Abstract
Background
Anemia is a common co-morbidity in older adults with heart failure and a preserved ejection fraction (HFPEF) and is associated with worse outcomes. We hypothesized that treating anemia with subcutaneous epoetin alfa (ESA) would be associated with reverse ventricular remodeling and improved exercise capacity and health status compared with placebo.
Methods and Results
Prospective, randomized, single blind, 24-week study with blinded endpoint assessment among anemic (average hemoglobin of 10.4±1 g/dl), older adult patients (n=56, 77±11 years, 68% female) with HFPEF (EF=63±15%, BNP of 431±366 pg/ml). Treatment with ESA resulted in significant increases in hemoglobin (p<0.0001). Changes in end diastolic volume (−6±14 vs. −4±16 ml, p=0.67) at 6 month did not differ between ESA and placebo but declines in stroke volume (−5±8 vs. 2±10 ml, p=0.09) without significant changes in LV mass were observed. Changes in six minute walk distance (16±11 vs. 5±12 meters, p=0.52) did not differ. While QOL improved by the KCCQ and MLHFQ in both cohorts, there were no significant differences between groups.
Conclusions
Administration of ESA to older adult patients with HFPEF compared with placebo did not change LVEDV, LV Mass nor improve submaximal exercise capacity or quality of life.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00286182.
Keywords: aging, heart failure, anemia
Half of heart failure (HF) patients residing in the community have a preserved left ventricular ejection fraction (HFPEF).1 These patients are often older adult women with hypertension 2 and several extra cardiac comorbidities including diabetes, obesity, anemia and chronic kidney disease (CKD) among others. 3 These co-morbid conditions have been associated with altered pressure volume relations and pump function in HFPEF4 and confer an adverse prognosis in patients with HFPEF1, 5, 6. Since large scale clinical trials for this population 7–9, have not demonstrated effectiveness of any specific therapy in HFPEF and in light of the high prevalence of important co-morbidities and their strong relationship to adverse outcomes, identification and aggressive treatment of these conditions has been proposed as an effective strategy for HFPEF, while awaiting for new HFPEF specific therapies to emerge.10
Among the co-morbidities prevalent in subjects with HFPEF, anemia has been shown to be highly prevalent, strongly associated with morbidity and mortality, often due to underlying chronic renal disease and among the most modifiable pharmacologically. Accordingly, the purpose of the current study was to evaluate if treating anemia with subcutaneous epoetin alfa (ESA) in patients with HFPEF would be associated with reverse ventricular remodeling and changes in non-invasively determined pressure volume relations, significant improvements in exercise capacity, and improved health status, as compared with placebo.
Methods
Study Design
This was single center, prospective randomized (with 1:1 allocation), single blind, twenty-four week study (NCT00286182) among community dwelling, older adult patients with anemia and HFPEF who received either ESA or placebo. To limit chance imbalances in a trial of this size and because the differences in hemoglobin levels between genders and the strong association of renal function with hemoglobin levels, randomization was stratified by gender and baseline renal function (dichotomized by cut point of an estimated Creatinine clearance of 40 ml/min). Study subjects were blinded to treatment assignment, which was maintained throughout the study, by providing weekly injections of ESA or placebo in unmarked syringes. Endpoints were performed by study personnel blinded to the result of randomization.
Study Subjects
The diagnosis of heart failure was based on the NHANES -CHF Criteria with a score ≥ 311 and study participants were considered to have a preserved ejection fraction if three dimensional echocardiographically determined ejection fraction was ≥40%. Anemia was defined as hemoglobin < 12 g/dL 12. Patients were excluded from the study if they had uncontrolled hypertension (SBP >160 mmHg and/or DBP > 90 mmHg, resting heart rate >120 bpm, baseline 6 minute walk > 450 meters, valvular heart disease greater than mild stenotic or greater than moderate regurgitant lesions by transthoracic echocardiography, infiltrative cardiac disease such as hemochromatosis and amyloidosis, hypertrophic cardiomyopathy, chronic pulmonary disease (FEV1 < 60% predicted), renal failure (GFR < 15 mL/min), hemoglobin < 9 g/dL, exercise limited by angina, claudication or neurological diseases, severe liver dysfunction, cardiac surgery less than 3 months prior, known iron deficiency anemia from chronic blood loss, significant alcohol or illicit drug use, known hypercoagulable state or an active hematologic disease. Patients were also excluded if they had a history of deep venous thrombosis or pulmonary embolus within 12 months before study entry, had a history of CVA or TIA within 6 months, or an acute coronary syndrome within 6 months of study entry, had an allergy or sensitivity to human serum albumin, or had a known hypersensitivity to mammalian cell-derived products. The study was approved by the Columbia University Medical Center IRB. Informed consent was obtained in all subjects.
