Abstract
Hepatic angiomyolipoma (AML) is rare. Based on its wide histomorphological range, several distinctive histological variants have been delineated. However, hepatic AML displaying predominantly or exclusively inflammatory pattern closely mimicking inflammatory pseudotumor (IPT) is exceptionally rare with only 7 cases reported so far. We herein describe a new case of hepatic inflammatory AML in a 51-year-old woman who presented with unexplained constitutional symptoms suggesting an infectious disease. A liver mass was detected during imaging examination and resected (4.3 cm in maximum diameter). The patient’s symptoms resolved completely after surgery. Currently, she is alive and well 7 years after surgery. She has no evidence of other organ manifestations of IgG4-related systemic disease. The tumor displayed a pure IPT-like histological pattern with dense infiltrates of plasma cells, lymphocytes and histiocytes admixed with scattered few adipocytes, irregularly distributed thick-walled vessels (some of them showed obliterative phlebitis) as well as aggregates and fascicles of histiocytoid and spindle-shaped myoid cells that were immunoreactive for HMB45 and Melan A with focal expression of alpha smooth muscle actin. Lesional cells were negative for desmin, protein S100, CD21, CD23, CD15, CD30, HepPar-1, pankeratin (KL-1), ALK1, and EBV in situ hybridization (EBER). The surrounding liver parenchyma showed striking lymphoplasmacytic non-destructive pericholangitis. Numerous scattered and aggregated IgG4 positive plasma cells were seen within the mass and in the peritumoral inflammatory lesions (mean, 37 cells/HPF; IgG4: IgG ratio = 28%). To our knowledge, this is the first report of hepatic inflammatory AML closely resembling IgG4-related IPT of the liver. A possible role for IgG4 seems likely to explain the peculiar histological features and the unusual clinical presentation in this case.
Keywords: Angiomyolipoma, liver, inflammatory, inflammatory pseudotumor, IgG4, cholangitis
Introduction
Hepatic angiomyolipoma (AML) is a rare benign tumor first described by Ishak in 1976 [1]. While earlier series comprised a higher number of autopsy cases [2], hepatic AML has been increasingly recognized during the last years as a result of wide-spread use of high resolution imaging techniques and many cases have been diagnosed by core needle biopsy of unclear liver masses [3]. As a consequence, several large case series appeared in the recent literature [4,5]. Owing to their significantly varied histological appearance, several histological variants of hepatic AML have been delineated (classical/ mixed, leiomyomatous, lipomatous, myelolipomatous, angiomatous/angiomyomatous, epithelioid, trabecular, oncocytic, pleomorphic and inflammatory variants) [3-6]. Recognition of these AML variants is necessary to avoid misinterpreting them as variants of hepatocellular carcinoma, melanoma or other primary or metastatic malignant neoplasms with prominent inflammation, particularly on core needle biopsy [3,7]. Otherwise these histological variants carry no particular clinical significance.
The inflammatory variant is the least common hepatic AML variant with 7 cases reported in the literature so far [8-10]. Most of these cases displayed minor focal conventional tumor component that represented a strong hint to diagnosis on H&E stained slides. However, the pathogenesis of the inflammatory reaction in hepatic AML remained poorly understood. We herein describe an unusual case of hepatic inflammatory AML that closely mimicked IPT. In addition, the tumor showed strikingly overlapping features with IgG4-related IPT including prominent plasma cell component, storiform sclerosis, obliterative phlebitis, increased tissue IgG4-plasma cells, and associated peritumoral non-destructive pericholangitis within adjacent liver parenchyma.
