Abstract
In the current WHO blue book, combined hepatocellular-cholangiocarcinoma (C-HCC-CC) was classified into two types; classical type and type with stem cell features. The latter is extremely rare, and is subcategorized into the following three subtypes; typical subtype, intermediate cell subtype, and cholangiocellular subtype. Recently, intrahepatic cholangiocarcinoma (ICC) with features of ductal plate malformations (DPM) have been reported, and the ICC with DPM was proposed as a subtype of ICC. The author herein reports a case of C-HCC-CC with stem cell features. Characteristically, the CC element showed features of DPM. A 51-year-old man of HBV carrier was found to have high AFP. A laboratory test showed an elevated AFP (395 ng/ml, normal 9-10) and hepatitis B virus-related antigens and antibodies. Liver and ductal enzymes and PIVKAII were within normal ranges. Imaging modalities including CT identified a small liver tumor. Hepatocellular carcinoma (HCC) was suspected, and the resection of the hepatic tumor was performed. Grossly, the liver tumor is well-defined white solid tumor measuring 22x16x23 mm. Microscopically, the tumor was a C-HCC-CC, and was composed of following three elements: well differentiated HCC, well differentiated cholangiocarcinoma (CC), and intermediate tumor element. Characteristically, the CC cells formed tortuous markedly irregular tubules with intraluminal cell projections, bridge formations, intraluminal tumor biliary cells; such features very resembled the ductal plate (DP) and DPM. Immunohistochemically, the cells of CC element were positive for stem cell antigens (KIT (CD117), CD56, EMA, CD34), HepPar1, EpCAM, cytokeratin (CK) CAM5.2, AE1/3, CK34BE12 (focal), CK7, CK8, CK18, CK19, CA19-9, p53, MUC1, MUC2, MUC5AC, MUC6, and Ki-67 (labeling=25%). They were negative for CEA, CK5/6, CK20, NSE, chromogranin, synaptophysin, and p63. No mucins were found by histochemically. The background liver showed chronic hepatitis B (a1, f3). Very interestingly, many DPMs were scattered in the non-tumorous parenchyma. This type of C-HCC-CC with DPM features has not been reported. The author herein proposes that this tumor should be included or added in the C-HCC-CC subtype as C-HCC-CC with stem cell features, DMP subtype.
Keywords: Combined hepatocellular cholangiocarcinoma, liver stem cells, ductal plate, ductal plate malformation, histopathology, immunohistochemistry
Introduction
According to Nakanuma [1], intrahepatic cholangiocarcinoma (ICC) is an intrahepatic malignancy with biliary epithelial differentiation. ICC can arise in any portion of the intrahepatic biliary tree, from the segmental and area ducts and their major branches to the smallest bile ducts and ductules. He also classified ICC into hilar and peripheral ones [1]. Most ICCs are adenocarcinomas with variable differentiation and fibroplasia. ICC arising in non-biliary cirrhosis frequently presents with bile ductile differentiation, possibly arising from the hepatic progenitor or stem cells [1]. He proposed several rare variants of ICC such as squamous cell carcinoma, adenosquamous carcinoma, mucinous carcinoma, signet ring cell carcinoma, clear cell carcinoma, mucoepidermoid carcinoma, lymphoepithelioma-like carcinoma, sarcomatous ICC, intraductal papillary carcinoma, and precursor lesions of BillN [1,2]. Very recently, Nakanuma [2,3] proposed a new subtype of ICC; i.e, ICC with predominant “ductal plate malformation (DPM)” pattern. This new subtype of ICC is characterized by well differentiated adenocarcinoma whose carcinoma cells very resemble DPM. The ductal plate (DP) implies progenitor biliary cells in the fetus livers characterized by a double-layered cylinder of precursor biliary cells with capacities of apoptosis and cell proliferation, and differentiation into fetal biliary cells, adult biliary cells, stem cells of fetus and postnatal livers, hepatoblasts, hepatocytes, pancreatic acinar cells, biliary cells with pancreatic digestive enzymes, and peribiliary glands [4-42]. DPM is the persistence of fetal DP in the postnatal liver, and DPM is characterized by markedly irregular tortuous tubules with bridge formations, biliary cell projections into the lumen, and intraluminal tumor cells somewhat reminiscent of fetal DP [4-42]. DMP is seen mainly in congenital hepatic fibrosis, polycystic liver and kidney diseases, congenital biliary atresia, von-Meyenburg complex, Caroli’s disease [43-49]. This condition is also called DPM disease [7,46] or hepatobiliary fibropolycystic disease [47,49]. DPM is also seen in ductular reactions in various hepatobiliary diseases [44,50-52]. DP is entirely different from DPM.
Although the author is only a diagnostic solo-pathologist in a low-grade hospital, the author examined many cases of ICC in the author’s young era [53-56]. The author had noticed the presence of ICCs whose carcinoma cells resembled DP or DPM about 20 years ago, but the author did not report the findings because the author thought that such ICCs were not a single clinicopathological entity. The author also had noticed the presence of combined hepatocellular carcinomas (HCC) and ICC in which the ICC resembled DP or DPM. The author also had not reported them because of the same reasons.
Herein reported is a case of combined HCC and ICC, in which the ICC cells resembled DP or DPM. The author herein proposes that this tumor should be included or added in the combined HCC and ICC subtype as combined hepatocellular-cholangiocarcinoma with stem cell features, DMP subtype.
Case report
A 51-year-old man of HBV carrier was found to have high serum AFP in the periodical follow-up. A laboratory test showed elevated AFP (395 ng/ml, normal 9-10), mild anemia (385x104 /μl, normal 450-550 x104), and high glucose (228 mg/dl, normal 70-110). Liver and ductal enzymes were within normal ranges. HBV-related antigens and antibodies were positive. PIVKAII was within normal limits (20 mAU, normal 0-30). Imaging modalities including CT and MRI identified a small tumor (2cm in diameter) of the S6 of the liver right lobe (Figure 1). HCC was suspected, and the resection of the tumor was performed.