Intervention
Erythropoietin alfa (Epoetin alpha, Ortho Biotec, Inc) was administered weekly by subcutaneous injection using a pre-specified dosing algorithm13 that made weekly adjustments based on the rate of rise (ROR) of the hemoglobin over a one week period, as well as the absolute hemoglobin value. Subjects were monitored every week and dose adjustments were made to avoid rapid increases in hemoglobin (greater than 0.4 g/dL) in any given weekly interval. All subjects randomized to active treatment initially received 7,500 units of ESA given weekly by subcutaneous injection, while placebo subjects received the same injection volume of normal saline. All subjects were given oral iron (Ferrous gluconate 325 mg orally twice a day). Placebo injection volumes were changed based on study algorithm during the study, to give the appearance that dose adjustments were occurring in the placebo group. Hemoglobin was measured weekly on a venous sample obtained in the patients home by a point of care system (Hemocue Inc. Sweeden), which was shown to be highly correlated with a hospital lab14.
Weekly Home Visits
Blood pressure measured by an automated cuff sphygmomanometer (Omron, Kyoko City, Japan) and weight by a digital scale (SECA, Hamburg, Deuschland) were measured weekly in the subjects home by trained research personnel (ST). During home visits, subjects also had brief review of symptoms, targeted physical exams to evaluate weight, blood pressure and volume status along with careful review of all medications including type, dose and frequency. Blood was obtained via venipuncture in order to assess weekly hemoglobin values which were used to determine dose of study drug.
Two and Three dimensional Echocardiography
Standard two-dimensional transthoracic echocardiography (2-DE) was performed on each subject. End-diastolic measurements of left ventricular internal dimension (LVID), septal thickness (IVS), and posterior wall thickness (LVPWT) were acquired according to the standards of the American Society of Echocardiography15. Doppler indices of the mitral inflow pattern including peak E wave velocity, peak A wave velocity and isovolumetric relaxation time (IVRT) as well as lateral mitral annual velocities (e′) were recorded for three beats and averaged. Left ventricular filling pressures were estimated by the formula EDP=11.96 +0.596 · E/e′16.
The equipment and procedures of freehand three-dimensional transthoracic echocardiography (3-DE) have been previously described in detail17, 18. 3-DE was performed using a conventional real-time echocardiograph, three-dimensional acoustic spatial locater, personal computer, and custom software. The data derived includes left ventricular chamber end-diastolic volume (EDV), myocardial volume (MV), stroke volume (SV), and the ejection fraction (EF = SV/EDV). Myocardial volume was multiplied by 1.05 gm/dl to determine ventricular mass. Echocardiograms were performed by study personnel blinded (SH) to clinical information.
Functional Assessment
A six minute walk test was performed in all subjects and a cardiopulmonary exercise test was performed in subjects able to exercise. Testing was performed at baseline and after 3 and 6 months of treatment. The total distance walked was recorded. Patients performed an upright bicycle exercise test, by study personnel blinded to treatment assignment, with the workload increased every three minutes by 25 Watts to a patient limited maximum level after 3 minutes of rest. Expired gas analysis was performed continuously with a Metabolic Cart (Medical Graphics). Peak VO2 was defined as the highest value of VO2 achieved in the final 30 seconds of exercise.