Case presentation
A 51-year-old woman presented with constitutional symptoms including episodes of unexplained fever followed by general illness and arthritis thought to be of infectious etiology but no specific focus of infection or inflammation could be found on thorough clinical workup. She suffered from hypochromic anemia for several years but previous investigations were limited to gynecological examination which revealed no cause for her anemia. Abnormal laboratory findings upon admission were: elevated erythrocyte sedimentation rate of 56 mm, C-reactive protein (CRP) of 11.32 mg/dl, serum iron of 13 μg/dl, Hemoglobin of 10.8 g/dl, ANA of 1:800, ASMA of 1:40 and pANCA titer of 1:16. All other findings including relevant liver function tests, rheumatoid factor, AMA and ferritin level were within normal ranges. Abdominal ultrasound and magnetic resonance imaging (MRI) showed a 4.3 cm circumscribed round mass within the left liver lobe (segment 2/4) without specific radiological features that would have permitted definitive diagnosis of AML. In addition, a 1.2 cm lesion typical of hemangioma was seen in segment VIII of the right lobe. Retrospective review of radiological images taken 5 years ago revealed no liver mass. Based on this and the observed gradual increase in the size of the mass, a decision was made for surgical resection of the mass. No other pathological findings were seen in the abdominal organs. All other clinical investigations (upper GI endoscopy, colonoscopy, magnetic resonance cholangiopancreatography/MRCP and abdominal MRI) revealed no further lesions. A segmental resection of the left liver lobe was performed. The patient recovered well after surgery. Her initial symptoms resolved completely and the serological inflammation parameters returned to normal at the time of release from hospital. Currently she is alive and well 7 years after surgery. Serum IgG4 level has not been investigated.
Material and methods
The resection specimen has been fixed in formalin and embedded routinely for histological examination. In addition to H&E stain, Perdiodic Acid Schiff (PAS), Prussian blue, Elastica van Gieson and Gömöri sliver impregnation methods were also used. Immunohistochemistry was performed on 3 μ sections cut from paraffin blocks using a Ventana automated system (Ventana Medical Systems Inc., Tucson, AZ, USA) according to the manufacturer’s instructions and the following antibodies: hepatocyte paraffin-1 (HepPar-1), pankeratin (KL-1), CK7, desmin, alpha-smooth muscle actin (ASMA), HMB45, Melan A, protein S100 (polyclonal), CD3, CD15, CD20, CD21, CD23, CD30, CD68, ALK-1, CD1a, CD117, Kappa/lambda light chains, rabbit polyclonal antibody against human IgG (1:2000, Dako) and mouse monoclonal antibody against human IgG4 (clone MCA2098G, 1:100, SeroTec, UK). For detection of Epstein Barr Virus (EBV) in situ hybridization was used to detect EBER1/2 using a kit purchased from Zytomed Systems, Ltd. (Berlin, Germany). Numbers of IgG4- and IgG-positive plasma cells were counted in three high power fields (HPF) and the ratio calculated using the method described previously [11].
Results
The resection specimen contained a 4.3 cm tumor with brown soft cut-surface. The tumor was grossly well demarcated from surrounding parenchyma but not encapsulated (Figure 1A). Histological examination showed a predominance of mixed inflammatory infiltrates composed of prominent plasma cells admixed with small mature lymphocytes, scattered neutrophils, histiocytes, fibroblasts and a few eosinophils. A prominent diffuse and storiform fine reticular sclerosis/hyalinosis was seen in the background with occasional focal accentuation (Figure 1B). Several small to medium-sized thick-walled veins within the lesion showed complete lumen obliteration by inflammatory cells and fibrosis (Figure 1C). The tumor contained scattered large mono-, bi- or multinucleated cells with voluminous pale to eosinophilic granular cytoplasm and overall histiocytoid appearance (Figure 1D), occasionally with central nucleoli superficially mimicking Reed-Sternberg cells (Figure 1E and 1F). Only isolated scattered fat vacuoles were seen (< 5% of lesional cells) but there were no areas of classical AML, aggregated adipocytes or tortuous dysplastic vessels. The interphase to the surrounding liver was partially sharp (Figure 1A). However, in several areas the tumor showed irregular borders infiltrating into the liver parenchyma with accentuation around portal tracts (Figure 2A). Here, focal obliterative angiitis involving thick-walled vessels (Figure 2B) and isolated portal tracts surrounded by prominent concentric periductal mononuclear inflammation associated with sclerosis (Figure 2C and 2D) and isolated HMB45 positive cells (Figure 2D) were seen. Otherwise, the liver parenchyma away from the mass showed no significant pathological changes, in particular there was no steatosis, cholestasis, iron overload or fibrosis. Immunohistochemical staining with HMB45 and Melan A highlighted numerous medium to large sized polygonal and predominantly slender spindled cells within the inflammatory background (Figure 3A). These cells displayed multiple dendritic-like cytoplasmic processes that were best highlighted with the HMB45 immunostaining, but were barely discernible on H&E stain (Figure 3B). ASMA stained many of these slender myoid cells in addition to stromal myofibroblasts in areas of sclerosis but most of HMB45 positive cells lacked this marker. Immunohistochemical staining with IgG4 antibody revealed numerous positive staining plasma cells that were mostly evenly scattered within the tumor but also showed occasional cluster formation (Figure 3C and D). The average number of IgG4 positive cells was 37 cells/HPF (range: 28-54; IgG4: IgG ratio = 28%). Although focal areas rich in IgG4 positive plasma cells were seen at the interphase with the surrounding liver parenchyma, the pericholangitis lesions away from the main mass did not contain increased numbers of IgG4 plasma cells. Kappa and lambda light chains showed polytypic plasma cells. All other markers listed above including EBER were negative.
