Abstract
Although hematological disorders with salient features of thrombocytopenia have been well documented in dengue patients, the role of CD61-expressing platelets and the megakaryocytic cell lineage in the pathogenesis of dengue virus (DENV) infection remains largely unexplored. A prospective observational study was performed using blood samples and PBMCs from dengue-confirmed patients, as well as from rhesus monkeys (RM) experimentally infected with DENV. Immunohistochemical staining and FACS techniques were applied to evaluate the frequencies of CD61+ cells that contained DENV antigen. Highly enriched population of CD61+ cells was also isolated from acute DENV-infected RM and assayed for DENV RNA by quantitative RT-PCR. Results revealed that DENV antigen was found in small vesicles of varying size, and more frequently in anucleated cells associated with platelets in dengue patients. The DENV antigen-containing cells were CD61+ and appeared to share characteristics of megakaryocytes. Kinetic profiles of CD61+ cells from DENV-infected RM revealed a transient increase in CD61+CD62P+ cells early after DENV infection. DENV RNA in a highly enriched population of CD61+cells from the infected RM was observed during acute stage. Our results indicate that virus containing CD61+ cells may be directly linked to the platelet dysfunction and low platelet count characteristics of dengue patients.
Keywords: Dengue, Viremia, Thrombocytopenia, Fever, Megakaryocytes, DF, DHF
Introduction
Dengue virus (DENV) infection has been recognized among one of the most important arthropod borne human diseases. Although dengue is mainly thought to be a disease confined to tropical and subtropical regions, more than 100 countries are endemic with about 50–100 million people at risk annually. Recent outbreaks in Key West, Florida, and Brazil serve as a vivid reminder that dengue represents an immediate threat to North America [1, 2]. Due to its high incidence and morbidity, dengue has become a global public health concern.
The clinical characteristics of DENV infection include abrupt high-grade persistent fever, imbalance of hemostasis, thrombocytopenia, leucopenia, atypical lymphocytosis, and to some extent, lymphocytopenia. Although the majority of the symptoms of dengue disease are similar to other febrile viral illnesses, which are self-limiting, some of the infected patients require hospitalization, suffering serious complications that can result in death [3]. The symptoms of severe forms of DENV infection include plasma leakage, bleeding, or severe dysfunction of various organs [4]. There are four distinct dengue serotypes (DENV-1 to DENV-4), each capable of inducing dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) [5].
Hematological disorders with salient low platelet counts have been well recognized as clinical manifestation of DENV infection in patients. Thus, thrombocytopenia is a consistent laboratory finding and has been viewed as one of the critical physiological hallmarks of abnormal hemostasis in dengue patients [6, 7]. Studies performed on natural DENV-infected humans reveal two prominent scenarios that account for the thrombocytopenia: bone marrow suppression, in particular early suppression of megakaryocytopoiesis, and peripheral platelet destruction during the late febrile and early convalescent phase of disease [8–10]. Both theories are supported by a number of post-prodromic studies that have documented the presence of viral antigens on the surface of platelets, immune-complex-containing platelets in skin biopsy specimens, detection of DENV-like particles in platelets, production of platelet-associated immunoglobulin M or G (PAIgM/PAIgG) and its correlation with thrombocytopenia, bone-marrow suppression with marked reduction of megakaryocytes, and circulating readily detectable levels of immune-complex in sera of dengue patients [11–17]. In addition, valuable information has been obtained from in vitro studies [18]. These reports suggest that megakaryocytic destruction may result in immune-mediated clearance accompanied by defective production of platelets that together contribute to thrombocytopenia. However, the precise mechanisms accounting for thrombocytopenia in DENV infections remain controversial due to the lack of direct in vivo evidence. These thoughts prompted us to carry out a more detailed study designed to provide further insights on the potential cause of thrombocytopenia in DENV-infected patients and experimentally DENV-infected rhesus macaques.
Methods
Dengue patient enrollment
A total of 167 patients hospitalized with suspected or confirmed dengue virus infection at the Siriraj Hospital, a large public tertiary care center in Bangkok, from November 2006 to September 2007 [16] and from March 2009 to October 2011 were eligible for enrollment in the study. The World Health Organization grading system on dengue fever and dengue hemorrhagic fever was used to define severity of disease. Confirmation of dengue virus infection based on the clinical data, serological data and viral serotyping was performed using procedures that have been previously reported [16]. Of the 167 patients, specimens from 34 dengue confirmed cases were included in this study. There was no treatment intervention in this study. The study was approved by the Siriraj Hospital Ethics Committee and conforms to the guidelines of the Ministry of Health, Government of Thailand. Prior informed consent was obtained from all participants or a parent of pediatric patients prior to enrollment into the study.
Dengue virus infection in nonhuman primates
Rhesus monkeys (Macaca mulatta) of Indian origin (designated RM#4, RM#5 and RM#6) were infected with 1 ml of dengue virus serotype 2 (16681 strain; 107 FFU/ml) as previously described [19]. Whole blood was collected in sodium citrate anti-coagulated tubes at different time points post infection and peripheral blood mononuclear cells (PBMCs) were isolated by utilizing lymphocyte separation medium according to the manufacturer’s instructions (Mediatech, Inc., Manassas, VA, USA). All experimental protocols and procedures were conducted following approval by the Emory Institutional Animal Care and Use Committee (IACUC), and all animals were housed at the Yerkes National Primate Research Center of Emory University and cared for in conformance to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council and the Health and Human Services [20].
Collection of megakaryocytic cells in circulation
Isolation of megakaryocytes from whole blood of dengue patients was carried out using a method developed by Wilde et al. [21]. Briefly, a 0.3 ml aliquot of whole blood was passed via gravity through an assembled syringe filter holder containing a nucleopore polycarbonate membrane of 5 μm pore diameter (Fisher Scientific, Pittsburgh, PA, USA) as shown by the schematic illustrated in Supplementary Figure 1. Following two washes with saline, the membrane was removed and left to dry thoroughly before staining.