Figure 1.
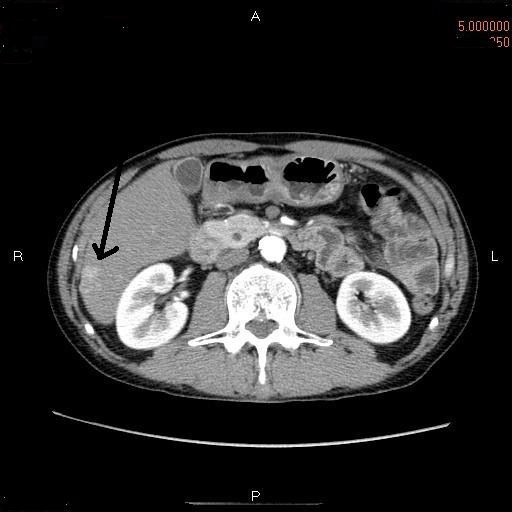
CT of the hepatic tumor. A small tumor (arrow) of 3 am in diameter is seen in the S6 of the liver right lobe.
Grossly, the tumor is well-defined white solid tumor measuring 22x16x23 mm (Figure 2). Microscopically, the tumor was a combined HCC and ICC (Figure 3A-G). The tumor cells were composed of the following three elements: well differentiated HCC (Figure 3B), well differentiated ICC (Figure 3C), and intermediate tumor element (Figure 3D). The HCC element was typical well-differentiated HCC of Edmondson’s grade II with compact and focally trabecular histologies without fibrous stroma (Figure 3B). The IHC element was well differentiated adenocarcinoma with abundant fibrous stroma (Figure 3C). Characteristically, all the ICC cells formed tortuous markedly irregular tubules with intraluminal projections of tumor cells, bridge formation of tumor cells, and intraluminal tumor cells islands, which were features of DP and DPM (Figure 3D-G). The intermediate tumor element had intermediate histological features of both HCC and ICC, and appeared a cancer stem cell population (Figure 3D). The intermediate tumor element showed frequent HCC differentiation and ICC differentiation (Figure 3D), thus compatible with cancer stem cells. There were gradual transitions between HCC element and intermediate tumor element, and between intermediate tumor element and ICC element (Figure 3A and 3D).
Figure 2.

Gross features of the resected hepatic tumor. The tumor is well-defined, white, firm, and solid tumor measuring 22x16x23 mm.
Figure 3.
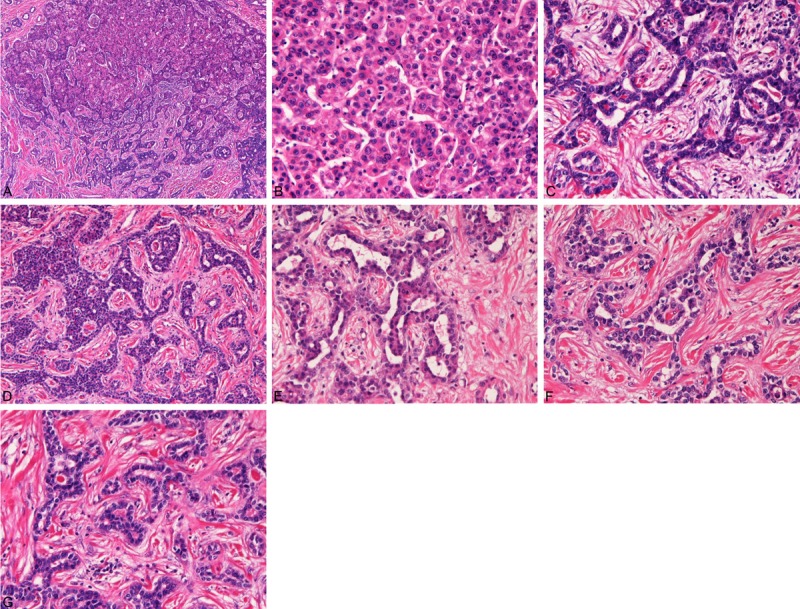
Histological findings of the combined hepatocellular-cholangiocellular carcinoma with stem cell features, DPM subtype. A: Very low power view of the tumor. The tumor is composed of hepatocellular carcinoma (HCC, upper), intermediate tumor cells (middle), and cholangiocarcinoma (CC) element (lower). There are gradual transitions between HCC element and intermediate tumor element and also between ICC element and intermediate tumor element. HE,x 40. B: The HCC element. The carcinoma element is apparent well differentiated HCC of Edmondson’s grade II. The tumor cells show thin trabecular pattern. HE, x200. C: High power view of the CC element of the tumor. The carcinoma element is apparent well differentiated CC with features of ductal plate malformations (DPM). The DPM features include toutous irregular tubules lined by biliary epithelial cells. Cell projections into the lumens, bridge formations, and intraluminal tumor cells are seen. HE, x200. D: High power view. The intermediate tumor cells show solid nests of cells of intermediate characters of HCC cells and CC cells. Hepatocellular differentiation (upper) and cholangiocellular differentiation (lower) are seen. This type of intermediate cells seems cancer tumor cells. HE, x100. E, F, G: High power view of the CC element of the tumor. The carcinoma element is apparent well differentiated CC with DPM. The DPM features include toutous irregular tubules lined by biliary epithelial cells. Cell projections into the lumens, bridge formations, and intraluminal tumor cells are seen. E, F, G: HE, x200.