Figure 1.
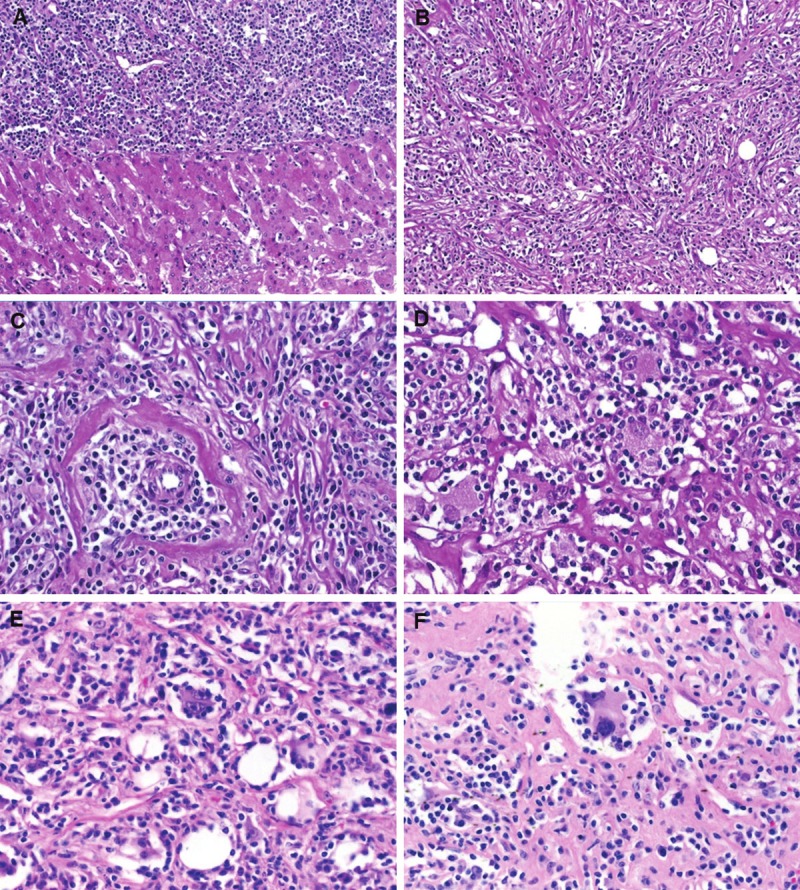
Representative images of IPT-like hepatic angiomyolipoma. A. The tumor was partially sharply circumscribed from adjacent liver parenchyma but non-encapsulated. B. prominent fine-reticular storiform sclerosis is seen at this magnification. C. Obliterative phlebitis with prominent plasmacytic perivascular infiltrates. D. Small clusters of histiocytoid cells with voluminous granular eosinophilic cytoplasm. E. Occasional reed-Sternberg-like binucleated cells were seen (note scattered fat vacuoles). F. Another binucleated giant cell showed slender dendritic-like cytoplasmic processes.
Figure 2.

Peritumoral changes within adjacent liver. A. Dense inflammatory infiltrates entrapping sheets of hepatocytes. B. A thick-walled vessel within adjacent liver showed prominent angiitis obliterating its lumen. C. Concentric fibrosis around a small bile duct associated with lymphoplasmacytic infiltrates but no evidence of epithelial destruction was seen. D. Another portal area showed both pericholangitis and obliterative angiitis. Isolated cells within perivascular/peribiliary aggregates strongly expressed HMB45 (D: inset).
Figure 3.