Immunostaining for dengue viral antigen and platelet/megakaryocyte markers
Smears of unfractionated whole blood and peripheral blood mononuclear cells (PBMCs) were prepared from freshly collected blood in a citrate- anti-coagulated tube. PBMCs were isolated by utilizing lymphocyte separation medium according to the manufacturer’s instructions (Mediatech, Inc., Manassas, VA, USA). Aliquots of the blood and PBMC were smeared onto slides and air-dried. Both blood smears and PBMC slides were subjected to immunofluorescence staining using an approach as previously described [17]. Images of the stained cells were captured with a Zeiss microscope equipped with an Axis 5 digital camera.
Immunohistochemical staining for the detection of dengue viral antigen in freshly isolated PBMCs was performed by employing the Vectastain ABC immunohistochemistry kits (Vector Laboratories, Inc., Burlingame, CA, USA) according to the manufacturer’s instructions. Mouse anti-E monoclonal antibody (clone 4G2), mouse anti-prM monoclonal antibody (clone 2H2) and isotype-matched control (IgG2a) antibody were utilized in the primary staining step. The stained samples were incubated with 3-amino-9-ethylcarbazole (AEC) or diaminobenzidine (DAB) as an enzyme substrate for peroxidase followed by mounting with DAPI (Invitrogen) or counterstaining with hematoxylin. For double staining of dengue viral antigen with a specific marker for platelets and megakaryocytes, freshly isolated PBMCs were fixed on slides with 4 % paraformaldehyde for 20 min and permeabilized with 0.2 % triton X-100 for 10 min at RT. The samples were treated with 0.6 % H2O2 for 30 min to block endogenous peroxidase followed by 30-min incubation with 10 % human AB serum. After two washes with PBS, the samples were treated with avidin and biotin solutions using the avidin/biotin blocking kit (Vector) according to the manufacturer’s instructions and then incubated with mouse anti-E monoclonal antibody (clone 4G2) or its isotype-matched control antibody at 4 °C overnight. The samples were washed three times with PBS and incubated with biotinylated horse anti-mouse immunoglobulins (Vector) at RT for 30 min followed by three washes with the same buffer. The samples were then incubated for 30 min each with Vectastain ABC reagent and AEC substrate (Vector Laboratories, Inc., Burlingame, CA, USA) for the development of peroxidase signals. Thereafter, the samples were washed three times with PBS, incubated with 10 % normal mouse serum for 30 min, and then labeled with FITC-conjugated mouse anti-human CD41 antibody (Genway Biotec, San Diego, CA, USA) for 1 h. Following washing with PBS, the samples were incubated with 1:250 dilution of rabbit anti-FITC antibody conjugated to alkaline phosphatase (Sigma Aldrich, St. Louis, MO, USA) and the signal was developed using Vector blue alkaline phosphatase substrate kit III in the presence of levamisole solution (Vector), an inhibitor of endogenous alkaline phosphatases. To perform double staining of megakaryocytes on the polycarbonate membranes, the staining procedure was performed as described above except that the membrane was fixed with acetone/methanol (50:50) for 90 s and 50 mM Tris, pH 7.5 was used as washing buffer. The stained membranes were mounted with DAPI reagents (Invitrogen) and the resulting images were captured with a Zeiss microscope equipped with an Axis 5 digital camera.
Flow cytometry and cell sorting from infected rhesus blood samples
In efforts to identify the kinetics by which changes that appeared in the cell lineages suspected to be targets of DENV infection, blood from rhesus macaques experimentally infected with DENV was collected in a sodium citrate tube at the indicated time points and stained with a panel of fluorochrome-conjugated antibodies with specificity for the following cell surface markers: CD45, CD3, CD20, CD14, CD41, CD61 and CD62P. The stained cells were subjected to polychromatic fluorescence-activated cell sorter (FACS) analysis using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). CD61 and CD62P (P-selectin) markers are cell surface markers expressed by platelets and megakaryocytes that also serve as markers of platelet activation, respectively. The frequency of CD61+CD62P+ cells was analyzed in the gated leukocyte subpopulation. Data were collected and analyzed using the FlowJo software (TreeStar Inc., Ashland, OR, USA).
In efforts to determine whether the CD61+ cells are the target of DENV infection, an aliquot of PMBCs from each of the three infected rhesus monkeys on days 3 and 5 post infection was stained with a panel of fluorochrome-labeled antibodies with specificity for CD3, CD20, CD14, CD16, CD41 and CD61. A FACSAria II (BD Biosciences) cell sorter was then utilized to isolate cells from the stained samples by gating out cells that expressed CD3, CD20, CD14 and CD16 and by selecting for cells that expressed CD41 and CD61. The cell sorting strategy is illustrated in Supplementary Figure 2. Aliquots of pre- and post-sorted cells were analyzed for the purity of the sorted population.
Quantitation of dengue viral RNA by real-time RT-PCR
RNA was extracted from the cell sorter-enriched population of CD41+CD61+ cells using RNeasy mini kit (QIA-GEN, Germantown, MD, USA). The resultant RNA was subjected to quantitative RT-PCR using the TaqMan RT kit (Perkin Elmer Applied Biosystem) and Bio-Rad iCycler system with a standard control for viral RNA quantitation similar to a previously described method [17, 19]. The limit of detection was approximately 100 copies of RNA equivalent viral genome per milliliter in this assay.