A mucin study was performed using mucicarmine stain, PAS stain, diastase-PAS (d-PAS) stain, Alcian blue (AB) stains at pH 2.5 and pH1.0, and combined d-PAS and AB technique. An immunohistochemical study was performed with the use of Dako Envision method (Dako Corp, Glostrup, Denmark), as previously described [57-60]. The author selected antibodies to liver stem cells/progenitor cells (KIT (CD117), CD34, CD56, cytokeratin (CK)14, antibodies showing hepatocellular lineage (HepPar1, AFP, CK8, CK CAM5.2, CK AE1/3, and CK18), antibodies showing cholangiocyte lineage (CK AE1/3, CK CAM5.2, CK7, CK8, CK18, CK19, CEA, CA19-9, MUC1, MUC2, MUC5AC, MUC6, and EpCAM). The author also used antibodies of neuroendocrine antigens (chromogranin, synaptophysin, NSE), CK34BE12, CK5/6, CK20, EMA, p63, p53 and Ki-67 antigen to evaluate their expression status.
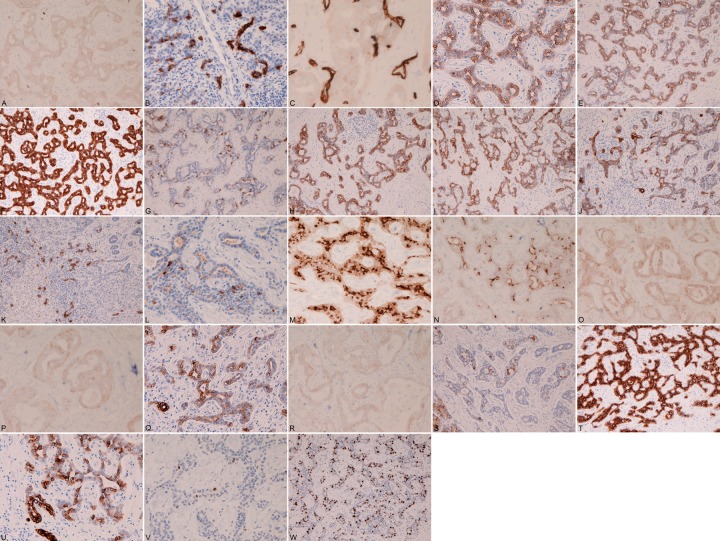
Immunohistochemically, the ICC cells were positive for all four liver stem cell markers, i.e, KIT (Figure 4A), CD56 (Figure 4B), CD34 (Figure 4C), and CK14 (Figure 4D). They were also positive for antigens showing cholangiocyte lineage; CK AE1/3 (Figure 4E), CK CAM5.2 (Figure 4F), CK7 (Figure 4G), CK8 (Figure 4H), CK18 (Figure 4I), CK19 (Figure 4J), CEA (Figure 4K), CA19-9 (Figure 4L), MUC1 (Figure 4M), MUC2 (Figure 4N), MUC5AC (Figure 4O), MUC6 (Figure 4P), and EpCAM (Figure 4Q). The cells of ICC also showed positive some antigens of hepatocellular lineage; HepPar1 (Figure 4R), AFP (Figure 4S) CK8 (Figure 4H), CK18 (Figure 4I) and CK CAM5.2 (Figure 4F). They were positive for EMA (Figure 4T), CK34BE12 (Figure 4U), p53 (focal, Figure 4V) and Ki67-antigen (Figure 4W) (labeling index= 40%). The cells of ICC element were negative for CK5/6, CK20, p63, chromogranin, synaptophysin and NSE. Mucins were negative histochemically.
Figure 4.
Immunohistochemical findings. The cholangiocarcinoma (CC) element of the present combined hepatocellular-cholangiocellular carcinoma are positive for four liver stem cell markers; KIT (A), CD56 (B), CD34 (C), and CK14 (D). The CC cell are were positive for antigens showing cholangiocyte lineage; CK AE1/3 (E), CK CAM5.2 (F), CK7 (G), CK8 (H), CK18 (I), CK19 (J), CEA (K), CA19-9 (L), MUC1 (M), MUC2 (N), MUC5AC (O), MUC6 (P), and EpCAM (Q). The cells of CC also show positive some antigens of hepatocellular lineage; HepPar1 (R), AFP (S) CK8 (H), CK18 (I) and CK CAM5.2 (F). The CC cells are positive for EMA (T), CK34BE12 (U), p53 (focal, V) and Ki67-antigen (W) (labeling index= 40%).
The cells of HCC element were positive for three liver stem cell markers, i.e, KIT, CD34, and CK14, but were negative for CD56. They were positive for antigens of hepatocellular lineage; HepPar1, AFP, CK8, CK18, and CK CAM5.2. They were also positive for same antigens showing cholangiocyte lineage; CK7, MUC1, MUC2, MUC5AC, MUC6, and EpCAM. They were also positive for p53 and Ki-67 (labeling index=37%). They were negative for CK AE1/3, CK34BE12, CK5/6, CK19, CK20, EMA, CEA, CA19-9, p63, chromogranin, synaptophysin and NSE. Mucins were negative histochemically.
The cells of intermediate tumor element showed intermediate immunophenotype. They were positive for all the four liver stem cell markers, i.e, KIT, CD56, CD34, and CK14. They were positive for antigens of hepatocellular lineage; HepPar1, AFP, CK8, CK18, and CK CAM5.2. They were also positive for same antigens showing cholangiocyte lineage; CK AE1/3, CK7, CK19, MUC1, MUC2, MUC5AC, MUC6, and EpCAM. They were also positive for EMA, p53 and Ki-67 (labeling index=32%). They were negative for CK34BE12, CK5/6, CK20, CEA, CA19-9, p63, chromogranin, synaptophysin and NSE. Mucins were negative histochemically.