Immunohistochemical findings in hepatic inflammatory angiomyolipoma. A. Large mono-, bi- and multinucleated histiocytoid myoid cells strongly expressed HMB45 (Note dendritic features of the larger cells on the right). B. Another area with several slender dendritic spindled cells with over all reticulum-like morphology highlighted by HMB45 immunostaining (note prominent sinusoidal vessels). C. several plasma cells expressed IgG4. D. IgG4-positive plasma cells formed dense aggregates at the interphase between tumor (upper left) and liver tissue (lower right).
Discussion
Owing to the poorly characterized nature of the “true lesional cell” in hepatic IPTs for decades, the term “hepatic IPT” has been historically applied to a variety of lesions of different histogenetic, etiological and clinical backgrounds, having in common the mere presence of a heavy tumefactive mononuclear predominantly lymphoplasmacytic and histiocytic mixed inflammatory infiltrate [12]. By definition, a well recognizable neoplastic component (epithelial, lymphoid or others) is lacking in hepatic IPT. However, similar to its extra-hepatic counterparts, the concept of hepatic IPT underwent continuous evolution and splitting as a result of recent developments that lead to a better understanding of underlying disease mechanisms and facilitated definition and recognition of the “true” neoplastic cell” in individual tumors [13]. A subset of hepatic IPT was found to harbor a clonal proliferation of EBV-infected follicular dendritic cells (FDCs). This currently well characterized true low-grade neoplasm has been redefined as IPT-like FDC tumor with a predilection for the liver and the spleen [14]. Furthermore, recognition of cytogenetic aberration involving the anaplastic lymphoma kinase-1 (ALK-1) in a subset of lesions within the spectrum of IPT facilitated their reproducible classification as inflammatory myofibroblastic tumor (IMT). IMT of the liver is less common but well documented in the literature [15,16].
Another variant in the rubric of “hepatic IPT” has been recently recognized as part of the emerging IgG4-related systemic fibrosclerosis [17]. IgG4-related IPT of the liver may occur either in isolation or as a hepatic manifestation of systemic disease with involvement of the hepatopancreatobiliary system and other organ systems [18-23]. Hepatic manifestation of IgG4 disease may vary greatly but the cardinal histological features of this disorder are: 1) a predominantly lymphoplasmacytic inflammatory infiltrate, 2) prominent reticular or storiform sclerosis, 3) occasional obliterative angiitis (phlebitis), 4) variable numbers of IgG4 positive plasma cells either scattered or forming dense aggregates, 5) elevated IgG4: IgG ratio within biopsy tissue, and 6) associated elevation of serum IgG4 level [17,23-26]. The diagnosis should therefore be based on a combination of these findings and can be particularly confirmed by the presence of other organ manifestations of the disorder [23-26].
The inflammatory variant of hepatic AML is exceedingly rare. Including our case, 8 cases have been reported to date [8-10] (Table 1). There is a striking female predilection (F: M = 7: 1). Patients’ mean age was 41 yrs (range: 21-63). Five of the lesions originated in the left liver lobe. None was associated with other liver diseases or cirrhosis and hepatitis B and C serology has been negative in two cases with available data. Unlike classical hepatic AML (of which ~10% were associated with the tuberous sclerosis complex (TSC)), none of the 8 reported hepatic inflammatory AML was associated with this disorder. Most were incidental findings on imaging examination and only two cases (including our case) presented with constitutional symptoms (unexplained fever or general illness) [8]. The tumor size ranged from 3 to 10 cm (mean: 6.5). None recurred or metastasized at a mean follow-up of 5.3 yrs (follow-up range: 2-9 yrs). All tumors showed a predominance of inflammatory pattern comprising more than 90% of the lesion and fatty cells represented < 5% of lesional cells in all cases. The presence of scattered adipocytes, sinusoidal or irregular thick-walled vessels and myoid cells were the clue to diagnosis of AML. Reviewing illustrations of reported inflammatory hepatic AML, we found that our case and the case reported by Kojima et al [8] were strikingly similar and both closely mimicked IPT including the presence of obliterative phlebitis. Our case showed in addition inflammatory lesions involving adjacent portal tracts.
Table 1.