Results
Dengue viral antigen detected in small vesicles
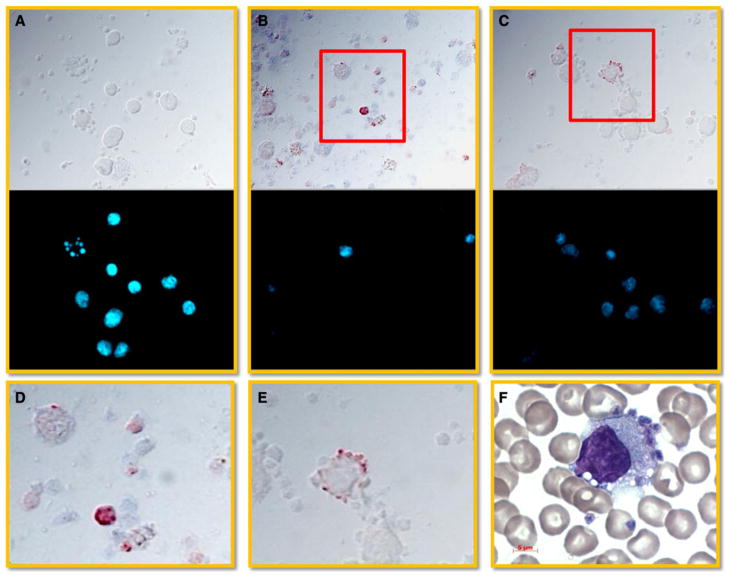
PBMCs were isolated from dengue patients, smeared onto slides and subjected to immunohistochemical staining as described in the “Methods”. Dengue viral antigen was predominantly observed within vesicle-like structures of anuclear cells of varying size (Fig. 1b, d). In addition, some of these dengue viral antigen-positive cells appeared either adherent to or associated with the surface of nucleated cells (Fig. 1c, e), presumably macrophages/monocytes (Fig. 1f and [19]. Isotype control antibody staining was used as a negative control (Fig. 1a).
Fig 1.
Detection of dengue viral antigen in small vesicles. PBMCs were isolated from dengue patients, smeared onto slides and subjected to immunohistochemical staining for the detection of dengue viral antigen. Briefly, the cells were successively incubated with either mouse anti-dengue E antibody (clone 4G2) or isotype-matched control antibody followed by horse anti-mouse immunoglobulins conjugated with biotin and developed by a streptavidin-HRP complex. 3-amino-9-ethylcarbazole (AEC) was utilized as a substrate. The stained cells were mounted with Hoechst dye and observed using a fluorescence microscope. a Isotype antibody staining. b Anuclear cells and small vesicles were the cells that were predominantly positive for dengue viral antigen. c Dengue viral antigen containing platelets/vesicles adhered onto the surface of a nuclear cell. d Enlarged view of the red rectangle area in b. e Enlarged view of the red rectangle area stained with c. f Wright’s stain revealed that platelets/vesicles adhered onto the surface with morphology similar to monocyte/macrophage. Results are shown in bright field (dengue viral antigen, red), and in fluorescent field (nucleus, blue) and are representative of 8 dengue confirmed specimens. Results obtained using specimens from healthy donors performed in parallel showed findings similar to that noted with the use of isotype-matched control antibody staining
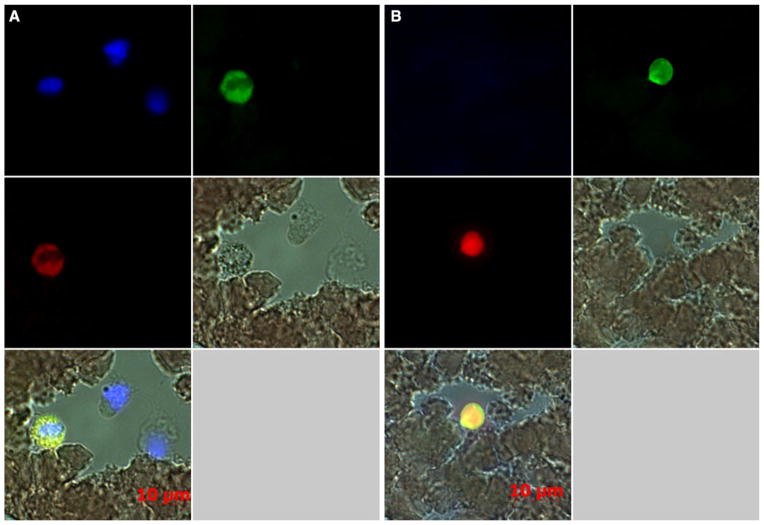
CD61+ cells were positive for dengue viral antigen
In attempts to identify the cell surface phenotype and the lineage of the cells that contained dengue viral antigen, a number of known specific surface markers were utilized to perform double staining with the blood smears from dengue patients as described in the “Methods”. To our surprise, cells that stained for CD61, a marker for platelets and megakaryocytes, were observed to be positive for dengue viral antigen (Fig. 2a, b). The nucleus of the CD61+ cells seemed to be granulated and perhaps degenerated (Fig. 2a and Supplementary Figure 3). The average number of cells with a visible nucleus in blood smears prepared from 20 randomly selected samples was determined to be 1153 ± 153 (mean ± SD). However, the frequencies of such cells were rare, with approximately 4–6 cells noted per blood smear slide from acute samples. These cells either are a unique and unidentified population of cells or are phagocytic cells that have engulfed dengue viral antigen such as monocytes/macrophages, which are also positive for CD41/CD61 [6, 19, 22]. We submit that the CD61+ anuclear cells could potentially be giant platelets that are positive for dengue viral antigen (Fig. 2b and Supplementary Figure 4A and B). As a whole, the percentage of cells positive for the dengue viral antigen varied from individual to individual ranging from 0.05 to 0.9 % (0.63 ± 0.26 %, Mean ± SD) among the CD61+ anuclear cells (Supplementary Figure 4C and D). This line of evidence further suggests that cells that express the cell surface CD61 marker are likely one of the targets during acute DENV infection.
Fig 2.
