Abstract
The physiological state of animals in many taxonomic groups can be modified via social interactions including simply receiving communication signals from conspecifics. Here, we explore whether the catecholaminergic system of female songbirds responds during social interactions that are limited to song reception. We measured the protein product of an immediate early gene (ZENK) within three catecholaminergic brain regions in song-exposed (n = 11) and silence-exposed (n = 6) female zebra finches (Taeniopygia guttata). ZENK-ir induction was quantified in catecholamine cells as well as within cells of unknown phenotypes in three brain regions that synthesize catecholamines, the ventral tegmental area, the periaqueductal gray and the locus coeruleus (LoC). Our results reveal that there are no significant differences in the overall number of cells expressing ZENK between song- and silence-exposed females. However, when we limited our measurements to catecholamine-containing cells, we noticed a greater number of catecholamine-containing cells expressing ZENK within the LoC in the song-exposed females compared to silence-exposed females. Furthermore, we measured five behaviors during the song- and silence-exposed period, as behavioral differences between these groups may account for differences in the coinduction of ZENK and TH-ir. Our results reveal that there were no statistically significant differences in the five measured behaviors between song- and silence-exposed females. Our study demonstrates that noradrenergic cells within the LoC are involved in the neural architecture underlying sound perception and that cells within the catecholaminergic system are modulated by social interactions, particularly the reception of signals used in animal communication.
Key Words: Songbird, Catecholamine, Noradrenaline, Dopamine, Communication, Immediate early gene
Introduction
Animal communication requires both the production of a species-typical communication signal as well as the reception of this signal, principally by conspecifics [Bradbury and Vehrencamp, 1998]. The reception of communication signals can trigger a behavioral change in the animal receiving the signal but, as increasingly more studies demonstrate, such behavioral changes only occur when the receiver's physiological state enables them [Wilczynski et al., 2005; Ball and Balthazart, 2008; Wilczynski and Lynch, 2011]. Furthermore, underlying physiological factors not only modulate the behavioral response during social interactions but are also modulated by social interactions such as receiving communication signals [Bentley et al., 2000; Chu and Wilczynski, 2001; Lynch and Wilczynski, 2006; Goymann, 2009; Maruska and Fernald, 2010]. This is the case even when communication signals are limited to a single sensory modality with which to receive the appropriate communication signals [Burmeister and Wilczynski, 2000; Lynch and Wilczynski, 2006]. While reproductive hormones have been repeatedly demonstrated to be a physiological factor that both modulates and is modulated by signal production and signal reception, it is very likely that other physiological systems can also be modified during communication. The catecholaminergic system is one such candidate.
Catecholamines, such as dopamine and noradrenaline, are evolutionary ancient and well conserved across many taxa [Smeets and Reiner, 1994]. These neuromodulators influence sensory processing, motor responses, and cognitive processes such as attention, arousal, motivation and decision making in a wide range of vertebrates [Robbins and Everitt, 1995; Berridge and Waterhouse, 2003; Cardin and Schmidt, 2004; Hurley et al., 2004; Aston-Jones and Cohen, 2005; Solis and Perkel, 2006; Riters and Pawlish, 2007; Gale et al., 2008; Lynch and Ball, 2008; St. Onge and Floresco, 2009; Winter et al., 2009; Mesce and Pierce-Shimomura, 2010; Jocham et al., 2011]. Here, we examine the responses of catecholamine-containing cells after exposure to male mate signals (i.e. song). Our aim is to ascertain whether these cell groups are responsive to the reception of sound. In this preliminary investigation, we examine this possibility by exploring whether these cells express an immediate early gene (IEG) after the reception of conspecific songs. Catecholamine cell groups that respond after reception of species-typical communication signals may provide fundamental insights into whether catecholamines act to enable behavior during critical animal communication events, such as sexual selection via mate choice.