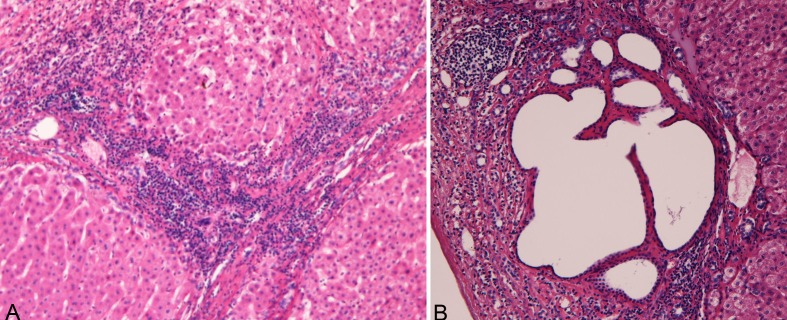
The background liver showed chronic hepatitis B (a1, f3) (Figure 5A). Very interestingly, many DPMs were scattered in the non-tumorous parenchyma (Figure 5B).
Figure 5.
Histologies of the non-tumorous parenchyma. A: The non-tumorous parenchyma shows chronic hepatitis B (a1, f3). B: Very interestingly, many DPMs are scattered in the non-tumorous parenchyma.
Discussion
Combined HCC and ICC is a relatively rare condition. According to Theise [61], most cases of combined HCC and ICC (C-HCC-CC) are derived from liver progenitor cells or stem cells. According to Theise [61], C-HCC-CC is defined as a tumor containing unequivocal, intimately mixed elements of both HCC and ICC. The C-HCC-CC should be distinguished from separate HCC and ICC arising in the same liver [61]. Such a tumor may be separated or intermixed (“collision tumor”). The present tumor is a single tumor composed of mixing HCC, ICC and intermediate tumor elements, thus fulfilling the criteria of C-HCC-CC of Theise [61].
Theise [61] classified C-HCC-CC into that of classical type and that of subtypes with stem-cell features. The latter was further categorized into the following three subtypes: CC-HCC-CC with stem cell features, typical subtype, CC-HCC-CC with stem cell features, intermediate subtype, and CC-HCC-CC with stem cell features, cholangiocellular subtype [61].
Most of the C-HCC-CC is C-HCC-CC of classical type [61]. This classical type of C-HCC-CC is characterized by the presence of ordinary HCC and ICC cells. The HCC element was immunohistochemically positive for HepPar1, CD10, rabbit polyclonal CEA, and AFP [61]. The ICC element is typical adenocarcinoma, shows fibrosis, and is positive for mucins [61]. It is immunohistochemically positive for biliary type CK (CK7 and CK19). In many cases of this classical type of C-HCC-CC there are foci of intermediate morphology at the interface of the HCC and ICC element. Immunohistochemistry often provides confirmatory evidence of mixed phenotypes in these regions. Cells having phenotypical and immunophenotypical features of stem cells/progenitor cells may be present [61]. However, if these predominate, C-HCC-CC of with stem cell feature type should be considered. The present case is not C-HCC-CC classical type, because the ICC element showed features of stem-cell type, as described later, and also because the present tumor showed stem cell features such as positive KIT, CK14, CD34 and CD56 (liver stem cell markers).
The C-HCC-CC of stem-cell type is very rare. A PubMed search could not detect such a case in the English literature. The present case belongs to this type of C-HCC-CC, because the tumor cells showed stem cells phenotypes and immunophenotypes positive KIT, CD34, CD56 and CK14. According to Theise [61], the current case may belong to C-HCC-CC with stem-cell features, cholangiocellular subtype. However, the ICC element of the present case resembles DP or DPM, and showed stem-cell antigens. The ICC element of the present case is very similar to ICC of DPM type of Nakanuma [2,3]. In the present case, the tumor cells showed liver stem cell phenotypes o Thus, the present tumor belong to Thus, the author believes that the biliary elements of the present C-HCC-CC show DMP. The author wants to emphasize that the present case is a new subtype of C-HCC-CC with stem cell features, DPM subtype, and the author want that the WHO adds this C-HCC-CC with stem cell features, DPM subtype in the list of variants of C-HCC-CC.
The C-HCC-CCC with stem-cell features, cholangiocellular subtype of Theise [61], the pictures of which is available in the WHO blue book [61], is somewhat similar to the bile duct structures [DPM] of the present case and to the DPM type of ICC of Nakanuma [2,3]. Therefore, there seems to be a strong association of these three entities proposed by different researchers. In the literature, ductular reactions of various chronic liver disease and focal nodular hyperplasia (FNH) have been recognized to have DPM features by Desmet [50-52] and by the author [44]. The first description of DPM was made by Jorgenson in 1977 [62]. The definition of DP is clear [10-39], but the definition of DPM is unclear. In the pioneer works of Jorgenson and Desmet of DPM [7-10,50-52,62], they showed that the DPM denotes simple tortuous tubules reminiscent of DP of fetal life. DPM is generally considered to be an aberrant biliary structures in the postpartum livers resembling fetal DP [7-10,43-48,50-52,62], and is considered to result from lack of DP remodeling during human intrahepatic bile duct development [7-39,43-48,50-52,62,63]. Recently, the pathogenesis of DMP has been studied, and it was found that the development of DPM is involved the molecular deficiencies or emergency of hepatocyte nuclear factor-6 (HNF6), HNF1β, cystin-1, and HNF1B/TCF2 mutation, and a new classification of DPM was proposed [64,65]. Immunohistochemically, DPM expresses CK7, CK8, CK19, but not CD34 [66]. The DPM of Nakanuma [2,3,48] seems to be an extreme examples of DPM showing very marked biliary cells abnormality. The author thinks, like Desmet and Jorgenson, that the ICC component of the present tumor is a DPM rather than cholangiocellular phenotype. Therefore, the author reported this case as C-HCC-CC with stem cell features, DMP subtype rather than C-HCC-CC with stem cell features, cholangiocellular subtype. The author also want to stress that there is a strong similarity between the C-HCC-CC with stem cell features cholangiocellular subtype of Theise [61] and ICC of DPM subtype of Nakanuma [2,3]. The tumor is equivalent for hepatic “stem cell malignancy” reported by Theise et al [67]. The author think that the C-HCC-CC with stem cell features, cholangiocellular subtype of Theise [61] may contain cases of C-HCC-CC with stem cell features, DPM subtype, because the picture of C-HCC-CC with stem cell features, cholangiocellular subtype of Theise in the WHO blue book somewhat resembles the present case of C-HCC-CC with stem cell features, DPM subtype. Much more studies of C-HCC-CC with stem cell features should be required. These confusions may result from the unclear definition of DPM. The DPM of Nakanuma showed marked irregularities of the DPM [2,3,48], while The DPM of Desmet [7-9,50-52], Jorgenson [62], Summerfield [49] and the author [43-47] includes biliary cell abnormalities with mild irregularities including bile ductular reactions and von-Meyenburg complex. Thus, the definition of DPM is different among researchers. The strict definition of DPM is need.