CD61+ cells were positive for dengue viral antigen. Blood smears prepared from dengue patients were subjected to double immunofluorescence staining for dengue viral antigen and CD61 as described in the “Methods” section. The stained cells were mounted with DAPI and observed using a Zeiss fluorescence microscope equipped with an Axis 5 digital camera. a Granular or degenerated cell with clumping nuclei was positive for dengue viral antigen and CD61 marker. b Anuclear cells were highly positive for dengue viral antigen and CD61. Results are representative of 20 specimens showing bright field, fluorescent field and merged images from the two different areas on the blood slides. Dengue viral antigen (red); CD61 (green); nucleus (blue); co-localization of dengue viral antigen with CD61 (yellow)
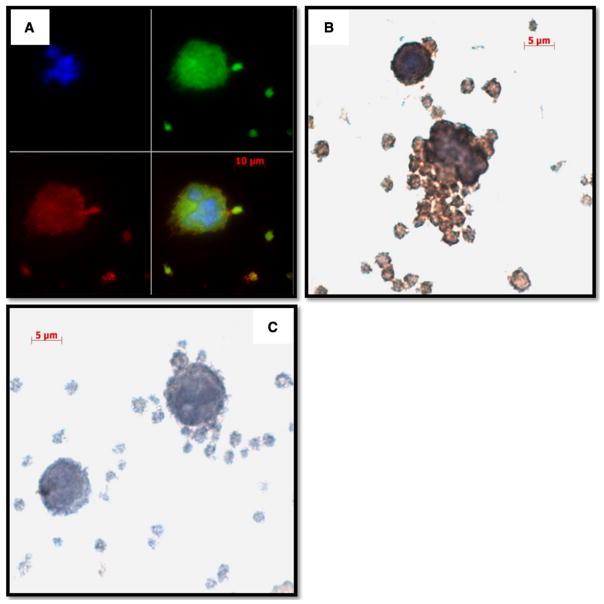
Dengue viral antigen containing megakaryocytic cells in freshly isolated PBMC
Dengue viral antigen has been reported to be detectable in the cytoplasm of anucleated cells in association with an increase in the number of megakaryocytes in the capillaries of various organs in studies of autopsy specimens [23]. In addition, it has been suggested that DENV may infect megakaryocytes and alter transcriptional events including differentiation and nucleic acid synthesis [24, 25]. As platelets are the product of megakaryocytes, it was reasoned that a study of markers of megakaryocytes in fresh PBMC specimens from dengue patients would thus be appropriate.
Immunofluorescence staining was thus utilized with antibodies specific for dengue viral antigen and cell surface markers of platelets. These studies revealed that cells in the process of releasing vesicles (presumably platelets) were positive for the viral antigen (Fig. 3a). These cells were undergoing platelet production and were also positive for dengue viral antigen (Fig. 3b). As expected, isotype control staining did not show the dengue viral antigen signal (Fig. 3c). Immunohistochemical staining also revealed cells with odd nuclei and loose cytoplasm as well as anucleated cells that were associated with platelets that appeared to contain dengue viral antigen (Supplementary Figure 5A and C). The specificity of dengue viral antigen detection was confirmed using an isotype control antibody (Supplementary Figure 5B and D). Moreover, double immunohistochemical staining for a platelet-specific marker and dengue viral antigen demonstrated that the PBMC specimens from dengue patients contained condensed cells with small cytoplasm-to-nucleus ratios (which were capable of binding platelets and pro-platelet formation) harbored dengue viral antigen (Supplementary Figure 6A and B, respectively). These were not observed in specimens stained with the antibody isotype control (Supplementary Figure 6C). The platelet-specific marker and dengue viral antigen were observed mainly at the outer edges of the cytoplasm and rarely inside the cell (Supplementary Figure 7A). Occasionally, cells with low cytoplasm-to-nucleus ratios were seen as well (Supplementary Figure 7B); the morphological appearances of these cells were apparently very similar to those observed in Figure 2a. These findings together strongly suggest that an association of dengue viral antigen with megakaryocytes exists.
Fig 3.
Dengue viral antigen containing CD61+ cells show characteristics of megakaryocytes. PBMC smears prepared from dengue patients were subjected to the processes as described in the “Methods”. a Double immunofluorescence staining for dengue viral antigen (red) and CD61 (green); b Immunohistochemical staining for dengue viral antigen (brown) in the presence of hematoxylin counterstaining (blue); and c staining with isotype-matched control antibody served as a negative control. Cells in the process of releasing vesicles and budding progenies were positive for dengue viral antigen and CD61. Representative images from 20 specimens are shown
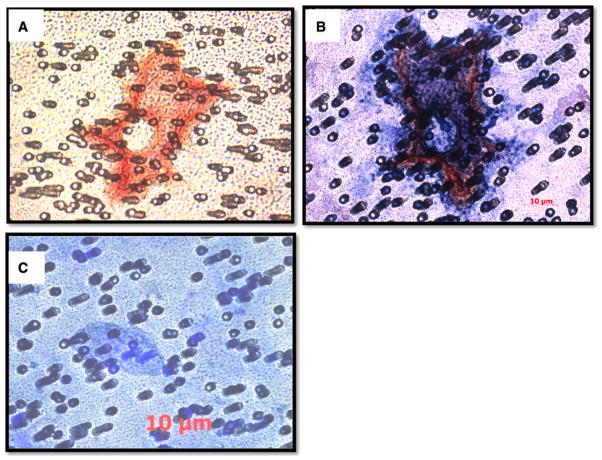
Detection of dengue viral antigen in circulating megakaryocytes
Initial attempts to isolate the megakaryocytes in circulation with frozen and thawed PBMC from dengue patients revealed that features such as membranes of megakaryocytic cells in the process of ongoing production of platelets were lost. Thus, special care was taken with freshly obtained samples in efforts to preserve the integrity of the cells due to the large cell volume and mass of megakaryocytes. A majority of these enriched population of cells appeared not to contain a visible nucleus, but some of them did stain positive for dengue viral antigen as described above. The finding of giant cells that stained positive for both the dengue viral antigen and the megakaryocytic cell surface marker CD41 was rare (Fig. 4a, b). The specificity of this detection was verified by staining with isotype control antibody (Fig. 4c). These results lend support to the early observations that large numbers of megakaryocytes are in the capillaries of dengue patients and that megakaryocytes could be infected by DENV. Furthermore, the results also suggest that alteration of the characteristics of megakaryocytes and the physiology of the body’s fluid composition secondary to DENV infection may result in the mobilization of young megakaryocytes from the bone marrow into the circulating peripheral blood.