Reported cases of hepatic inflammatory angiomyolipoma (n=8)
| No | Author | Age/sex | Size cm | Symptoms | Signs of TSC | Classic component | IHC + | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Site | |||||||||
| 1 | Kojima et al [8] | 21/F | 7.3 | PUO, leucocytosis (11.000/mm?3), HBV & HCV negative. | No | <5% (scattered fat cells, myoid cells & thick-walled vessels) | ASMA, MSA, HMB45 | Resection left liver lobe | 5 yrs 5 months ANED |
| Left lobe | |||||||||
| 2 | Shi et al [9] | 21/F | 5.5 | Hepatic mass | No | <50%, fat less 5% | HMB45, ASMA, S100 | Hemihepatectomy | 5 yrs ANED |
| Left lobe | |||||||||
| 3 | Shi et al [9] | 42/F | 7.5 | Hepatic mass | No | <50%, fat less 5% | HMB45, ASMA | Hemihepatectomy | 7 yrs ANED |
| Right lobe | |||||||||
| 4 | Shi et al [9] | 48/M | 8 | Abdominal discomfort & hepatic mass | No | <50%, fat less 5% | HMB45, ASMA, desmin | Hemihepatectomy | 4 yrs ANED |
| Left lobe | |||||||||
| 5 | Shi et al [9] | 40/F | 6.3 | Hepatic mass | No | <50%, fat less 5% | HMB45, ASMA | Hemihepatectomy | 3 yrs ANED |
| Left lobe | |||||||||
| 6 | Shi et al | 45/F | 10 | Abdominal discomfort & pain, hepatic mass | No | <50%, fat less 5% | HMB45, ASMA, desmin | Hemihepatectomy | 9 yrs ANED |
| Right lobe | |||||||||
| 7 | Liu et al [10] | 63/F | 3 | Incidental on routine US. AFP & CEA normal, HBV & HCV negative | No | <20%, fat <5%(thick-walled vessels & fat cells peripherally) | HMB45; ASMA; Melan A | Hemihepatectomy | 2 yrs ANED |
| Right lobe | |||||||||
| 8 | Current case | 51/F | 4.3 | Fever, arthritis, anemia, general illness | No | <1% (isolated fat cells) | HMB45, (ASMA) | Segmental resection | 7 yrs ANED |
| Left lobe |
F = female, M = male, PUO = pyrexia of unknown origin, HBV = hepatitis B virus, HCV = hepatitis C virus, AFP = alpha fetoprotein, CEA = carcinoembryonic antigen, US = ultrasound, IHC+ = positive on immunohistochemistry, ASMA = alpha smooth muscle actin, MSA = muscle-specific actin, ANED = Alive with no evidence of disease.
However, the current case features unusual findings that merit special presentation and discussion. Without the scattered fatty vacuoles and immunohistochemistry for HMB45, the lesion would have been classified as IPT. Notably, the lesion showed clear-cut features of IgG4-related IPT including prominent fine storiform reticular sclerosis, prominent plasma cell population, presence of obliterative phlebitis and increased number of IgG4-positive tissue plasma cells. Furthermore, adjacent liver showed a peculiar type of pericholangitis associated with fibrosclerosis and isolated HMB45 positive cells. On the other hand, the presence of isolated fatty cells within the lesion and the strong characteristic granular cytoplasmic expression of melanocytic markers are strong clues for a diagnosis of inflammatory AML.
Thus, the classification of this lesion raises several questions. Is this really a hepatic inflammatory AML or an IPT with secondary “non-specific or metaplastic” myomelanocytic immunophenotype? Is the adjacent inflammatory liver change a secondary associated phenomenon or a precursor lesion that ultimately would be incorporated into the growing tumor? What is the role and clinical significance of IgG4 in this lesion?
Regarding the first question, a diagnosis of AML seems justified on the basis of histological features (fatty cells), immunoprofile (HMB45+) and similarity to previously reported cases that showed in addition a classical component. Adjacent liver lesions showed actually identical appearance as the main lesions but differed only by virtue of their periductal concentric accentuation. Presence of isolated HMB45 staining cells within them makes a histogenesis similar to the main lesion more likely.