In a wide range of vertebrates including songbirds, catecholamines are synthesized within the ventral tegmental area (VTA; previously referred to as the area ventralis of Tsai; group A10 in the nomenclature) [Reiner et al., 2004], the periaqueductal gray (PAG; previously referred to as midbrain central gray; group A11), and the locus coeruleus (LoC; group A6). The brainstem region chiefly responsible for noradrenaline production is the LoC, whereas the midbrain regions (VTA and PAG), primarily synthesize dopamine (fig. 1a, b) [Smeets and Reiner, 1994]. These catecholamine-containing cell groups have a profound influence on a large range of higher order processes including auditory responses to song [Cardin and Schmidt, 2004; Lynch and Ball, 2008]. In female canaries (Serinus canaria) deficits in noradrenaline result in increased errors in behavioral responses to song such that the bird tends to miss the detection of relevant song types [Appeltants et al., 2002]. This error in behavioral response is mirrored by deficits in auditory responses to song (as measured by IEG induction) in the auditory forebrain, the putative secondary auditory cortex [Lynch and Ball, 2008]. In female European starlings (Sturnus vulgaris), deficits in noradrenaline cause females to increase time spent near nest boxes broadcasting male songs [Riters and Pawlisch, 2007], and noradrenaline reduction decreases estradiol-dependent selectivity in behavioral responses to song in female zebra finches [Vyas et al., 2009]. These studies indicate a clear role for noradrenaline in regulating female behavioral and auditory responses to song. Here, we explore whether the reverse is also the case by exploring whether catecholamines are regulated by the reception of conspecific communication signals. To that end, we use communication signals to probe the song-responsive properties of catecholamine-containing cell groups within the female brain.
Fig. 1.
a, b Three catecholaminergic brain regions investigated in this study. a VTA and PAG in a coronal plane of section. b LoC in a coronal plane of section. Scale bar = 1 mm.
We investigate whether catecholaminergic cell groups respond during signal reception in female zebra finches (Taeniopygia guttata) by colocalizing an IEG within catecholaminergic cells. IEG expression, specifically zenk mRNA (an acronym for zif-268,egr-1, ngfi-a, and krox 24) or its protein product, ZENK, are frequently used in studies of animal communication including studies in songbird communication [Mello et al., 1992; Mello and Riberio, 1998; Gentner et al., 2001; Bailey et al., 2002; Sockman et al., 2002; Leitner et al., 2005]. We examined the expression of the ZENK protein product within three catecholamine-producing brain regions, i.e. VTA, PAG and LoC in song- and silence-exposed females.
Materials and Methods
Female zebra finches were purchased from a local supplier and placed in single-sex housing at Johns Hopkins University, Baltimore, Md., USA in cages measuring 49 × 95 × 51 cm with no more than 6 birds per cage. The birds were fed finch food, provided with water ad libitum and kept on a light schedule of 14L:10D. The females were randomly placed into one of two groups: a negative control (silence) and treatment (conspecific song exposure). Song stimuli were recorded from 5 unfamiliar males at 20 kHz using a Realistic microphone (Iotech DaqBoard 2000) and custom software written by Amish Dave. The male zebra finches were placed into acoustic isolation chambers to record song, such that songs were recorded with no background noise. However, because the males were isolated, the recorded songs were undirected song, which can be less attractive to female zebra finches as compared to directed song [Woolley and Doupe, 2008]. We captured hundreds of examples of song from each male. No single song appeared more than once within the 30-min exposure time. Song stimuli were assembled using Adobe Audition 1.5 with approximately 20 s of song per minute. The total duration of song presentation was roughly 10 min whereas the total silence was approximately 20 min. Songs were broadcast into the acoustic chamber using RCA speakers at 82 dB SPL, as measured 30 cm from the speaker. Song presentation took place in a controlled acoustic environment measuring 42.5 × 60 × 57.5 cm (Industrial Acoustic Company, Bronx, N.Y., USA) with acoustic foam lining the interior to reduce reverberation. The females were visually and acoustically isolated from all other birds for approximately 24 h prior to testing. This allowed the females to become habituated to the sound chamber prior to the onset of the experiment. We chose this time period to ensure that females became habituated to their new surroundings while simultaneously minimizing the time spent in social isolation. The song-exposed females heard 30 min of conspecific zebra finch song (n = 11) whereas the silence-exposed females (n = 6) did not. Ninety minutes after the onset of song playback (or silence), the females were sacrificed via rapid decapitation. All procedures were approved by Johns Hopkins University Animal Care and Use Committee.