Fig 4.
Detection of dengue viral antigen in megakaryocytic cells in the circulation. Megakaryocytic cells in whole blood from dengue patients were collected on a nucleopore polycarbonate membrane of 5 μm pore diameter as described in the “Methods”. The membrane was then processed for double immunofluorescence staining for dengue viral antigen (red) and CD41 (dark blue), a marker for platelets and megakaryocytes. Representative results from 26 dengue-confirmed specimens are shown. a Cells stained for dengue viral antigen only. b Cells stained for both dengue viral antigen and CD41. c Cells stained with isotype-matched control antibody which served as negative control
Kinetic profile of CD61+ cells and their association with dengue viral RNA following dengue virus infection
Since it is difficult to perform kinetic studies of the frequencies of CD61+ cells during acute infection on dengue patients, we took advantage of samples from the recently described nonhuman primate dengue model reported by our lab [19] and performed the kinetic studies. Whole blood from three DENV-infected rhesus monkeys was subjected to staining and analysis by flow cytometry using the gating strategy as shown in Supplementary Figure 8. Results showed that CD61+ cells particularly in the activated form (CD61+CD62P+) were detected predominantly within the gated monocyte population and to a lesser extent within the gated granulocyte and lymphocyte populations, respectively (Supplementary Table 1). The frequency of these CD61+CD62P+ cells appeared to be increased in all the gated cell populations early after DENV infection (Supplementary Table 1).
To verify the presence of dengue viral materials in these CD61+ cells, the CD61+ cells were cell sorter purified from PBMC obtained post days 3 and 5 of infection from the rhesus monkeys. The strategy for the cell sorting is shown in Supplementary Figure 2. The purity of the sorted cells ranged from 85 to 95 % as determined by FACS (data not shown). Detection of dengue viral RNA from the sorted cells was performed as described in the “Methods”. The contents of the viral RNA in these isolated CD61+ cells were on average, 100 copies per cell on day 3, while almost undetectable on day 5 after infection (Table 1). These results suggest that the CD61+ cells could serve to promote virus dissemination in dengue patients that may be followed by subsequent engulfment by phagocytic cells [6, 19].
Table 1.
Dengue viral RNA in CD41+ CD61+ sorted cells from infected PBMCa
| Days | Category | RM#4 | RM#5 | RM#6 |
|---|---|---|---|---|
| 3 | Total cells | 3,433 | 1,076 | 837 |
| Total viral RNA copies | 3.38 × 105 | 1.4 × 105 | 7.65 × 104 | |
| RNA copies/cell | 98.5 | 93 | 91.4 | |
| 5 | Total cells | 7,839 | 6,230 | 5,937 |
| Total viral RNA copies | 9.75 × 103 | 9.1 × 103 | 4.5 × 103 | |
| RNA copies/cell | 1.24 | 1.5 | 0.76 |
PBMCs were collected from three dengue virus-infected rhesus monkeys (designated RM#4, RM#5 and RM#6) on days 3 and 5 post infection and subjected to fluorescence-activated cell sorting for CD41+CD61+cells. The sorted cells were enumerated and determined for the presence of dengue viral RNA by real-time RT-PCR
Discussion
Dengue is a timing disease that is conceivably a consequence of immune-mediated illness. Patients with dengue do not die at the time of viremia but rather at a later point as a result of the post-viremic shock, which occurs at a variable time point once the platelet count nadirs. The role of hematological imbalance in disease development remains unclear.
The loss of platelets in dengue disease could result from enhanced immune-complex mediated complement lysis, IgG-Fcγ II-mediated phagocytosis by macrophage or dendritic cells, direct engagement with virus, or anti-platelet IgG-induced fragmentation via the induction of reactive oxygen species [16, 17, 26–29]. Recent results show that platelet–monocyte aggregation is one of the most significant events during the defervescent stage [22]. In addition, an increased level of phagocytosis of DENV-induced apoptotic platelets by macrophages was observed during secondary DENV infection [30], and dengue viral antigen containing platelets were engulfed by phagocytic cells [6]. For these reasons, the timing of immune-complex formation and the development of cytokine storms in the context of disease severity need further study.
The significance of circulating immune complexes in dengue has been well established [11, 32]. As generation of immune complexes between antibody and antigen is dependent on non-covalent forces, which are highly temperature-sensitive [31], it is possible that the formation of circulating immune complexes found in the plasma of dengue patients could occur more efficiently at normal body temperature than at higher temperatures. Interestingly, the maximum levels of immune complexes are found in patients when the fever subsides and platelet counts reach a nadir [33], while higher numbers of platelets and viral titers were observed during the high fever period [34]. In addition, PAIgM/PAIgG has been investigated [35] and found in acute dengue patients and declines to undetectable levels after viremia has resolved [36]. The presence of DENV-like particles in platelets of dengue patients and in infected rhesus monkeys has also been documented [16, 17]. These data suggest that the direct attack of platelets by DENV may be possible and that the PAIgM/PAIgG may react to dengue viral antigen expressed on the surface of platelets [13, 27, 35]. Mitrakul et al. [37] demonstrated that radio-labeled platelets showed increased localization to the liver rather than in the spleen of dengue patients. In addition, the deposition of dengue viral antigen, human immunoglobulin, and C3 have each been shown on the surface of platelets in dengue patients [14, 38], suggesting that immune complexes may be alternatively transported by red blood cells carrying CR1 on their surface to the liver or spleen for destruction by phagocytes [39]. These findings together suggest that the immune-mediated injury is the underlying mechanism of platelet destruction in peripheral blood of dengue patients. This may explain why recipients who were transfused with blood components such as RBC and platelets from donors prior to the donor’s manifesting symptoms of DENV infection led to the incidence of severe dengue disease [40], and may partially account for the low recovery of infectious DENV in platelets isolated from dengue patients at the stage of shock [41], despite the high percentage of dengue viral RNA detected during the fever phase [12, 16].
