Abstract
Cytokeratin (pan) and epithelial membrane antigen (EMA) were considered as commonly useful epithelial markers to distinguish cancer from lymphoma, but the expression of cytokeratin (pan) and EMA had also been seen in some lymphomas. Here, we presented an unusual case of anaplastic large cell lymphoma (ALCL) absence of some lymphoid markers (Including CD3, CD20, Pax-5, CD45Ro) and positive for cytokeratin (pan) and EMA, which was misdiagnosed as metastatic carcinoma. Our case suggested that the epithelial markers (including cytokeratin and EMA) show an overlap with that of ALCL, which represented a diagnostic pitfall for confusing ALCL with metastatic carcinoma.
Keywords: Anaplastic large cell lymphoma, anaplastic lymphoma kinase, immunohistochemistry
Introduction
Cytokeratins are proteins of keratin-containing intermediate filaments which are useful markers for epithelial cells, Previous studies have shown that cytokeratin can also been expressed in tumors of non-epithelial origin such as epithelioid sarcoma, epithelioid angiosarcoma [1], synovial sarcoma, mesothelioma, chordoma and some lymphomas [2]. In this paper, we reported a case of cytokeratin-positive anaplastic large cell lymphoma in lymph node, which was misdiagnosed as a metastatic carcinoma.
Case description
A 35-year-old Chinese man presented with enlarged right axillary nodes without other systemic symptoms for 3 months. Physical examination: the enlarged lymph node is about 2cm in diameter, with smooth surface, clear boundary and medium texture. The patient had no history of malignancy and lack of fever, night sweat and rash on body. Serum tumor markers including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA125) were all within the normal range.
Immunohistochemical studies
The biopsy specimen was fixed in 10% buffered formalin solution and embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Immunohistochemical staining was performed by using the streptavidin-peroxidase system (Ultrasensitive, MaiXin Inc, Fuzhou, China) according to the manufacturer’s instruction. Heat-induced epitope retrieval was performed. The following antibodies (MaiXin Inc, China, prediluted) were used: Cytokeratin (pan), EMA, CD3, CD20, Pax-5, CD45RO, S-100, HMB45, MelanA, Desmin, MyoD1, PLAP, CD117, CD15, CD30, ALK/P80 and the Ki-67. Positive and negative controls were evaluated appropriately for each procedure.
Pathological findings
The patient was diagnosed as lymphadenopathy and a lymph node biopsy was carried out. Hematoxylin and eosin sections showed that the normal structure of lymph node was destroyed and substituted by atypia tumor cell nests (Figure 1A, 1B). In some regions, tumor cells grew in sheets (Figure 1C) and infiltrated lymph node sinuses (Figure 1D). Immunohistochemical stainings showed that the tumor cells were positive for cytokeratin (pan) (Figure 2A) and EMA, but negative for CD3 (Figure 2C), CD20 (Figure 2D), Pax-5 (Figure 2E), CD45RO, PLAP, CD117, S-100, HMB45, MelanA, Desmin and MyoD1. The Ki-67 (Figure 2F) labeling index was about 70%. The tumor cells showed some similar characteristics of carcinoma and expression of epithelial cells marker. Therefore, the patient was diagnosed as metastatic carcinoma and then was referred to the department of oncology to search for the origin. Because non-malignant tumors were found in other organs by PET-CT scan, the oncologist advised a revision of the pathological diagnosis.
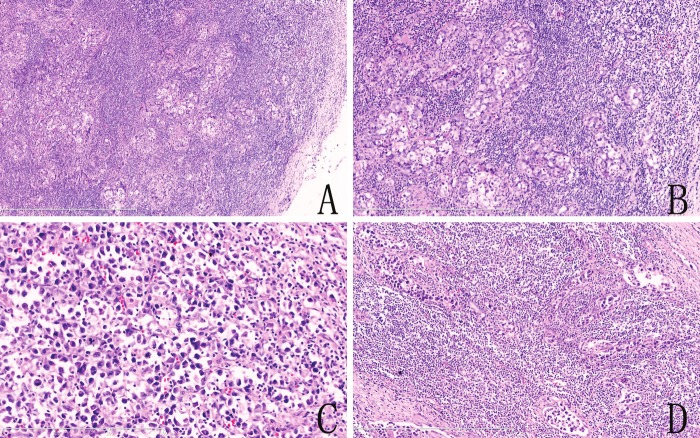
Figure 1.