Tissue Processing
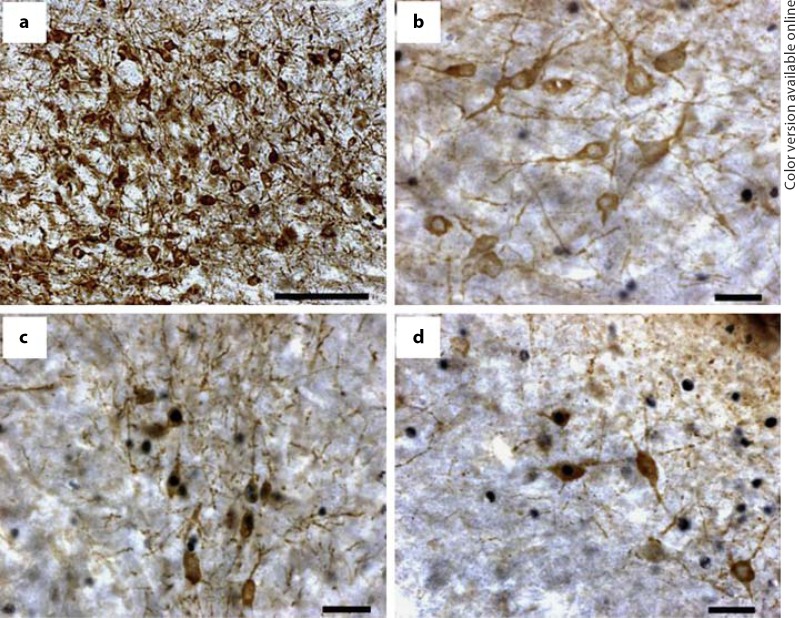
Brain tissue was fixed in 5% acrolein and cryoprotected in 30% sucrose until cut into three series of 40-µm coronal free-floating sections. We conducted double-label immunocytochemistry using a tyrosine hydroxylase (TH) antibody and a ZENK antibody. TH is a rate-limiting enzyme necessary for the synthesis of catecholamines, and is commonly used to detect catecholaminergic neurons [Ball et al., 2003; Bharati and Goodson, 2006; Lynch et al., 2008]. We conducted the double-label immunocytochemistry by performing two sequential free-floating reactions following standard avidin-biotin complex procedures, as described in Lynch et al. [2008]. An abbreviated description is as follows: we incubated the tissue in ZENK antibody (1:2,000 dilution of polyclonal Egr-1 antibody raised in rabbit; Santa Cruz Biotechnology, Santa Cruz, Calif., USA; cat. No. sc-189) at 4°C for 48 h followed by incubation in biotinylated goat anti-rabbit secondary antibody (1:250 dilution; Vector Laboratories, Burlingame, Calif., USA) for 1 h at room temperature. Antibody bound to ZENK protein was then visualized using a 1:200 dilution of Vectastain ABC Elite kit (Vector Laboratories) and nickel-enhanced 3,3′-diaminobezidene tetrahydrochloride chromagen, yielding a black reaction product (DAB; Polysciences, Warrington, Pa., USA). We continued by incubating the tissue in a TH antibody (1:10,000 dilution of TH monoclonal antibody raised in mouse; Immunostar, Hudson, Wisc., USA; cat. No. 22941). This antibody is isolated and purified from rat cells and has wide species cross-reactivity because it recognizes a midregion of the TH molecule where extensive homology exists. Primary antibody incubation was done at 4°C for 48 h. The final incubation was a biotinylated horse anti-mouse secondary antibody (1:250 dilution; Vector Laboratories) for 1 h at room temperature. The antibody bound to TH was visualized as described above except this DAB reaction lacked nickel enhancement, which yielded a brown reaction product (see fig. 2). The tissue was then mounted onto slides and coverslipped using Permount.