Quality of Life, Depression and Pain
The Kansas City Cardiomyopathy Questionnaire, (KCCQ)19 and the Minnesota Living with Heart Failure Questionnaire, (MLWFQ)20 were employed as two valid, reliable, self-administered disease specific assessments of quality of life in patient with heart failure. Finally, we employed the 30 item geriatric depression scale21 and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)22, to assess depressive symptoms and pain, respectively. All questionnaires were administered at baseline at after 3 and 6 months of follow-up.
Blood volume analysis
Blood volume was determined at baseline and after 6 months of therapy by intravenous administration of iodine131–labeled albumin (Volumex, Daxor Corp., New York City, New York) as previously described23. Plasma volume was determined as the volume of distribution of the radiolabeled albumin obtained by semi-logarithmic extrapolation of values measured from at least 3 samples drawn twelve minutes after injection at 6-minute intervals. Plasma radioactivity of each sample was measured in a semi-automated counter (BVA-100 Blood Volume Analyzer, Daxor Corp). Blood volume and red blood cell volumes were calculated from the plasma volume measurement and then compared with normal values for age, gender, height, and weight based on the subject’s ideal weight to determine the percent deviation from normal24.
Statistical analyses
SAS for Windows (Version 9.1.3, SAS Institute Inc., Cary, North Carolina) was used for all analyses. Results are expressed as mean ± standard deviation unless otherwise noted. The primary endpoint was the change in left ventricular end diastolic volume measured by three-dimensional echocardiography. This was selected as the primary endpoint because: (1) anemia can result in a high output state characterized by increases in left ventricular volumes25, (2) previous data show left ventricular size was actually increased in subjects with HFPEF and this was associated with several comorbid conditions including anemia3, (3) pilot data had demonstrated efficacy of ESA with regards to this endpoint26 and (4) given the highly accurate and reproducible nature of measuring LV volumes with freehand three dimensional echocardiography, the sample size needed to test the hypothesis was feasible to recruit in a single center study. Secondary endpoints included NYHA class, submaximal exercise capacity as assessed by six minute walk, maximal exercise capacity as assessed by cardiopulmonary exercise testing with cycle ergometry, quality of life as assessed by the KCCQ and Minnesota Living with Heart Failure Questionnaire and blood volume measurements.
The power calculation for this longitudinal study involving three time points (baseline, 3 and 6 months) was based on the method of Diggle27. According to this method, given an anticipated dropout rate of 15% and assuming a high correlation of serial end diastolic volume (EDV) measured by freehand three dimensional echocardiography (r=0.98), 28 subjects per group would ensure at least 80% power to detect a reduction in EDV of 20 ml (~ 20% decline) in subjects receiving ESA compared with placebo after 6 months of therapy at the usual two-sided 5% level of significance.
Comparisons between subjects randomized to ESA and placebo regarding baseline demographic and clinical characteristics were evaluated by chi-squared test for dichotomous variables, by un-paired student’s t-test for continuous variables having a normal distribution or by Kruskall-Wallis test for non-normally distributed continuous variables.
To evaluate the differences in primary endpoint of change in end diastolic volume over time, a longitudinal data analysis was performed using the generalized estimating equations (GEE) 28. A similar strategy was followed for the secondary endpoints as well. Additionally, graphical displays were made for change in EDV, change in hemoglobin, NYHA class, 6-minute walk distance, peak VO2 and KCCQ summary score.
For endpoints that were not specified as primary or secondary but rather were exploratory, p-values for these tests were adjusted for multiple testing in the False Discovery Rate (FDR) context 29.