Detection of bone marrow components, such as megakaryocytic cells, in peripheral blood of dengue patients is of interest, since hypocellularity during the early stages of infection in the bone marrow of acute dengue patients has been previously documented [8, 9]. Our kinetic studies using bone marrows from DENV-infected rhesus monkeys [17] demonstrated a transient surge of bone marrow cellularity, together with temporarily increased CD41+CD61+ cells during the course of acute DENV infection. In addition, we observed the presence of monocytes that had previously engulfed activated platelets containing dengue viral antigen [19]. Furthermore, the kinetics of dengue virus replication in highly enriched population of cell sorter purified CD41+CD61+ cells revealed that dengue viral RNA was readily detectable on day 3, but declined by day 5 after infection, suggesting that CD41+CD61+ cells with megakaryocytic characteristics may be among the initial target cells for the amplification and/or dissemination of dengue virus during the early phase of infection, and these cells are cleared off from the circulation by phagocytic cells such as monocytes during the late phase of infection. Therefore, these particular cells may serve as one of the early primary targets for DENV infection and may be responsible for thrombocytopenia in dengue disease. A potential role of megakaryocytes as targets for a number of other viruses has also been reported previously. For example, a study on Junin virus infection which induces acute thrombocytopenia in Argentine hemorrhagic fever showed typical Junin viral particles that were seen only in platelet demarcation channels of megakaryocytes, but not in other bone marrow cells in infected guinea pigs [42]. Hematopoietic progenitor cells (HPC) normally reside in the bone marrow, but can be mobilized into the peripheral blood by stimulation with cytokines/chemokines [43, 44]. These cytokine/chemokine-induced signals are produced by cells directly in response to invading pathogens. CD41+CD61+ cells, most likely belonging to the megakaryocytic cell lineage, normally account for 1 % of the bone marrow but can change dramatically in certain diseases or infections, causing their propulsion into the peripheral circulation. The results of this study are consistent with the autopsy observations described in the early 1960s [45, 46]. This phenomenon of the mobilization of bone marrow resident cells into the blood may likely be stimulated by the levels and combinations of cytokines/chemokines and may possibly be related to the bone pain, or the so-called breakbone fever, in dengue patients. These findings suggest that DENV-like particles or antigen-positive platelets are likely derived from the megakaryocytes or one of their progenitor cells. However, this hypothesis needs further study.
It has been suggested that there is an ambivalent relationship between platelets and viruses. Platelets may serve as a shelter beneficial for the spreading of virus, but on the other hand, they may facilitate the destruction of dengue and the clearance of platelets [47–50]. Interestingly, recent results showed the presence of DENV-like particles inside platelets of dengue patients [16] and in infected rhesus monkeys [17], suggesting to some extent that some of the events, important for the DENV life cycle, may occur in platelets or in its precursor cells, megakaryocytes. However, it has been known that platelets are not only able to engulf foreign particles, such as latex beads, bacteria, and viruses [51–53], but platelets readily associate and adhere to immune cells, in particular, monocytes or macrophages [54]. Moreover, the engulfment of platelets by monocytes has been shown to occur in samples from both dengue patients and experimentally dengue-infected nonhuman primates [6, 19, 22], and that infected leukocytes were always present on the last day or the day after disappearance of the virus from plasma [55]. A model, therefore, can be drawn from these observations: platelets may provide a perfect shield for the DENV during the early time period of infection, and PAIgM/PAIgG production is likely involved during later time points of the infection to assist host immune defense mechanism to clear the body of DENV. However, whether PAIgM/PAIgG antibodies enhancing the clearance accounting for the shorter duration of viremia in secondary infection remains to be demonstrated. In addition, our findings could have a significant impact on the evaluation of safety and efficacy of live-attenuated DENV vaccine that has predominantly relied on the levels of viremia and the levels of virus neutralization antibody titers, respectively. However, the window for DENV detection in plasma is very short and the antibody neutralizing titer is detected late after the second dose in clinical trials, potentially due to the fact that the cellular niche for circulating virus is not well defined [56]. Consequently, our findings of dengue viral products in the platelets may suggest a new strategy for the development of dengue vaccine, and potentially assist in the evaluation of DENV vaccine safety and efficacy.
Supplementary Material
Acknowledgments
We would like to thank Korakot Polsrila at the Center of Excellence for Flow Cytometry and clinical staffs at the Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, for sample collections and laboratory confirmation of dengue. The research was supported in part by Emory SOM start-up fund, Thailand Research Fund for Senior Research Scholar, Robert E. Shop International Fellowship, the U19 Pilot Project Funds U19 AI057266 (RFA-AI-02-042), NIH/SERCEB, Emory URC grants, and the NCRR p51 support to the Yerkes National Primate Research Center DRR000165.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12185-012-1175-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no competing financial interests.
Contributor Information
Sansanee Noisakran, Department of Pathology and Laboratory Medicine, Emory Vaccine Center, Emory University School of Medicine, Dental School Building, Room 429, 1462 Clifton Road, Atlanta, GA 30322, USA. Medical Biotechnology Research Unit, National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Pathumthani 12120, Thailand.
Nattawat Onlamoon, Center of Excellence for Flow Cytometry, Mahidol University, Bangkok, Thailand.
Kovit Pattanapanyasat, Center of Excellence for Flow Cytometry, Mahidol University, Bangkok, Thailand.
Hui-Mien Hsiao, Department of Pathology and Laboratory Medicine, Emory Vaccine Center, Emory University School of Medicine, Dental School Building, Room 429, 1462 Clifton Road, Atlanta, GA 30322, USA.
Pucharee Songprakhon, Center of Excellence for Flow Cytometry, Mahidol University, Bangkok, Thailand. Office for Research and Development, Mahidol University, Bangkok, Thailand.