Fig. 2.
a VTA cells stained for TH. Scale bar = 10 µm. b TH cells with no ZENK-ir induction within the nucleus. Scale bar = 1 µm. c, d Cells with ZENK-ir within the nucleus of a TH cell. Scale bars = 1 µm.
Quantification
We examined two immunoreactive patterns: colocalization of TH and ZENK-ir (double-labeled cells), and cells expressing only ZENK-ir. A Zeiss Axioskop microscope (Carl Zeiss, Thornwood, N.Y., USA) with a 100× objective was used to count double-labeled cells. ZENK-ir alone and TH-ir cells were counted using a 40× objective. Openlab 3.1.7 software (Improvision, Lexington, Mass., USA) captured photomicrographs of the whole region for each region of interest (see fig. 1a, b). These were used for a manual count of each immunoreactive pattern of interest (see fig. 2). The number of sections quantified depended on the region itself. The LoC was the smallest region ranging from two to four sections and the PAG and VTA were larger regions, ranging from four to six sections. Tissue sections that were folded or damaged were excluded. We calculated the mean number of cells expressing either ZENK-ir or ZENK and TH-ir for each subject.
Because there was no a priori reason to predict that the number of TH-ir cells would rapidly change over a short period due to song or silence exposure, we randomly chose a subset of subjects in which to estimate the overall TH cell number (song-exposed, n = 8; silence-exposed, n = 4). As described above, the mean number of TH-ir cells was calculated for each region.
Behavioral Measurements
We recorded the behavior of the female zebra finches using a camera and a VHS recorder. We measured five behaviors during the first 10 min of song to determine if song onset caused a behavioral change in the song-exposed group as compared to the silence-exposed group. These behaviors included the following: time spent near speaker, time spent feeding/drinking, number of movements across the perch, number of grooming behaviors (preening, bill wipes) and number of flights (either to a new perch or to the ground). The side in which the speaker was placed was switched to control for possible side biases. A bird was considered near the speaker if she moved within 15 cm of the speaker. The perch was deliberately placed outside this range so that the female would need to move off the perch in order to be near the speaker. Time spent near the speaker and time spent feeding/drinking is expressed as total number of seconds whereas all other variables were measured by counting the number of occurrences. The time spent near the speaker provides a rough estimate of the female's interest in the song or speaker and time spent feeding/drinking may provide an estimate of lack of interest in the song or speaker. The number of perch movements, grooming behaviors and flights can provide an estimate of overall motor responses during the experiment. Because responses of catecholaminergic cells can be related to motor activity, we used these measures to estimate motor activity. The behavior of 1 female in the silence group was not recorded due to video tape failure during behavioral observations. Therefore, the final sample sizes for behavioral measurements were n = 11 for the song-exposed group and n = 5 for the silence-exposed group. Lights went on at 8 a.m. and experiments (song playback or silence along with behavioral recordings) started at approximately 10 a.m. every day. Four females were tested on each day. The start of the experiment was staggered by a maximum of 15 min so that at the end of song playback (or silence) there was sufficient time to sacrifice each bird and place the brain into a fixative. Therefore, all behavior was almost simultaneously recorded for both the silence- and song-exposed females.
Statistical Analyses
Separate two-way analyses of variance were used to determine if the number of cells with either colocalized TH/ZENK-ir or ZENK-ir alone was significantly different among the brain regions (main effect) or between song exposure groups (main effect). These tests were followed by different post hoc analyses. A Tukey post hoc test explored whether the brain regions exhibited a statistical difference in the number of cells colocalizing TH and ZENK-ir or ZENK-ir alone. However, this post hoc analysis revealed that the noradrenergic region (LoC) was statistically dissimilar from the other two regions that were primarily dopaminergic in nature (VTA and PAG). Therefore, pairwise comparisons were done using independent t tests so that we could examine dopaminergic regions separate from the noradrenergic regions. These comparisons determined whether any of these brain regions exhibited a significantly different double label as a consequence of the treatment group. We used a Bonferroni correction for multiple comparisons when examining the dopaminergic brain regions as they appeared to be statistically similar to one another. The alpha value for the post hoc pairwise comparison of dopaminergic brain regions was p ≤ 0.025, whereas the alpha value for the single noradrenergic region was set at 0.05.