The role of IgG4 in this peculiar lesion remains obscure. Recent studies have demonstrated significant numbers of IgG4 positive plasma cells as a constituent of intra- and peritumoral inflammatory response in different histological subtypes of carcinomas from various organs [11]. However, the overall histological similarity of the tumor in the current case to the IgG4-related lesions strongly suggests a role for IgG4 in the pathogenesis of the inflammatory infiltrate and the associated vascular and stromal changes. Excessive IgG4 production by the tumor-associated inflammatory cells might be responsible for the rheumatoid-like clinical symptoms and the general illness in our patient. This is strengthened by complete resolution of her clinical symptoms after tumor resection. Unfortunately, preoperative serum IgG4 level was not available in our case as this special aspect of her lesion was recognized in a retrospective way. However, absence of extra-hepatic manifestations in our patient and the uneventful follow-up for the next 7 years indicates a localized disease with paraneoplastic (probably IgG4-associated) symptoms and argues against a systemic disorder.
High counts of IgG4 plasma cells have been detected in synovial biopsies from patients with rheumatoid arthritis [11]. Furthermore, IgG4 has been implicated in the pathogenesis of rheumatoid diseases [27]. Thus, the arthritis featured by our case might have been IgG4-induced as it resolved after tumor resection. Straub et al. reported a case of cholangiocarcinoma associated with an IgG4-related pseudotumor in the same liver resection specimen where both lesions formed a single bilobated gross mass [28]. More recently, the presence of IgG4 plasma cells has been implicated in the prominent stromal sclerosis commonly seen in a subset of mucoepidermoid carcinomas [29]. All these observations and the current case make it likely that increased numbers of IgG4 producing plasma cells my accompany different neoplasms thereby causing unusual sclerosing and/or inflammatory appearance and they might be responsible for paraneoplastic phenomena related to hyper-IgG4 status that would resolve after removal of the tumor. Constitutional symptoms have been observed in cases of IPT/inflammatory myofibroblastic tumor of different sites [30], but the role of IgG4 in these older cases remains unknown.
Given that HMB45 staining might represent the only clue to diagnosis and that most hepatic IPT reported earlier have not been stained with HMB45, this variant of hepatic AML might be under-recognized. Thus, it is highly recommended to include HMB45 and Melan A in the marker panel used for classifying lesions in the spectrum of “hepatic IPT”. It would be of interest to test hepatic IPT in other settings (particularly those lesions with features of IgG4-related disease) for HMB45 expression as some of them might display overlapping features with inflammatory AML as in our case.
In summary, we reported a unique case of hepatic inflammatory AML showing hybrid features of IgG4-related pseudotumor. Recognition of this peculiar lesion in the rubric of hepatic IPT should enhance delineation of this entity and help to clarify its pathogenesis and particularly the role of IgG4.
Conflict of interest statement
None to declare.
References
- 1.Ishak KG. Mesenchymal tumors of the liver. In: Okuda K, Peters RL, editors. Hepatocellular Carcinoma. New York: John Wiley & Sons; 1976. [Google Scholar]
- 2.Goodman ZD, Ishak KG. Angiomyolipomas of the liver. Am J Surg Pathol. 1984;8:745–50. doi: 10.1097/00000478-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Agaimy A, Vassos N, Croner RS, Strobel D, Lell M. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int J Clin Exp Pathol. 2012;5:512–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Z, Zhang JM, Ying JQ, Ge YP. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis. 2011;20:65–9. doi: 10.1007/s11749-010-0230-2. [DOI] [PubMed] [Google Scholar]
