Abstract
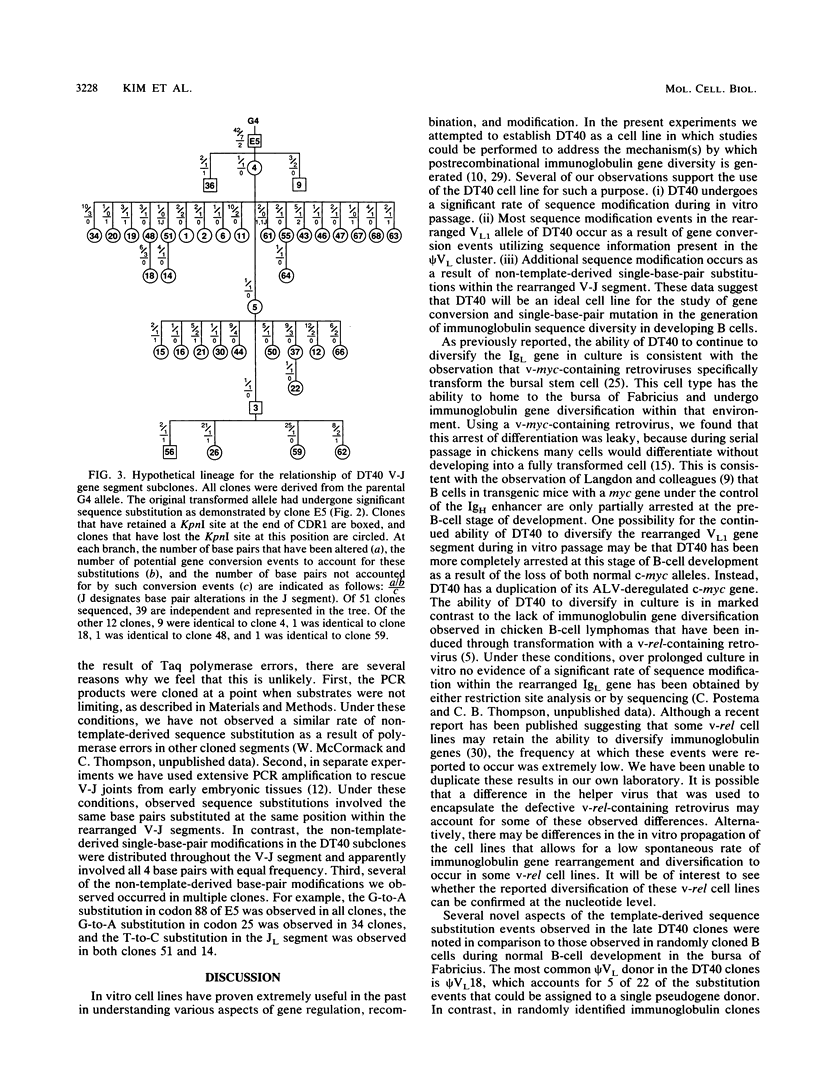
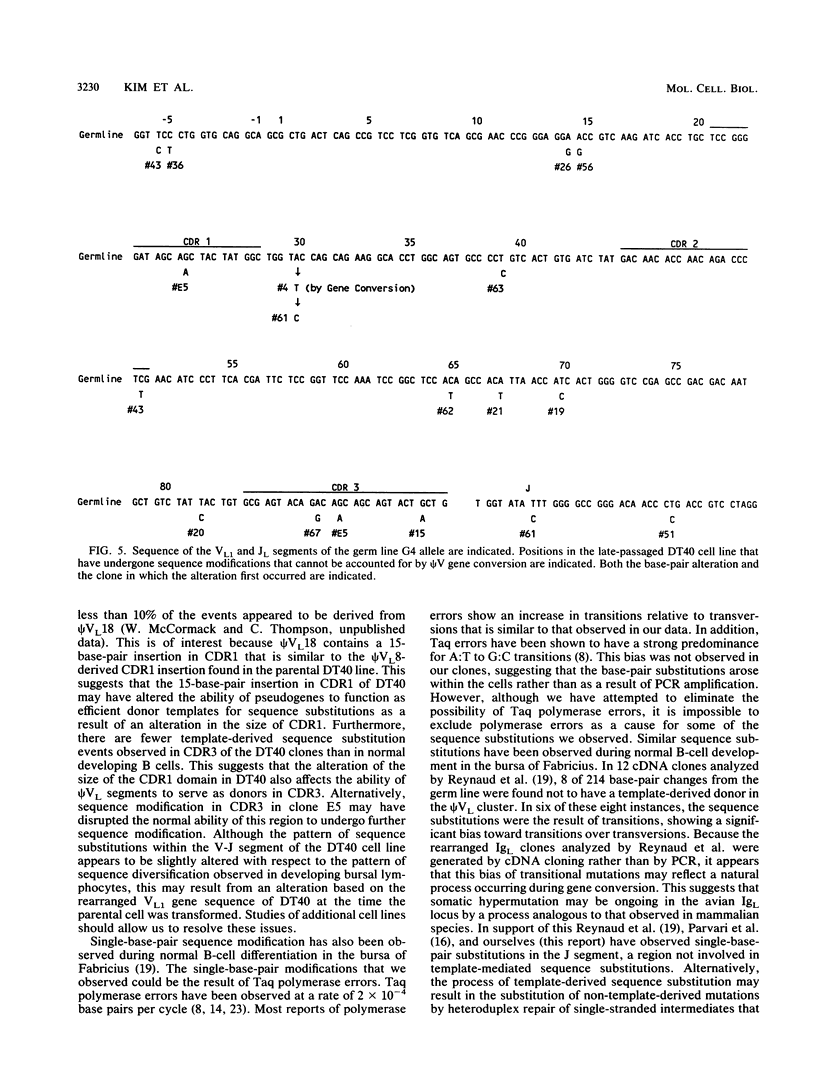
The chicken immunoglobulin light-chain gene (IgL) encodes only a single variable gene segment capable of recombination. To generate an immune repertoire, chickens diversify this unique rearranged VL gene segment during B-cell development in the bursa of Fabricius. Sequence analysis of IgL cDNAs suggests that both gene conversion events derived from VL segment pseudogene templates (psi VL) and non-template-derived single-base-pair substitutions contribute to this diversity. To facilitate the study of postrecombinational mechanisms of immunoglobulin gene diversification, avian B-cell lines were examined for the ability to diversify their rearranged IgL gene during in vitro passage. One line that retains this ability, the avian leukosis virus-induced bursal lymphoma cell line DT40, has been identified. After passage for 1 year in culture, 39 of 51 randomly sequenced rearranged V-J segments from a DT40 population defined novel subclones of the parental tumor. All cloned V-J segments displayed the same V-J joint, confirming that the observed diversity arose after V-J rearrangement. Most sequence variations that we observed (203 of 220 base pairs) appeared to result from psi VL-derived gene conversion events; 16 of the 17 novel single nucleotide substitutions were transitions. Based on these data, it appears that immunoglobulin diversification during in vitro passage of DT40 cells is representative of the diversification that occurs during normal B-cell development in the bursa of Fabricius.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Giroir B. P., Humphries E. H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology. 1985 Jul 15;144(1):139–151. doi: 10.1016/0042-6822(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A. 1985 Jan;82(1):213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C. F., Humphries E. H. Expression of v-rel induces mature B-cell lines that reflect the diversity of avian immunoglobulin heavy- and light-chain rearrangements. Mol Cell Biol. 1988 Dec;8(12):5358–5368. doi: 10.1128/mcb.8.12.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerstedde J. M., Reynaud C. A., Humphries E. H., Olson W., Ewert D. L., Weill J. C. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 1990 Mar;9(3):921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Maizels N. Might gene conversion be the mechanism of somatic hypermutation of mammalian immunoglobulin genes? Trends Genet. 1989 Jan;5(1):4–8. doi: 10.1016/0168-9525(89)90004-8. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Barth C. F., Carlson L. M., Petryniak B., Humphries E. H., Thompson C. B. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989 Jun;3(6):838–847. doi: 10.1101/gad.3.6.838. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Carlson L. M., Petryniak B., Barth C. F., Humphries E. H., Thompson C. B. Chicken IgL gene rearrangement involves deletion of a circular episome and addition of single nonrandom nucleotides to both coding segments. Cell. 1989 Mar 10;56(5):785–791. doi: 10.1016/0092-8674(89)90683-1. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Gehly E. B., Carlson L. M., Cotter R. C., Thompson C. B. Bursal stem cells as targets for myc-induced preneoplastic proliferation and maturation arrest. Curr Top Microbiol Immunol. 1988;141:67–74. doi: 10.1007/978-3-642-74006-0_10. [DOI] [PubMed] [Google Scholar]
- Parvari R., Ziv E., Lentner F., Tel-Or S., Burstein Y., Schechter I. Analyses of chicken immunoglobulin light chain cDNA clones indicate a few germline V lambda genes and allotypes of the C lambda locus. EMBO J. 1987 Jan;6(1):97–102. doi: 10.1002/j.1460-2075.1987.tb04724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Anquez V., Weill J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989 Oct 6;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Ripley L. S., Clark A. Frameshift mutations produced by proflavin in bacteriophage T4: specificity within a hotspot. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6954–6958. doi: 10.1073/pnas.83.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley L. S., Clark A., deBoer J. G. Spectrum of spontaneous frameshift mutations. Sequences of bacteriophage T4 rII gene frameshifts. J Mol Biol. 1986 Oct 20;191(4):601–613. doi: 10.1016/0022-2836(86)90448-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schubach W., Groudine M. Alteration of c-myc chromatin structure by avian leukosis virus integration. Nature. 1984 Feb 23;307(5953):702–708. doi: 10.1038/307702a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Humphries E. H., Carlson L. M., Chen C. L., Neiman P. E. The effect of alterations in myc gene expression on B cell development in the bursa of Fabricius. Cell. 1987 Nov 6;51(3):371–381. doi: 10.1016/0092-8674(87)90633-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Gefter M. L. Gene conversion and the generation of antibody diversity. Annu Rev Biochem. 1989;58:509–531. doi: 10.1146/annurev.bi.58.070189.002453. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Bargmann W., Bose H. R., Jr Rearrangement and diversification of immunoglobulin light-chain genes in lymphoid cells transformed by reticuloendotheliosis virus. Mol Cell Biol. 1989 Nov;9(11):4970–4976. doi: 10.1128/mcb.9.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]