Independent t tests were also used to compare the behavioral variables between song- and silence-exposed females. We used Kolmogorov-Smirnov tests to assess normality and a Levene test to assess equality of variances. Where variances were unequal, the Levene corrected statistics were reported for all pairwise comparisons. The means ± standard errors are reported throughout these analyses.
Results
ZENK-ir
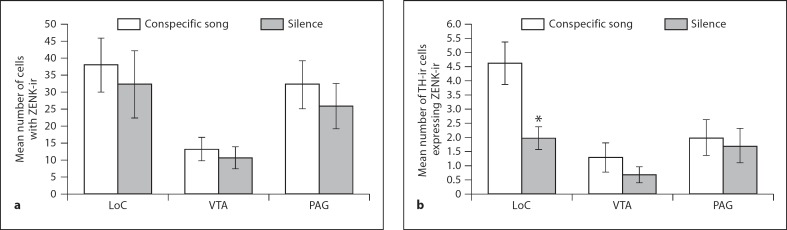
There was a significant difference in the number of cells expressing ZENK-ir as a consequence of brain region (F = 5.93; d.f. = 2, 51; p = 0.005; fig. 3a) but not as a consequence of treatment group (F = 1.17; d.f. = 1, 51; p = 0.284; fig. 3a). There was no significant interaction between brain region and treatment (F = 0.86; d.f. = 2, 51; p = 0.92). Tukey's post hoc comparison revealed a significant difference in the number of cells expressing ZENK-ir between VTA and PAG (p = 0.047), and between LoC and VTA (p = 0.002) whereas LoC exhibited no significant difference in the number of ZENK-ir cells compared to PAG (p = 0.45).
Fig. 3.
a Mean number of ZENK-ir cells within three catecholaminergic nuclei of song-exposed (n = 11) and silence-exposed female zebra finches (n = 6). b Mean number of cells with colocalized TH and ZENK-ir within three catecholaminergic nuclei of song- and silence-exposed female zebra finches. All means are reported ± SEM. Asterisk denotes significant differences between groups.
TH-ir Cells Expressing ZENK-ir
There was a significant difference in the number of cells with colocalized TH/ZENK-ir among the brain regions (F = 6.14; d.f. = 2, 51; p = 0.004; fig. 3b) as well as a significant effect of song exposure treatment (F = 4.64; d.f. = 1, 51; p = 0.037; fig. 3b). There was no significant interaction between brain region and treatment (F = 1.84; d.f. = 2, 51; p = 0.171). Tukey's post hoc comparison revealed no significant difference in the number of cells with colocalized TH/ZENK-ir between the dopaminergic brain regions (VTA and PAG; p = 0.42), whereas LoC exhibited a significantly greater number of colocalized TH/ZENK-ir cells compared to both of the dopaminergic regions (VTA, p = 0.0001; PAG, p = 0.02). Independent t tests revealed there was no significant difference in the number of cells expressing TH/ZENK-ir as a consequence of treatment within either of the dopaminergic brain regions (PAG, t = 0.29, d.f. = 15, p = 0.78; VTA, t = 0.8, d.f. = 15, p = 0.43). However, there were significantly more cells with colocalized TH/ZENK-ir within the LoC in song-exposed females compared to silence-exposed females (LoC, t = 2.46, d.f. = 15, p = 0.026; fig. 3b).
TH-ir
The differences in colocalized TH/ZENK-ir expression were not related to possible differences in the overall number of TH cells present between the song exposure treatments. While there was a significant difference in the number of cells expressing TH-ir as a consequence of brain region (F = 37.4; d.f. = 2, 51; p = 0.00), there was no difference as a consequence of song exposure treatment (F = 2.47; d.f. = 1, 51; p = 0.13). Tukey's post hoc comparison revealed a significant difference in the number of cells expressing TH-ir between VTA and PAG (p = 0.00), and between LoC and VTA (p = 0.000) whereas LoC exhibited no significant difference in the number of TH-ir cells compared to PAG (p = 0.11).