Hematoxylin and eosin staining. A. The normal structure of lymph node is destroyed and replaced by nests of infiltrating tumor cells (hematoxylin-eosin, original magnification ×50). B. The tumor cells presented some similar characteristics of carcinoma cells. (hematoxylin-eosin, original magnification ×100). C. The tumor cells showed in a patchy distribution and exhibited typical hallmark cells (hematoxylin-eosin, original magnification ×200). D. Showed the tumor cells infiltrates lymph node sinuses. (hematoxylin-eosin, original magnification ×100).
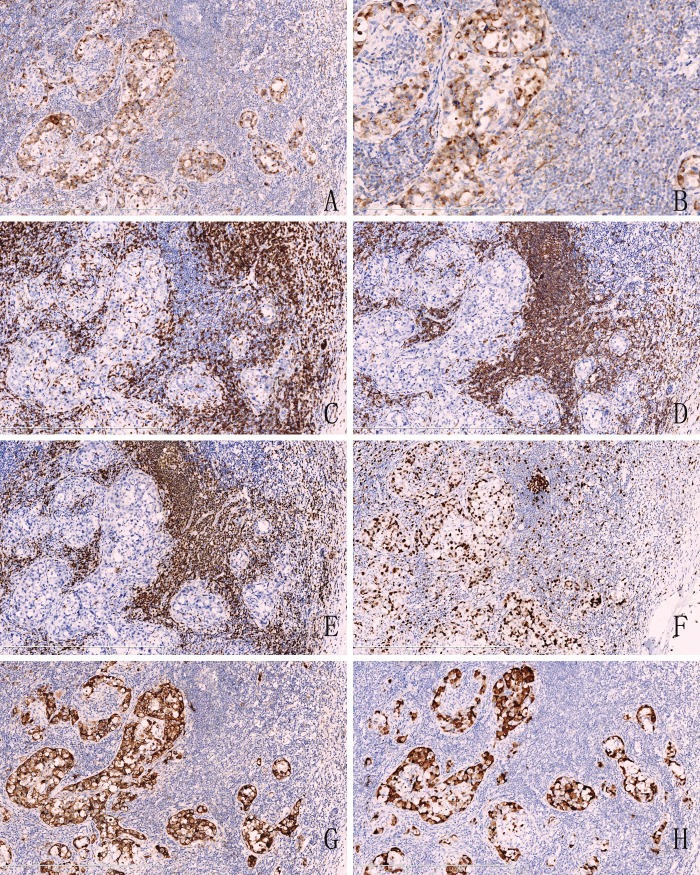
Figure 2.
Immunohistochemical analysis. A. The tumor cells were positive for cytokeratin(pan), roblastic reticulum cells showed a typical dendritic shape (Original magnification ×100). B. High magnification showed that positive reactivity of cytokeratin(pan) with Golgi-associated dot-like staining pattern. (Original magnification ×200). C. The tumor cells were negative for CD3, but T lymphocytes showed a nuclear staining (Original magnification ×100). D. CD20, E. Pax-5 were also negative in atypia tumor cells and positive for B lymphocytes. (Original magnification ×100). F. showed the high Ki-67 labeling index. (Original magnification ×100). G. The large lymphocytes are strongly CD30 positive with a membrane and Golgi pattern of staining. (Original magnification ×100). H. ALK/P80 immunohistochemistry shows strong cytoplasmic staining in the neoplastic cells. (Original magnification ×100).
Revision was performed in the Department of Pathology in The First Affiliated Hospital of China Medical University. The hematoxylin and eosin-stained sections showed that the atypical tumor cells were medium size, with abundant cytoplasm, cell nuclear was enlarged and irregular, kidney-shaped or horseshoe-shaped nuclei, and prominent smaller eosinophilic nucleoli, termed “hallmark cells” (Figure 1C). The revision confirmed that the specimen was positive for cytokeratin (pan) with a dot-like perinuclear positive staining (Figure 2B), the infiltrated cells had a strong positive reaction to CD30 (Figure 2G) and the anaplastic large cell kinase ALK/p80 protein (Figure 2H). CD15 was negative. Based on the morphologic and immunophenotypic findings, we concluded that this was a ALK-positive systemic anaplastic large cell lymphoma.