Results
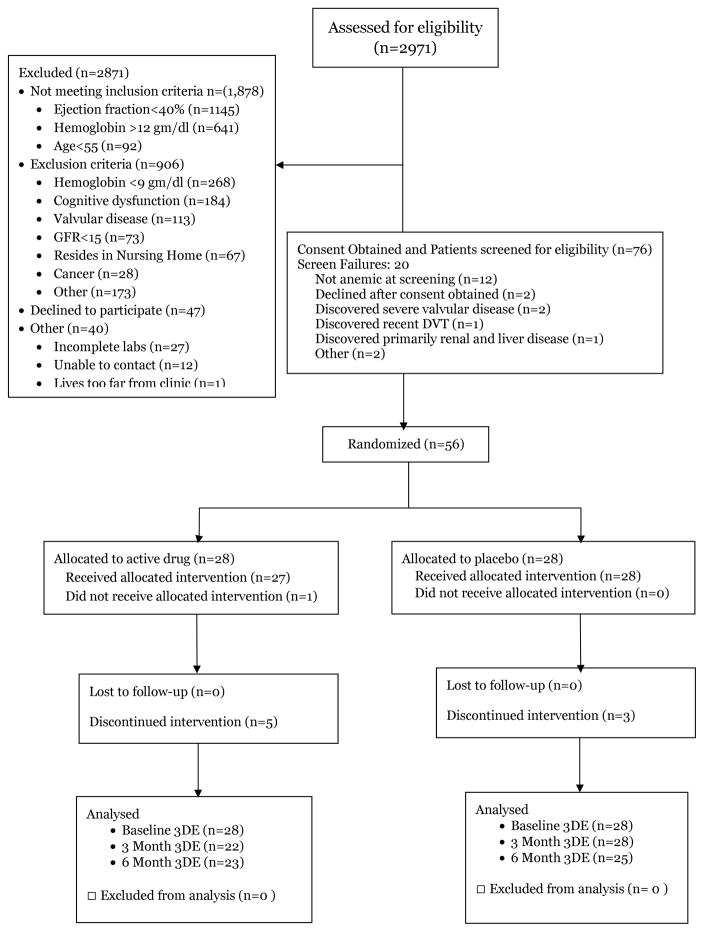
Fifty six subjects were randomized out of 2,971 screened. The major reasons for exclusion were the presence of a reduced ejection fraction (n=1145) absence of anemia or too severe anemia (n=909), concomitant cognitive dysfunction making informed consent unobtainable (n=184), significant valve disease (n=113), age<55 years (n=92) and other exclusion criteria (Figure 1). Of those subjects eligible to participate (n=123), 47 declined enrollment given the requirements of the trial study visits and an additional 20 who signed consent did not meet criteria for inclusion on more formal evaluation, in large part because their hemoglobin did not meet study criteria.
Figure 1.
The CCORT chart delineating the flow of patients through the trial.
The cohort studied (Table 1) were older adults (77±11 years, 43% over the age of 80 years), predominately women with multiple co-morbid conditions (hypertension, obesity, coronary artery disease, osteoarthritis and chronic renal disease) and depressive symptoms as well as chronic pain which are characteristic of patients with HFPEF.3, 10, 30. Subjects had isolated systolic hypertension with a widened pulse pressure and were taking on average 3.2 antihypertensive medications. At baseline, subjects randomized to ESA compared to placebo were more often diabetic but were well matched regarding other demographic features, clinical symptoms and findings to define HFPEF, co-morbid conditions, functional capacity and quality of life (Table 1). The overall ejection fraction was preserved (58±10%) in both cohorts with 46 of the 56 subjects recruited had LVEF > 50%. Of those with an LVEF< 50%, 5 had EF between 46–50% and 5 had LVEF between 40–45%. Average tissue Doppler velocities were low (~6–7 cm/sec) and E/E′ ratio was increased compatible with elevated estimated filling pressures (estimated LVEDP of 23 mm Hg). Consistent with this phenotype, B-type natriuretic peptides were elevated in both cohorts and did not differ between them at baseline.
Table 1.