A significant percentage of C-HCC-CC, in particular that with stem-cell feature type, is thought to arise from liver stem cells or progenitor cells [61,68-70]. This is also thought to be true in cases of other liver carcinomas such as HCC and ICC [61]. However, HCC usually arises from hepatocytes or dysplastic nodules [71-73], and cholangiocarcinoma from cholangiocytes [53-56]. Liver stem cells/progenitor cells are located in the ductules and Herring ducts next to liver parenchyma [61,74,75]. The antigens or markers of these liver stem cells are KIT (CD117), CD34, CD56, OV6, Thy-1 (CD90), CK14, CD133, ALDH, and M2PK [14-16,69,70,74,75]. In the current study, the author used KIT, CK14, CD34, and CD56 as markers of liver stem cells/liver progenitor cells. CK7, CK8, CK18, CK19, EMA, CEA, CA19-9, EpCAM, mucins, MUC apomucins are markers of cholangiocytes. The expression of CD8, CK18, AFP, and HepPar1 shows the hepatocellular lineage.
The present study is the first case using very wide ranges of antigens in the human combined HCC and ICC. It is very interesting in the current case that the all the HCC, ICC and intermediate tumor elements in a single C-HCC-CC with stem cell features showed immunoreactive liver stem cell markers (KIT, CD34 and CK14) strongly suggesting that the current tumor is entirely derived from liver stem cells/progenitor cells. The other one stem cell marker CD56 was positive in ICC and intermediate tumor elements but negative in HCC element. Taken together, it is suggested that the whole tumor of the present case are derived from liver stem cells. Thus, the present tumor is liver stem cell malignancy.
The MUC apomucins’ expression in the combined HCC and ICC has not been studied. The present study for the first time demonstrated the distribution of MUC1, MUC2, MUC5AC, and MUC6. Of very interest, these four MUC apomucins were expressed in the HCC element and intermediate tumor element in addition to ICC element. These are novel findings. These data suggest the homogenous natures of the current tumor, and imply that HCC and intermediate tumor elements had features of cholangiocytes in current tumor.
In the current case, CK expression was examined in a wide range of CKs. The expression of biliary type CK (CK7, 8, 18 and 19) and hepatocellular type of CK (CK8, 18) was the same of previous studies [10,19,28]. The new findings of the present study were that CK34BE12, a high molecular weight CK, was expressed in the ICC element of the current case. The negative expression of CD5/6, a high molecular weight CK in combined HCC and ICC is also a new finding. The negative CK20 in ICC and HCC is well recognized, and this negative CK20 is useful for differentiation from metastatic carcinomas of the liver.
The present case is the first that examine CEA and CA19-9 immunoreactivities in C-HCC-CC with stem cell features. CEA and CA19-9 expression was seen in the ICC element but not seen in HCC element and intermediate tumor element. The data suggest that CEA and CA19-9 may be present only in ICC element of this C-HCC-CC with stem cell features, DPM subtype.
The expression of EMA was different in the current tumor. EMA was expressed in the ICC element and intermediate tumor element, but not in HCC element. The lack of EMA immunoreactivity in hepatocytes and HCC is well known. The findings of the present study shows the expression of EMA in C-HCC-CC with stem cell features, DPM subtype is not different from other liver malignancies. The presence of EMA in the intermediate tumor element may suggest that EMA expression disappears from HCC during ICC transition to HCC process.
It is very interesting that AFP and HepPar1, both of which are antigens of hepatocytes and neoplastic hepatocytes, were expressed in ICC cells in the current case. This fact suggest that the tumor is derived from liver stem cells, and that ICC element of C-HCC-CC with stem cell features express hepatocellular lineage, i.e, AFP and HepPar1.
Chromogranin, synaptophysin, and NSE have not been investigated in C-HCC-CC with stem cell features. Expression of these molecules were not seen in the present case, suggesting that neuroendocrine characteristics or neuroendocrine differentiation were absent in C-HCC-CC with stem cell features. CD56 (NCAM) which is an antigen for both liver stem cells and neuroendocrine cells was expressed in the ICC and intermediate tumor elements but not in HCC element. In this case, the positive CD56 expression may not reflect the neuroendocrine features but demonstrate the liver stem cell nature of the tumor cells.