Nasikarn Angkasekwinai, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Kulkanya Chokephaibulkit, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Francois Villinger, Department of Pathology and Laboratory Medicine,Emory Vaccine Center, Emory University School of Medicine, 954 Gatewood Road, Atlanta, GA 30329, USA. Division of Pathology, Yerkes National Primate Research Center, 954 Gatewood Road, Atlanta, GA 30329, USA.
Aftab A. Ansari, Department of Pathology and Laboratory Medicine, Emory Vaccine Center, Emory University School of Medicine, 101 Wooddruff Circle, Atlanta, GA 30322, USA
Guey Chuen Perng, Email: gperng@emory.edu, Department of Pathology and Laboratory Medicine, Emory Vaccine Center, Emory University School of Medicine, Dental School Building, Room 429, 1462 Clifton Road, Atlanta, GA 30322, USA.
References
- 1.Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA. 2008;299:214–6. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Locally acquired Dengue—Key West, Florida, 2009–2010. MMWR. 2010;59:577–581. [PubMed] [Google Scholar]
- 3.Gregory CJ, Santiago LM, Arguello DF, et al. Clinical and laboratory features that differentiate dengue from other febrile illnesses in an endemic area—Puerto Rico, 2007–2008. Am J Trop Med Hyg. 2010;82:922–9. doi: 10.4269/ajtmh.2010.09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Dengue: guidelines for diagnosis, treatment, prevention and control. 2009. [PubMed] [Google Scholar]
- 5.WHO. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. World Health Organization; Geneva: 2008. [Google Scholar]
- 6.Tsai J-J, Liu L-T, Chang K, et al. The importance of hematopoietic progenitor cells in dengue. Ther Adv Hematol. 2011;3(1):59–71. doi: 10.1177/2040620711417660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srichaikul T, Nimmannitya S. Haematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol. 2000;13:261–76. doi: 10.1053/beha.2000.0073. [DOI] [PubMed] [Google Scholar]
- 8.Nelson ER, Bierman HR, Chulajata R. Hematologic findings in the 1960 hemorrhagic fever epidemic (Dengue) in Thailand. Am J Trop Med Hyg. 1964;13:642–9. doi: 10.4269/ajtmh.1964.13.642. [DOI] [PubMed] [Google Scholar]
- 9.La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249–70. doi: 10.1016/s0950-3536(05)80240-9. [DOI] [PubMed] [Google Scholar]
- 10.Na-Nakorn S, Suingdumrong A, Pootrakul S, Bhamarapravati N. Bone-marrow studies in Thai haemorrhagic fever. Bull World Health Organ. 1966;35:54–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Theofilopoulos AN, Wilson CB, Dixon FJ. The Raji cell radio-immune assay for detecting immune complexes in human sera. J Clin Invest. 1976;57:169–82. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito M, Oishi K, Inoue S, et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin Exp Immunol. 2004;138:299–303. doi: 10.1111/j.1365-2249.2004.02626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi K, Saito M, Mapua CA, et al. Dengue illness: clinical features and pathogenesis. J Infect Chemother. 2007;13:125–33. doi: 10.1007/s10156-007-0516-9. [DOI] [PubMed] [Google Scholar]
- 14.Boonpucknavig S, Vuttiviroj O, Bunnag C, et al. Demonstration of dengue antibody complexes on the surface of platelets from patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 1979;28:881–4. [PubMed] [Google Scholar]
- 15.Noisakran S, Chokephaibulkit K, Songprakhon P, et al. A reevaluation of the mechanisms leading to dengue hemorrhagic fever. Ann N Y Acad Sci. 2009;1171(Suppl 1):E24–35. doi: 10.1111/j.1749-6632.2009.05050.x. [DOI] [PubMed] [Google Scholar]
- 16.Noisakran S, Gibbons RV, Songprakhon P, et al. Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health. 2009;40:253–62. [PubMed] [Google Scholar]
- 17.Noisakran S, Onlamoon N, Hsiao HM, et al. Infection of bone marrow cells by dengue virus in vivo. Exp Hematol. 2012;40(250–259):e254. doi: 10.1016/j.exphem.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, He R, Patarapotikul J, et al. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology. 1995;213:254–7. doi: 10.1006/viro.1995.1567. [DOI] [PubMed] [Google Scholar]
- 19.Onlamoon N, Noisakran S, Hsiao HM, et al. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115:1823–34. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Research CoLS, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academic Press; 1996. [Google Scholar]
- 21.Wilde NT, Burgess R, Keenan DJ, et al. The effect of cardio-pulmonary bypass on circulating megakaryocytes. Br J Haematol. 1997;98:322–7. doi: 10.1046/j.1365-2141.1997.2373055.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsai JJ, Jen YH, Chang JS, et al. Frequency alterations in key innate immune cell components in the peripheral blood of dengue patients detected by FACS analysis. J Innate Immun. 2012;3:59–71. doi: 10.1159/000322904. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Summaries of Papers Presented at the WHO Inter-Regional Seminar on Mosquito-borne Haemorrhagic Fevers in the South-East Asia and Western Pacific Regions. Bulletin. 1966:35. [Google Scholar]
- 24.Bierman HR, Nelson ER. Hematodepressive virus diseases of Thailand. Ann Intern Med. 1965;62:867–84. doi: 10.7326/0003-4819-62-5-867. [DOI] [PubMed] [Google Scholar]
- 25.Nelson ER, Tuchinda S, Bierman HR, Chulajata R. Haematology of Thai haemorrhagic fever (dengue) Bull World Health Organ. 1966;35:43–4. [PMC free article] [PubMed] [Google Scholar]
- 26.Nardi M, Tomlinson S, Greco MA, et al. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551–61. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 27.Honda S, Saito M, Dimaano EM, et al. Increased phagocytosis of platelets from patients with secondary Dengue virus infection by human macrophages. Am J Trop Med Hyg. 2009;80:841–5. [PubMed] [Google Scholar]