- 5.Nonomura A, Enomoto Y, Takeda M, Takano M, Morita K, Kasai T. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology. 2012;61:863–80. doi: 10.1111/j.1365-2559.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsui WM, Colombari R, Portmann BC, Bonetti F, Thung SN, Ferrell LD, Nakanuma Y, Snover DC, Bioulac-Sage P, Dhillon AP. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23:34–48. doi: 10.1097/00000478-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Zhong DR, Ji XL. Hepatic angiomyolipoma-misdiagnosis as hepatocellular carcinoma: A report of 14 cases. World J Gastroenterol. 2000;6:608–612. doi: 10.3748/wjg.v6.i4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima M, Nakamura S, Ohno Y, Sugihara S, Sakata N, Masawa N. Hepatic angiomyolipoma resembling an inflammatory pseudotumor of the liver. A case report. Pathol Res Pract. 2004;200:713–6. doi: 10.1016/j.prp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Cao D, Wei L, Sun L, Guo A. Inflammatory angiomyolipomas of the liver: a clinicopathologic and immunohistochemical analysis of 5 cases. Ann Diagn Pathol. 2010;14:240–6. doi: 10.1016/j.anndiagpath.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Wang J, Lin XY, Xu HT, Qiu XS, Wang EH. Inflammatory angiomyolipoma of the liver: a rare hepatic tumor. Diagn Pathol. 2012;7:122. doi: 10.1186/1746-1596-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localized non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64:237–243. doi: 10.1136/jcp.2010.085613. [DOI] [PubMed] [Google Scholar]
- 12.Shek TW, Ng IO, Chan KW. Inflammatory pseudotumor of the liver. Report of four cases and review of the literature. Am J Surg Pathol. 1993;17:231–238. doi: 10.1097/00000478-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 14.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721–731. doi: 10.1097/00000478-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Chen ST, Lee JC. An inflammatory myofibroblastic tumor in liver with ALK and RANBP2 gene rearrangement: combination of distinct morphologic, immunohistochemical, and genetic features. Hum Pathol. 2008;39:1854–1858. doi: 10.1016/j.humpath.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Solomon GJ, Kinkhabwala MM, Akhtar M. Inflammatory myofibroblastic tumor of the liver. Arch Pathol Lab Med. 2006;130:1548–1551. doi: 10.5858/2006-130-1548-IMTOTL. [DOI] [PubMed] [Google Scholar]
- 17.Cheuk W, Chan JKC. IgG4-related Sclerosing Disease. A Critical Appraisal of an Evolving Clinicopathologic Entity. Adv Anat Pathol. 2010;17:303–332. doi: 10.1097/PAP.0b013e3181ee63ce. [DOI] [PubMed] [Google Scholar]
- 18.Nakanuma Y, Tsuneyama K, Masuda S, Tomioka T. Hepatic inflammatory pseudotumor associated with chronic cholangitis: report of three cases. Hum Pathol. 1994;25:86–91. doi: 10.1016/0046-8177(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 19.Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 20.Kanno A, Satoh K, Kimura K, Masamune A, Asakura T, Unno M, Matsuno S, Moriya T, Shimosegawa T. Autoimmune pancreatitis with hepatic inflammatory pseudotumor. Pancreas. 2005;31:420–3. doi: 10.1097/01.mpa.0000179732.46210.da. [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007;20:884–94. doi: 10.1038/modpathol.3800836. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K, Satoi S, Miyoshi H, Hachimine D, Ikeura T, Shimatani M, Matsushita M, Takaoka M, Takai S, Ashida K, Okazaki K. Inflammatory pseudotumors of the pancreas and liver with infiltration of IgG4-positive plasma cells. Intern Med. 2007;46:1409–12. doi: 10.2169/internalmedicine.46.6430. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Smyrk TC. Autoimmune pancreatitis and IgG4-related systemic diseases. Int J Clin Exp Pathol. 2010;3:491–504. [PMC free article] [PubMed] [Google Scholar]
- 24.Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, Nishino T, Ko SB, Mizuno N, Hamano H, Kanno A, Notohara K, Hasebe O, Nakazawa T, Nakanuma Y, Takikawa H Research Committee of IgG4-related Diseases; Research Committee of Intractable Diseases of Liver and Biliary Tract; Ministry of Health, Labor and Welfare, Japan; Japan Biliary Association. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536–42. doi: 10.1007/s00534-012-0521-y. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–92. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 26.Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–7. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelmann R, Brandt J, Eggert M, Karberg K, Krause A, Neeck G, Mueller-Hilke B. IgG1 and IgG4 are the predominant subclasses among auto-antibodies against two citrullinated antigens in RA. Rheumatology (Oxford) 2008;47:1489–92. doi: 10.1093/rheumatology/ken336. [DOI] [PubMed] [Google Scholar]
- 28.Straub BK, Esposito I, Gotthardt D, Radeleff B, Antolovic D, Flechtenmacher C, Schirmacher P. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch. 2011;458:761–765. doi: 10.1007/s00428-011-1073-2. [DOI] [PubMed] [Google Scholar]
- 29.Tian W, Yakirevich E, Matoso A, Gnepp DR. IgG4(+) plasma cells in sclerosing variant of mucoepidermoid carcinoma. Am J Surg Pathol. 2012;36:973–9. doi: 10.1097/PAS.0b013e318258f018. [DOI] [PubMed] [Google Scholar]
- 30.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]