Behavior
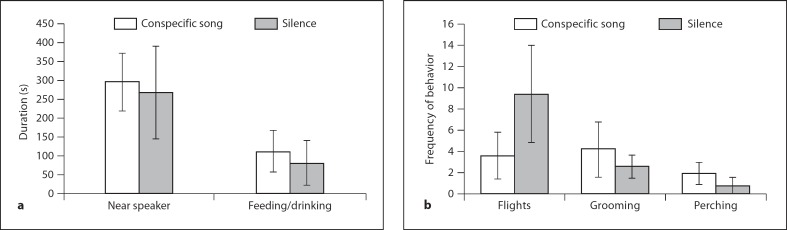
There were no significant correlations between the number of double-labeled cells and behavior for any of the five behaviors we measured. In addition, we examined whether there was a difference in any of the five behaviors between treatment groups. There were no significant differences in any of the behavioral measurements between the treatments (fig. 4). The time spent near the speaker and the time spent feeding/drinking were not significantly different between song-exposed and silence-exposed females (t = 0.18, d.f. = 16, p = 0.85; t = 0.30, d.f. = 16, p = 0.76, respectively). The frequency of short flights, grooming behaviors or perch movements were not significantly different between song- and silence-exposed females (t = 1.3, d.f. = 16, p = 0.21; t = 0.38, d.f. = 16, p = 0.71; t = 0.65, d.f. = 16, p = 0.53, respectively).
Fig. 4.
a Comparison between the amount of time spent near the speaker and the time spent feeding or drinking between song-exposed (n = 11) and silence-exposed (n = 5) female zebra finches. A bird was considered to be spending time near the speaker if she moved to the same side and corner as the speaker and within approximately 15 cm. b Comparison of the number of flights (either to a new perch or to the ground), grooming behaviors (bill wipes, preening) and movements across the perch between song- and silence-exposed female zebra finches.
Discussion
These results reveal that only one group of catecholaminergic cells responds (as measured by IEG expression within TH-labeled cells) after exposure to conspecific songs in female songbirds. These cells are primarily noradrenergic, reside within the LoC, and exhibit greater ZENK-ir expression in song-exposed females compared to silence-exposed females. In contrast, there is no song-dependent increase in ZENK-ir within noncatecholaminergic cells in any of the brain regions, nor is there elevated song dependent ZENK-ir in brain regions that are primarily dopaminergic in nature. Moreover, elevated IEG induction in catecholamine cells within the LoC following song or silence exposure is not related to behavioral responses to song or to the overall number of TH cells. This lack of behavioral differences indicates that ZENK expression within the LoC in song-exposed females is likely a consequence of song reception rather than behavioral responses to song.
Our results indicate that a group of primarily noradrenergic cells tends to respond when a female zebra finch hears sound. However, because we only presented females with conspecific songs (many different examples of conspecific song, yet still only conspecific songs), it is not clear whether catecholamine neurons in the LoC respond selectively to conspecific songs. It is possible that these neurons are not song-selective, in which case they may express ZENK to sound in general including heterospecific communication signals. However, these results do indicate that a group of cells that are primarily noradrenergic tend to respond when a female zebra finch hears sound. Future studies will examine the selectivity of this response to ascertain whether noradrenaline cells express ZENK in response to differences within conspecific species (less attractive, undirected songs vs. attractive, directed songs) as well as differences between species (conspecific song vs. heterospecific song).
It has been repeatedly demonstrated that ZENK-ir induction in the auditory forebrain, specifically the caudal medial nidopallium and the caudal medial mesopallium, exhibits selective responses to song [Mello et al., 1992]. Such selectivity in these auditory regions is modified by catecholamine deficits [Lynch et al., 2008] and moreover, catecholamine innervation to these auditory regions is estradiol-dependent [Matragrano et al., 2011]. Interestingly, noradrenergic-specific lesions stimulate female European starlings to approach nest boxes with broadcasted song, which may indicate a decrease in song selectivity if these females are broadening the range of songs to which they would attend as compared to untreated females [Riters and Pawlish, 2007]. In addition, noradrenergic deficits decrease estradiol-dependent selectivity in behavioral responses to song in female zebra finches [Vyas et al., 2009]. Consequently, it is possible that estrogen-dependent changes in catecholamine innervation to some regions of the auditory forebrain result in modified responses in these auditory regions, which may in turn influence the female's behavioral responses to song. Thus, future studies will examine the relationship between ZENK-ir induction in these higher auditory regions and ZENK-ir induction within the midbrain and brainstem regions examined here. Overall, however, our results are consistent with the hypothesis that cells within the LoC are involved in the neural circuitry underlying conspecific song or sound reception, at least in female zebra finches.