- 28.Avirutnan P, Mehlhop E, Diamond MS. Complement and its role in protection and pathogenesis of flavivirus infections. Vaccine. 2008;26(Suppl 8):I100–7. doi: 10.1016/j.vaccine.2008.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boonnak K, Slike BM, Burgess TH, et al. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol. 2008;82:3939–51. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonzo MT, Lacuesta TL, Dimaano EM, et al. Platelet apoptosis and apoptotic platelet clearance by macrophages in secondary dengue virus infections. J Infect Dis. 2012;205(8):1321–9. doi: 10.1093/infdis/jis180. [DOI] [PubMed] [Google Scholar]
- 31.Lipschultz CA, Yee A, Mohan S, et al. Temperature differentially affects encounter and docking thermodynamics of antibody–antigen association. J Mol Recognit. 2002;15:44–52. doi: 10.1002/jmr.559. [DOI] [PubMed] [Google Scholar]
- 32.Sobel AT, Bokisch VA, Muller-Eberhard HJ. C1q deviation test for the detection of immune complexes, aggregates of IgG, and bacterial products in human serum. J Exp Med. 1975;142:139–50. doi: 10.1084/jem.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruangjirachuporn W, Boonpucknavig S, Nimmanitya S. Circulating immune complexes in serum from patients with dengue haemorrhagic fever. Clin Exp Immunol. 1979;36:46–53. [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 35.Oishi K, Inoue S, Cinco MT, et al. Correlation between increased platelet-associated IgG and thrombocytopenia in secondary dengue virus infections. J Med Virol. 2003;71:259–64. doi: 10.1002/jmv.10478. [DOI] [PubMed] [Google Scholar]
- 36.Lin CF, Wan SW, Cheng HJ, et al. Autoimmune pathogenesis in dengue virus infection. Viral Immunol. 2006;19:127–32. doi: 10.1089/vim.2006.19.127. [DOI] [PubMed] [Google Scholar]
- 37.Mitrakul C, Poshyachinda M, Futrakul P, et al. Hemostatic and platelet kinetic studies in dengue hemorrhagic fever. Am J Trop Med Hyg. 1977;26:975–84. doi: 10.4269/ajtmh.1977.26.975. [DOI] [PubMed] [Google Scholar]
- 38.Phanichyakarn P, Pongpanich B, Israngkura PB, et al. Studies on dengue hemorrhagic fever. III. Serum complement (C3) and platelet studies. J Med Assoc Thai. 1977;60:301–6. [PubMed] [Google Scholar]
- 39.Emlen W, Carl V, Burdick G. Mechanism of transfer of immune complexes from red blood cell CR1 to monocytes. Clin Exp Immunol. 1992;89:8–17. doi: 10.1111/j.1365-2249.1992.tb06869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tambyah PA, Koay ES, Poon ML, et al. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med. 2008;359:1526–7. doi: 10.1056/NEJMc0708673. [DOI] [PubMed] [Google Scholar]
- 41.Scott RM, Nisalak A, Cheam-U-Dom U, Seridhoranakul S, Nimmannitya S. A preliminary report on the isolation of viruses from the platelets and leukocytes of dengue patients. J Infect Dis. 1980;141(1):1–6. doi: 10.1093/infdis/141.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Carballal G, Rodriguez M, Frigerio MJ, et al. Junin virus infection of guinea pigs: electron microscopic studies of peripheral blood and bone marrow. J Infect Dis. 1977;135:367–73. doi: 10.1093/infdis/135.3.367. [DOI] [PubMed] [Google Scholar]
- 43.Shang X, Cancelas JA, Li L, et al. R-Ras and Rac GTPase crosstalk regulates hematopoietic progenitor cell migration, homing, and mobilization. J Biol Chem. 2011;286:24068–78. doi: 10.1074/jbc.M111.226951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 45.Bhamarapravati N, Boonyapaknavik V, Nimsomburana P. Pathology of Thai haemorrhagic fever: an autopsy study. Bull World Health Organ. 1966;35:47–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Piyaratn P. Pathology of Thailand epidemic hemorrhagic fever. Am J Trop Med Hyg. 1961;10:767–72. doi: 10.4269/ajtmh.1961.10.767. [DOI] [PubMed] [Google Scholar]
- 47.Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clemetson KJ. Platelets and pathogens. Cell Mol Life Sci. 2010;67:495–8. doi: 10.1007/s00018-009-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flaujac C, Boukour S, Cramer-Borde E. Platelets and viruses: an ambivalent relationship. Cell Mol Life Sci. 67:545–556. doi: 10.1007/s00018-009-0209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satchell CS, Cotter AG, O’Connor EF, et al. Platelet function and HIV: a case–control study. AIDS. 2010;24:649–57. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 51.Zucker-Franklin D. The effect of viral infections on platelets and megakaryocytes. Semin Hematol. 1994;31:329–37. [PubMed] [Google Scholar]
- 52.White JG, Clawson CC. Effects of small latex particle uptake on the surface connected canalicular system of blood platelets: a freeze-fracture and cytochemical study. Diagn Histopathol Publ Assoc Pathol Soc Great Britain Irel. 1982;5:3–10. [PubMed] [Google Scholar]
- 53.Youssefian T, Drouin A, Masse JM, et al. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–9. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 54.Michelson AD. Platelets. San Diego, CA: Academic Press, Elsevier Inc; 2007. [Google Scholar]
- 55.Marchette NJ, Halstead SB, Falkler WA, Jr, et al. Studies on the pathogenesis of dengue infection in monkeys. 3. Sequential distribution of virus in primary and heterologous infections. J Infect Dis. 1973;128:23–30. doi: 10.1093/infdis/128.1.23. [DOI] [PubMed] [Google Scholar]
- 56.Hombach J, Cardosa MJ, Sabchareon A, et al. Scientific consultation on immunological correlates of protection induced by dengue vaccines report from a meeting held at the World Health Organization 17–18 November 2005. Vaccine. 2007;25:4130–9. doi: 10.1016/j.vaccine.2007.02.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.