Baseline Demographic, Clinical and Laboratory Characteristics
| Parameter | Placebo (n=28) | Epoetin Alfa (n=28) | P value |
|---|---|---|---|
| Age (years) | 79 (11) | 74 (9) | 0.052 |
| Gender (% Female) | 64% | 71% | 0.33 |
| Ethnicity (% Hispanic) | 67% | 54% | 0.27 |
| Race | 0.59 | ||
| White | 68% | 68% | |
| Black | 29% | 32% | |
| Asian | 4% | 0% | |
| Body Size | |||
| Height (cm) | 156 (9) | 158 (7) | 0.27 |
| Weight (kg) | 75 (15) | 83 (19) | 0.11 |
| BSA (m2) | 1.8 (0.2) | 1.9 (0.2) | 0.07 |
| BMI (kg/m2) | 31 (5) | 34 (7) | 0.10 |
| Co Morbid Conditions (%) | |||
| Hypertension | 100% | 100% | 1.0 |
| Diabetes | 50% | 82% | 0.01 |
| COPD | 11% | 18% | 0.45 |
| Coronary Artery Disease | 54% | 68% | 0.3 |
| Obesity | 54% | 68% | 0.3 |
| Chronic Kidney Disease | 50% | 68% | 0.2 |
| Hemodynamics | |||
| Systolic BP (mm Hg) | 140 (15) | 146 (17) | 0.14 |
| Diastolic BP (mm Hg) | 65 (11) | 67 (11) | 0.55 |
| Mean BP (mm Hg) | 90 (10) | 93 (10) | 0.37 |
| Pulse Pressure (mm Hg) | 75 (15) | 79 (19) | 0.41 |
| Heart Rate (bpm) | 74 (14) | 67 (13) | 0.35 |
| Medications | |||
| ACE inhibitors | 14 (50%) | 9 (32%) | 0.17 |
| ARBs | 6 (21%) | 11 (39%) | 0.15 |
| Beta Blockers | 22 (79%) | 17 (61%) | 0.15 |
| Calcium Channel Blocker | 13 (46%) | 15 (54%) | 0.6 |
| Aldosterone Antagonists | 4 (14%) | 5 (18%) | 0.7 |
| Loop Diuretics | 23 (82%) | 19 (68%) | 0.2 |
| Thiazide Diuretics | 4 (14%) | 8 (29%) | 0.19 |
| Laboratory Assessment | |||
| Hemoglobin (gm/dl) | 10.4 (1) | 10.6 (1.2) | 0.46 |
| Platelet count (x10(9)/L) | 232 (64) | 233 (68) | 0.8 |
| Albumin (gm/dl) | 3.7 (0.5) | 3.8 (0.4) | 0.5 |
| Potassium (mmol/L) | 4.5 (0.6) | 4.6 (0.5) | 0.3 |
| Creatinine (mg/dl) | 1.51 (0.8) | 1.58 (0.7) | 0.7 |
| Estimated GFR (ml/min/m2) | 48 (18) | 46 (19) | 0.6 |
| Blood urea nitrogen (mg/dl) | 37 (16) | 33 (17) | 0.2 |
| B-type natriuretic peptide (pg/ml) | 373 (336) | 488 (392) | 0.2 |
| Iron (ug/dl) | 60 (38) | 61 (49) | 0.7 |
| Ferritin (ng/L) | 85 (73) | 87 (79) | 0.9 |
| Transferrin Saturation (%) | 20 (13) | 20 (14) | 0.8 |
| Pain and Depression | |||
| Geriatric Depression Scale (0–30) | 7±5 | 10±7 | 0.16 |
| WOMAC Scale (0–96) | 23±16 | 26±16 | 0.57 |
WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
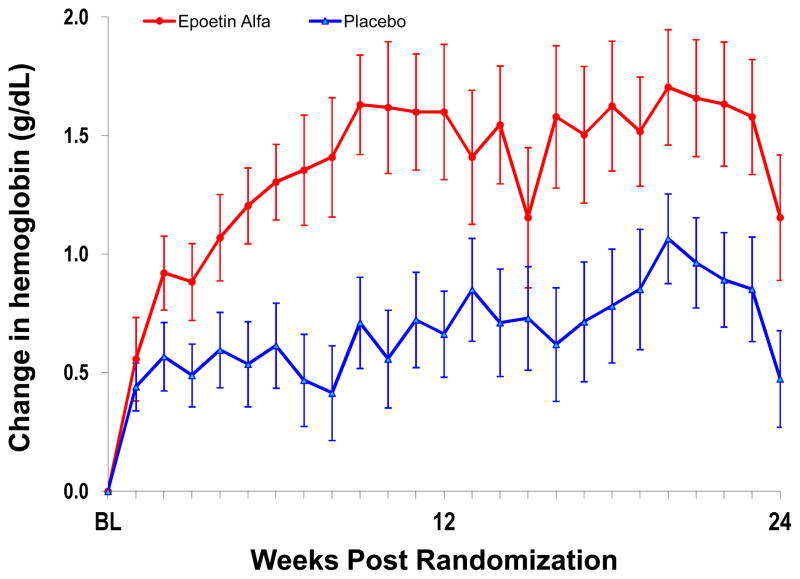
During the course of the trial, weekly hemoglobin rose in both the subjects assigned to ESA and placebo (Figure 2). Subjects assigned to ESA achieved on average increase in hemoglobin of +1.5 gm/dL. However, subjects assigned to placebo, also had a rise in hemoglobin, p<0.0001 for the difference. The blood volume analysis revealed that subjects randomized to ESA had improvement in their red cell deficit (p=0.07) and declines in plasma volume (p=0.04) compared to those who received placebo, while changes in total blood volume did not differ between the cohorts.