P53 and Ki-67 expression has not been examined in C-HCC-CC with stem cell features. In the present study, tumor cells of all the three elements were positive for p53 and Ki-67. The labeling index of Ki-67 was high. These findings suggest positive p53 gene mutations and high cell proliferative fraction in C-HCC-CC with stem cell features.
Morphologically, there were gradual transition between HCC element and intermediate tumor element and also between ICC element and intermediate tumor element in the current C-HCC-CC. Positive liver stem cell antigens strongly suggest that the present tumor is derived from liver stem cells/progenitor cells. It is very interesting; if one considers that the intermediate tumor element of the current case is composed of tumor cell stem cell. If so, it is conceivable that the cancer stem cells (intermediate tumor cells) may give rise to the HCC cells and ICC cells in the current C-HCC-CC. This concept seems to be supported by the present board immunohistochemical data. In the current study, the intermediate tumor cell element frequently showed HCC differentiation and ICC differentiation, strongly suggesting that the intermediate tumor cells element is a cancer stem cell population. Further studies of cancer stem cells in C-HCC-CC remain to be elucidated.
Interestingly, the present C-HCC-CC with stem cell features, DPM subtype was seen in chronic hepatitis B. Hepatitis B virus (HBV) is known to give rise to ICC in addition to HCC [1,61,76], though the reason is unclear with regard to ICC. However, the author previously suggested the role of hepatitis virus in the development of ICC [77], and showed that chronic hepatitis and cirrhosis may facilitate the development of ICC [77]. Therefore, the present tumor my be associated with chronic hepatitis B and HBV infection.
Of particular interest in the current study is that many benign DPM were seen in the non-tumorous parenchyma. Some DPMs, in particular von-Meyenburg complex, are known to show malignant transformation or associated with ICC [78] and HCC [79]. Therefore, there is a possibility that the present combined HCC and ICC was derived from the DPM.
In summary, the author reported a case of apparent C-HCC-CC with stem cell features. The ICC element showed apparent features of DPM. The author wants that this tumor should be included or added in the C-HCC-CC subtype as C-HCC-CC with stem cell features, DMP subtype. These statements were very strengthened by the broad immunohistochemical study done in the current tumor.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Calssification of tumours of the digestive system. Lyon: IARC; 2010. pp. 217–224. [Google Scholar]
- 2.Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419–427. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Arrizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012;36:1629–1635. doi: 10.1097/PAS.0b013e31826e0249. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Kitamura Y, Ashida K, Matsunaga Y, Ohta T. Expression of pancreatic digestive enzymes in normal and pathologic epithelial cells of the human gastrointestinal system. Virchows Arch. 1997;431:195–203. doi: 10.1007/s004280050088. [DOI] [PubMed] [Google Scholar]
- 5.Terada T, Nakanuma Y. Immunohistochemical demonstration of pancreatic α-amylase and trypsin in intrahepatic bile ducts and peribiliary glands. Hepatology. 1991;14:1129–1135. [PubMed] [Google Scholar]
- 6.Terada T, Nakanuma Y. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum Pathol. 1992;23:483–490. doi: 10.1016/0046-8177(92)90124-l. [DOI] [PubMed] [Google Scholar]
- 7.Desmet VJ. Congenital disease of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 8.Desmet VJ, Van Eyken P, Sciot R. Cytokeratins for probing cell lineage relationships in developing liver. Hepatology. 1990;12:1249–1251. doi: 10.1002/hep.1840120530. [DOI] [PubMed] [Google Scholar]
- 9.Desmet VJ. Intrahepatic bile duct under the lens. J Hepatol. 1985;1:545–559. doi: 10.1016/s0168-8278(85)80752-2. [DOI] [PubMed] [Google Scholar]
- 10.Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin immunohistochemical study. Hepatology. 1988;8:1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]
- 11.Shah KD, Gerber MA. Development of intrahepatic bile ducts in humans: immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med. 1989;113:1135–138. [PubMed] [Google Scholar]
- 12.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476–481. doi: 10.1002/hep.510230312. [DOI] [PubMed] [Google Scholar]
- 13.Shar KD, Gerber MA. Development of intrahepatic bile ducts in humans: possible role of laminin. Arch Pathol Lab Med. 1990;114:597–600. [PubMed] [Google Scholar]
- 14.Carpentier R, Suner RS, Van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Arhouri Y, Cord S, Jacquemin P, Lemaigre F, Leclercq IA. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki M, Nakanuma Y, Terada T, Kim YS. Biliary epithelial expression of MUC1, MUC2, MUC3, and MUC5/6 apomucins during human intrahepatic bile duct development and maturation: An immunohistochemical study. Am J Pathol. 1995;147:574–579. [PMC free article] [PubMed] [Google Scholar]
- 18.Terada T. Differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling ductal plate in human fetuses. Hepatology. 2012;56:2004–2005. doi: 10.1002/hep.25750. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Nakanuma Y. Development of human intrahepatic peribiliary glands: Histological, keratin immunohistochemical and mucus histochemical analyses. Lab Invest. 1993;68:261–269. [PubMed] [Google Scholar]
- 20.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: A lectin-histochemical and immunohistochemical study. Hepatology. 1993;18:529–536. [PubMed] [Google Scholar]
- 21.Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994;25:143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology. 1994;20:388–397. [PubMed] [Google Scholar]