Noradrenaline regulates a wide range of effects from sensory and motor processes to cognitive processes such as attention, arousal, motivation and decision-making among many taxa [Robbins and Everitt, 1995; Berridge and Waterhouse, 2003; Cardin and Schmidt, 2004; Aston-Jones and Cohen, 2005; Solis and Perkel, 2006; Lynch et al., 2008]. Thus, alternative possibilities exist for explaining sound-dependent regulation of noradrenergic cells within the LoC. For instance, it is possible that these sound-related responses may be a consequence of arousal due to the sudden onset of sound or the onset of attention-related mechanisms [Robbins and Everitt, 1995]. Alternatively, it may be possible that sound reception induces responses from these cells in order to modulate sensory systems. There are a number of studies providing evidence for the latter possibility. In songbirds, for instance, the auditory forebrain regions receive robust noradrenergic innervation [Reiner et al., 1994; Mello et al., 1998], and chemical lesions of noradrenergic cells remove the differential ZENK induction in forebrain auditory regions that typically occurs in response to hearing conspecific versus heterospecific songs in female zebra finches [Lynch and Ball, 2008]. Moreover, noradrenaline release into a single region of the auditory forebrain, the nucleus interfacialis of the nidopallium, modifies auditory processing in a state-dependent manner [Cardin and Schmidt, 2004]. In mammals, depletion of noradrenergic innervation reduces sensory-induced expression of IEGs [Cirelli and Tononi, 2004]. It is distinctly possible that sound-related regulation of these cells within the LoC is due to attention and arousal processes that ultimately influence auditory neurons within the forebrain. Distinguishing between these alternative hypotheses may provide more insight into the exact role noradrenaline plays in regulating neural responses to song.
The overall number of LoC cells expressing ZENK-ir within TH-labeled cells suggests that it may only require a few cells to produce a biological effect. Furthermore, this effect may be species-specific. For instance, while we saw only a few ZENK-labeled cells within the LoC, we were, nonetheless, able to clearly detect cells with colocalized expression. In contrast, there were no ZENK-positive cells identified in TH-labeled cells after song exposure in the LoC of female white-throated sparrows (Zonotrichia albicollis) [LeBlanc et al., 2007]. Differences in coinduction of ZENK-ir within TH cells in the LoC between zebra finches and white-throated sparrows may be a consequence of species differences or possibly experience-related differences as LeBlanc et al. [2007] tested wild-caught birds whereas the birds in the present study were born and raised in captivity. Importantly, although the overall number of LoC cells coexpressing ZENK and TH in the current study is small, the difference between the song exposure groups is significant. Thus, it is possible that activity in only a few noradrenaline cells may produce robust neuromodulatory activity. Following this reasoning, tract-tracing studies of catecholaminergic projections from the LoC to HVC (a telencephalic nucleus in the song control system in the songbird brain) report only a small number of cells projecting from the brainstem to the telencephalon [Appeltants et al., 2000], yet there are high noradrenaline concentrations [Barclay and Harding, 1988; Barclay and Harding, 1990], high rates of noradrenaline turnover [Harding et al., 1998] and high densities of α2-adrenergic receptors in HVC [Riters and Ball, 2002; Riters et al., 2002]. Thus, it is possible that responses of only a few catecholamine cells are needed to produce a significant biological effect.
