Solcia E. Nomenculature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization Calssification of Tumours of the Digestive System. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 24.Oshiro H, Matsuo K, Mawatari H, Inayama Y, Yamanaka S, Nagahama K, Endo I, Shimada H, Nakajima A, Kubota K. Mucin-producing gallbladder adenocarcinoma with focal small cell and large cell neuroendocrine differentiation associated with pancreaticobiliary maljunction. Pathol Int. 2008;58:780–786. doi: 10.1111/j.1440-1827.2008.02311.x. [DOI] [PubMed] [Google Scholar]
- 25.Fetissof F, Dubois MP, Lanson Y, Jobard P. Endocrine cells in renal pelvis and ureter, an immunohistochemical analysis. J Urol. 1986;135:420–421. doi: 10.1016/s0022-5347(17)45656-4. [DOI] [PubMed] [Google Scholar]