Discussion
Recent studies showed that the expression of cytokeratin (pan) had also been seen in some lymphoma, such as plasmacytoma [3], ALCL, diffuse large B-cell lymphoma [4]. Gustmann C [5] demonstrated that about 27% (5/18) ALCL showed positivity for cytokeratin. While both ALCL and metastatic carcinoma express EMA, therefore, cytokeratin (pan) and EMA immunohistochemical studies could not be utilized as markers to distinguish ALCL from metastatic carcinoma. Our case has identified that ALCL exhibits a unique dot-like staining pattern for cytokeratin (pan). Whether or not this staining pattern may be an useful aspect to definitive diagnosis of metastatic carcinoma need more case studies to illustrate.
Morphologically, the sinusoidal growth pattern is another deceptive feature of ALCL,While this is also a typical pattern of metastatic carcinoma or melanoma. If the neoplastic cells are noncohesive and contain irregular, hyperchromatic nuclei with minimal cytoplasm, it suggests the possibility of lymphoma. In this case, we need to detect ALK/P80 and CD30 to confirm the diagnosis of ALCL.
ALCL was classified as a T cell lymphoma by the World Health Organization classification [6]. However, 20-30% of ALCL showed antigen deletion of T-cell markers, especially CD3, CD5 and CD7, which was reported previously as “null cell” (expressing neither B nor T cell specific antigens). Recent studies have found that nearly all the ALCL could demonstrate T-cell receptor gene rearrangement even if the immunohistochemical analysis of T-cell markers were negative [7,8]. But this rare and diagnostically confusing immunophenotype (epithelial markers positive, B or T cell specific antigens were negative) may easily lead to misdiagnosis of the tumor as metastatic carcinoma rather than lymphoma. Pathologists must be aware of this phenomenon to avoid misdiagnosis.
In summary, this case was misdiagnosed with metastatic carcinoma because of the similarities in morphology and the positive immunoreaction toward epithelial cells markers and negative immunoreactivity of lymphocytes. However, the presence of hallmark cells and CD30, ALK/P80 positivity confirms the diagnosis of ALCL. This case emphasizes that cytokeratin expression can be a potential pitfall for confusing ALCL with metastatic carcinoma.
Acknowledgements
Authors’ contributions: Qingfu Zhang drafted the manuscript and performed the literature review, acquired photomicrographs, Jian Ming, Siyang Zhang, Bo Li, and Xue Han revised the manuscript for important intellectual content; Xueshan Qiu and Qingfu Zhang gave and reviewed the final histopathological diagnosis. All authors read and approved the final manuscript.
Conflicts of interest and source of funding
The author(s) indicated no significant relationships with, or financial interest in any commercial companies pertaining to this article.
References
- 1.Agaimy A, Kirsche H, Semrau S, Iro H, Hartmann A. Cytokeratin-positive epithelioid angiosarcoma presenting in the tonsil: a diagnostic challenge. Hum Pathol. 2012;43:1142–1147. doi: 10.1016/j.humpath.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 2.McCluggage WG, el-Agnaff M, O’Hara MD. Cytokeratin positive T cell malignant lymphoma. J Clin Pathol. 1998;51:404–406. doi: 10.1136/jcp.51.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JS, Stopyra GA, Warhol MJ, Multhaupt HA. Plasmacytoma with aberrant expression of myeloid markers, T-cell markers, and cytokeratin. J Histochem Cytochem. 2001;49:791–792. doi: 10.1177/002215540104900613. [DOI] [PubMed] [Google Scholar]
- 4.Lasota J, Hyjek E, Koo CH, Blonski J, Miettinen M. Cytokeratin-positive large-cell lymphomas of B-cell lineage. A study of five phenotypically unusual cases verified by polymerase chain reaction. Am J Surg Pathol. 1996;20:346–354. doi: 10.1097/00000478-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Gustmann C, Altmannsberger M, Osborn M, Griesser H, Feller AC. Cytokeratin expression and vimentin content in large cell anaplastic lymphomas and other non-Hodgkin’s lymphomas. Am J Pathol. 1991;138:1413–1422. [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH International Agency for Research on Cancer, World Health Organization. World Health Organization classification of tumours. Fourth Edition. IARC press; 2008. WHO classification of tumours of haematopoietic and lymphoid tissues; pp. 301–319. [Google Scholar]
- 7.Goldsby RE, Carroll WL. The molecular biology of pediatric lymphomas. J Pediatr Hematol Oncol. 1998;20:282–296. doi: 10.1097/00043426-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Foss HD, Anagnostopoulos I, Araujo I, Assaf C, Demel G, Kummer JA, Hummel M, Stein H. Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood. 1996;88:4005–4011. [PubMed] [Google Scholar]