- 23.Terada T, Ohta T, Nakanuma Y. Expression of transforming growth factor-α and its receptor during human liver development and maturation. Virchows Archiv. 1994;424:669–675. doi: 10.1007/BF00195783. [DOI] [PubMed] [Google Scholar]
- 24.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995;146:67–74. [PMC free article] [PubMed] [Google Scholar]
- 25.Terada T, Nakanuma Y. Expression of pancreatic enzymes (α-amylase, trypsinogen and lipase) during human liver development and maturation. Gastroenterology. 1995;108:1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 26.Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development: A possible role in biliary cell migration. Am J Pathol. 1995;147:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 27.Terada T, Kato M, Horie S, Endo K, Kitamura Y. Expression of pancreatic alpha-amylase protein and messenger RNA on hilar primitive bile ducts and hepatocytes during human fetal liver organogenesis: An immunohistochemical and in situ hybridization study. Liver. 1998;18:313–319. doi: 10.1111/j.1600-0676.1998.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 28.Terada T, Kitamura Y, Nakanuma Y. Normal and abnormal development of the intrahepatic biliary system: A review. Tohoku J Exp Med. 1997;181:19–32. doi: 10.1620/tjem.181.19. [DOI] [PubMed] [Google Scholar]
- 29.Terada T, Nakanuma Y, Ohta G. Glandular elements around the intrahepatic bile ducts in man: Their morphology and distribution in normal livers. Liver. 1987;7:1–8. doi: 10.1111/j.1600-0676.1987.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 30.Terada T, Nakanuma Y. Solitary cystic dilation of the intrahepatic bile duct: Morphology of two autopsy cases and a review of the literature. Am J Gastroenterol. 1987;82:1301–1305. [PubMed] [Google Scholar]
- 31.Terada T, Nakanuma Y. Morphological examination of intrahepatic bile ducts in hepatolithiasis. Virchows Arch A Pathol Anat Histopathol. 1988;413:167–176. doi: 10.1007/BF00749679. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8:139–149. doi: 10.1016/0168-8278(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 33.Terada T, Nakanuma Y. Innervation of intrahepatic bile ducts and peribiliary glands in normal livers, extrahepatic biliary obsrtruction and hepatolithiasis: an immunohistochemical study. J Hepatol. 1989;9:141–148. doi: 10.1016/0168-8278(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 34.Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: Heterotopic pancreas in the liver. Gastroenterology. 1990;98:1333–1337. doi: 10.1016/s0016-5085(12)90353-4. [DOI] [PubMed] [Google Scholar]
- 35.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: II. A possible source of cholangiocarcinoma. Hepatology. 1990;12:92–97. doi: 10.1002/hep.1840120115. [DOI] [PubMed] [Google Scholar]
- 36.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: III. Survey of necroinflammation and cystic dilatation. Hepatology. 1990;12:1229–1233. doi: 10.1002/hep.1840120525. [DOI] [PubMed] [Google Scholar]
- 37.Kato M, Shinozawa T, Kato S, Terada T. Divergent expression of midkine in the human fetal liver and kidney: an immunohistochemical analysis of developmental changes in hilar primitive bile ducts and hepatocytes. Liver. 2000;20:475–481. doi: 10.1034/j.1600-0676.2000.020006475.x. [DOI] [PubMed] [Google Scholar]
- 38.Kato M, Shinozawa T, Kato S, Terada T. Immunohistochemical localization of truncated midkine in developing human bile ducts. Histol Histopathol. 2003;18:129–134. doi: 10.14670/HH-18.129. [DOI] [PubMed] [Google Scholar]
- 39.Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of E-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development. J Hepatol. 1998;28:263–269. doi: 10.1016/0168-8278(88)80013-8. [DOI] [PubMed] [Google Scholar]
- 40.Terada T, Kono N, Nakanuma Y. Immunohistochemical and immunoelectron microscopical analyses of α-amylase isozymes in the intrahepatic biliary epithelium and hepatocytes. J Histochem Cytochem. 1992;40:1627–1635. doi: 10.1177/40.11.1431051. [DOI] [PubMed] [Google Scholar]
- 41.Terada T, Nakanuma Y. Intrahepatic cholangiographic appearance simulating primary sclerosing cholangitis in hepatobiliary diseases: A postmortem cholangiographic and histolopathological study in 154 autopsy livers. Hepatology. 1995;22:75–81. [PubMed] [Google Scholar]
- 42.Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express α-amylase isozymes, trypsin and pancreatic lipase: An immunohistochemical analysis. Hepatology. 1993;18:803–808. doi: 10.1002/hep.1840180409. [DOI] [PubMed] [Google Scholar]
- 43.Terada T. Hepatic nodular hamartoma containing liver cysts, ductal plate malformations and peribiliary glands. Hepatol Res. 2011;41:93–98. doi: 10.1111/j.1872-034X.2010.00746.x. [DOI] [PubMed] [Google Scholar]
- 44.Terada T. Projected focal nodular hyperplasia (FNH) of the liver with pronounced atypical ductular reaction resembling ductal plate and expressing KIT. Hepatol Res. 2012;42:721–726. doi: 10.1111/j.1872-034X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 45.Terada T, Nakanuma Y. Congenital biliary dilatation in autosomal dominant adult polycystic disease of the liver and kidneys. Arch Pathol Lab Med. 1988;112:1113–1116. [PubMed] [Google Scholar]
- 46.Terada T, Moriki T. Monolobar ductal plate malformation disease of the liver. Pathol Int. 2010;60:407–12. doi: 10.1111/j.1440-1827.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 47.Terada T, Moriki T. Monolobar hepatobiliary fibropolycystic disease. Pathol Oncol Res. 2011;17:159–165. doi: 10.1007/s12253-010-9285-3. [DOI] [PubMed] [Google Scholar]