Modulation of physiology via social interaction, particularly the reception of communication signals, has been demonstrated in a variety of taxa, especially with respect to hormones [Bentley et al., 2000; Burmeister and Wilczynski, 2000; Chu and Wilczynski, 2001; Lynch and Wilczynski, 2006]. Here, we demonstrate that modulation of catecholamine-containing cells in the LoC is not related to differences in motor behaviors between song- and silence-exposed birds. Rather, the responses of these cells are related to the reception of song. Our study supports the hypothesis that noradrenergic cells within the LoC are involved in the neural architecture underlying sound perception. Overall, the results of this study reveal that catecholamine-containing cells within the LoC are responsive to conspecific sociosexual signals and consequently, it is likely that these cells are part of the neural architecture underlying the reception of signals used in animal communication and possibly the decision to respond.
Acknowledgements
We thank Andrea Wallace, Amanda Dios and Dr. Christina Castelino for their assistance. This study was supported by the National Institutes of Health (NIH/NINDS RO1 NS 35467).
References
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002;113:221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann NY Acad Sci. 2003;1007:211–231. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philos Trans R Soc Lond B Biol Sci. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Res. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Wingfield JC, Morton ML, Ball GF. Stimulatory effects on the reproductive axis in female songbirds by conspecific and heterospecific male song. Horm Behav. 2000;37:179–189. doi: 10.1006/hbeh.2000.1573. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Introduction to animal communication; in Principles of Animal Communication. Sunderland, Sinauer Associates. 1998:pp 1–15. [Google Scholar]
- Burmeister S, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Cardin J, Schmidt M. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Wilczynski W. Social influences on androgen levels in the southern leopard frog, Rana sphenocephala. Gen Comp Endocrinol. 2001;121:66–73. doi: 10.1006/gcen.2000.7563. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol. 2008;508:824–839. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goymann W. Social modulation of androgens in male birds. Gen Comp Endocrinol. 2009;163:149–157. doi: 10.1016/j.ygcen.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–346. [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Ullsperge M. Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J Neurosci. 2011;31:1606–1613. doi: 10.1523/JNEUROSCI.3904-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol. 2008;72:207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93:870–876. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social regulation of plasma estradiol concentration in a female anuran. Horm Behav. 2006;50:101–106. doi: 10.1016/j.yhbeh.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm Behav. 2010;58:230–240. doi: 10.1016/j.yhbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. Eur J Neurosci. 2011;34:416–425. doi: 10.1111/j.1460-9568.2011.07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Mello CV, Riberio S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;93:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesce KA, Pierce-Shimomura JT. Shared strategies for behavioral switching: understanding how locomotor patterns are turned on and off. Front Behav Neurosci. 2010;4((pii)):49. doi: 10.3389/fnbeh.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Karle EJ, Anderson KD, Medina L, Catecholamines in avian brain . Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. In: Smeets WJ, Reiner A, editors. Cambridge, Cambridge University Press. 1994. p. p 148. [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Sex differences in the densities of alpha 2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J Chem Neuroanat. 2002;23:269–277. doi: 10.1016/s0891-0618(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha (2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Pawlisch BA. Evidence that norepinephrine influences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Arousal systems and attention . The Cognitive Neurosciences. In: Gazzaniga M, editor. Cambridge, MIT Press. 1995. pp. pp 703–720. [Google Scholar]
- Smeets W, Reiner A, Phylogenetic aspects of catecholamine systems in the CNS of vertebrates . Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. In: Smeets WJ, Reiner A, editors. Cambridge, Cambridge University Press. 1994. pp. pp 1–5. [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc Biol Sci. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MM, Perkel DJ. Noradrenergic modulation of activity in a vocal control nucleus in vitro. J Neurophysiol. 2006;95:2265–2276. doi: 10.1152/jn.00836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, Borg L, Bogdan D. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches' behavioral responses to songs. Horm Behav. 2009;55:50–59. doi: 10.1016/j.yhbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS. Female sexual arousal in amphibians. Horm Behav. 2011;59:630–636. doi: 10.1016/j.yhbeh.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior and neurobiology. Horm Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S, Dieckmann M, Schwabe K. Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behav Brain Res. 2009;198:206–213. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]