- 48.Nakanuma Y, Terada T, Ohta G, Matsubara F, Kurachi M. Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver. 1982;2:346–354. doi: 10.1111/j.1600-0676.1982.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 49.Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases: a clinical and histological review of 51 patients. J Hepatol. 1986;2:141–156. doi: 10.1016/s0168-8278(86)80073-3. [DOI] [PubMed] [Google Scholar]
- 50.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011;458:271–279. doi: 10.1007/s00428-011-1050-9. [DOI] [PubMed] [Google Scholar]
- 51.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261–270. doi: 10.1007/s00428-011-1049-2. [DOI] [PubMed] [Google Scholar]
- 52.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 53.Terada T, Kida T, Nakanuma Y, Noguchi T. Extensive portal tumor thrombi with portal hypertension in an autopsy case of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 1992;87:1513–1518. [PubMed] [Google Scholar]
- 54.Terada T, Nakanuma Y. Cell kinetic analyses and expression of carcinoembryonic antigen, carbohydrate antigen 19-9 and DU-PAN-2 in hyperplastic, preneoplastic and neoplastic lesions of intrahepatic bile ducts in livers with hepatoliths. Virchows Arch A Pathol Anat Histopathol. 1992;420:327–335. doi: 10.1007/BF01600212. [DOI] [PubMed] [Google Scholar]
- 55.Terada T, Sasaki M, Nakanuma Y, Takeda Y, Masunaga T. Hilar cholangiocarcinoma (Klatskin tumor) arising from intrahepatic peribiliary glands. J Clin Gastroenterol. 1992;15:79–81. [PubMed] [Google Scholar]
- 56.Terada T, Nakanuma Y. An immunohistochemical survey of amylase isoenzymes in cholangiocarcinoma and hepatocellular carcinoma. Arch Pathol Lab Med. 1993;117:160–162. [PubMed] [Google Scholar]
- 57.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 58.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 59.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 60.Terada T, Takeuchi T, Taniguchi M. Hepatobiliary cystadenocarcinoma with cystadenoma elements of the gall bladder in an old man. Pathol Int. 2003;53:790–795. doi: 10.1046/j.1440-1827.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 61.Theise ND, Nakashima O, Park YN, Nakanuma Y. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Calssification of tumours of the digestive system. Lyon: IARC; 2010. pp. 225–227. [Google Scholar]
- 62.Jorgensen MJ. The ductal plate malformation. Acta Pathol Microbiol Scand Suppl. 1977;257:1–87. [PubMed] [Google Scholar]
- 63.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–635. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 64.Raynaud P, Tate J, Callen C, Cordi S, Vandersmissen P, Carpentier R, Sempoux C, Devuyst O, Pierreux CE, Courtoy P, Daham K, Delbecque K, Lepreux S, Pontoglio M, Guary-Woodford L, Lemaigre FP. A classification of ductal plate malformations based on distinct pathogenic mechanisms of biliary dysmorphogenesis. Hepatology. 2011;53:1959–1966. doi: 10.1002/hep.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huppert SS. A new set of classification for ductal plate malformations. Hepatology. 2011;53:1795–1797. doi: 10.1002/hep.24404. [DOI] [PubMed] [Google Scholar]
- 66.Awasthi A, Das A, Srinivasan R, Joshi K. Morphological and immunohistochemical analysis of ductal plate malformation: correlation with fetal liver. Histopathology. 2004;45:260–267. doi: 10.1111/j.1365-2559.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 67.Theise ND, Yao JL, Harada K, Hytriroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic “stem cell” malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 68.Sell S, Dunsfold HA. Evidence for stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 69.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 70.Allison MR. Liver stem cells: implication for hepatocarcinogenesis. Stem cell Rev. 2005;1:253–260. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 71.Terada T, Hoso M, Nakanuma Y. Distribution of cytokeratin 19-positive biliary cells in cirrhotic nodules, hepatic borderline nodules (atypical adenomatous hyperplasia) and small hepatocellular carcinoma. Mod Pathol. 1995;8:371–379. [PubMed] [Google Scholar]
- 72.Terada T, Nakanuma Y. Arterial elements and perisinusoidal cells in borderline hepatocellular nodules and small hepatocellular carcinomas: an immunohistochemical study using anti-α-smooth muscle actin antibody. Histopathology. 1995;27:333–339. doi: 10.1111/j.1365-2559.1995.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 73.Terada T, Nakanuma Y. Multiple occurrence of borderline hepatocellular nodules in human cirrhotic livers: suggestion of muticentric origin of hepatocellular carcinoma. Virchows Archiv. 1995;427:379–383. doi: 10.1007/BF00199386. [DOI] [PubMed] [Google Scholar]
- 74.Roskams T, De Vos R, Desmet VJ. Undifferentiated progenitor cells in focal nodular hyperplasia of the liver. Histopathology. 1996;28:291–299. doi: 10.1046/j.1365-2559.1996.d01-438.x. [DOI] [PubMed] [Google Scholar]
- 75.Strain AJ, Crosby HA. Hepatic stem cells. Gut. 2000;46:743–745. doi: 10.1136/gut.46.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komuta M, Spree B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiocarcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 77.Terada T, Kida T, Nakanuma Y, Kurumaya H, Doishita K, Takayanagi N. Intrahepatic cholangiocarcinomas associated with nonbiliary cirrhosis: A clinicopathological study. J Clin Gastroenterol. 1994;18:335–342. doi: 10.1097/00004836-199406000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Jain D, Sarode VR, Abdul-Karim FW, Homer R, Robert ME. Evidence for the neoplastic transformation of Von-Meyenburg complexes. Am J Surg Pathol. 2000;24:1131–1139. doi: 10.1097/00000478-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Jian D, Nayak NC, Saigal S. Hepatocellular carcinoma arising in association with von-Meyenburg’s complexes: an incidental finding or precursor lesion?: A clinicopathological study of 4 cases. Ann Diagn Pathol. 2010;14:317–320. doi: 10.1016/j.anndiagpath.2010.04.003. [DOI] [PubMed] [Google Scholar]