Figure 2.
Weekly changes in hemoglobin from baseline during study period in subjects randomized to ESA (red circles) or placebo (blue triangles). Data represents mean ± standard error.
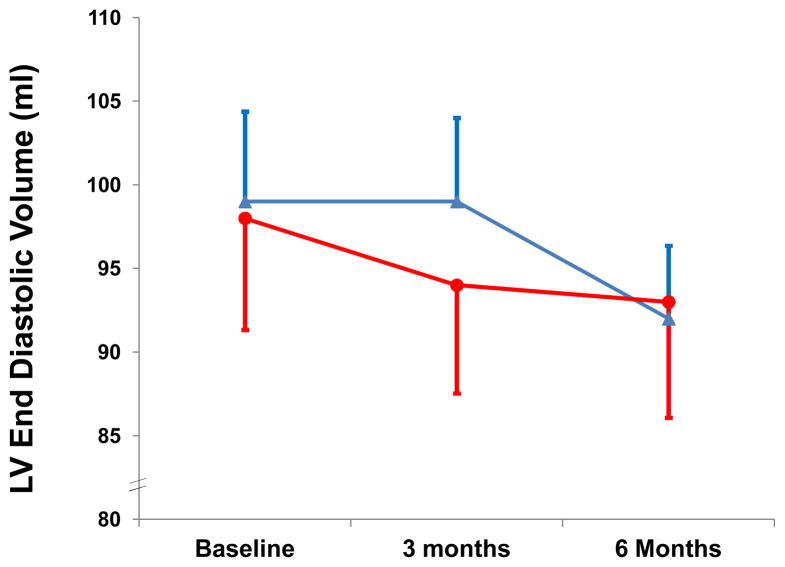
There was no significant difference in the primary endpoint of change in end diastolic volume (−6±14 vs. −4±16 ml, p=0.67; 95% CI: (−43.67, 39.67)) from baseline to 6 months in those assigned to ESA or placebo (Figure 3). Declines in stroke volume (−5±8 vs. +2±10 ml, p=0.09) tended to be greater in those receiving ESA without significant changes in LV mass (Table 2). Stroke work declined in subjects treated with ESA as compared with placebo after 6 months (−454.5± 675 vs. +211±896 ml*mmHg, p=0.09).
Figure 3.
Left ventricular end diastolic volume derived from three dimensional echocardiography, the primary endpoint of the study in subjects randomized to ESA (red circles) or placebo (blue triangles). Data represents mean ± standard error.
Table 2.
Changes in Parameters during Trial
| Placebo
|
Epoetin Alfa
|
Adjusted P Value Between Group Comparison* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | n | Change at 3 months | n | Change at 6 months | n | Baseline | n | Change at 3 months | n | Change at 6 months | ||
| Hemodynamics | |||||||||||||
| Systolic BP (mm Hg) | 28 | 140 (15) | 27 | −4.8 (25) | 22 | −5.86 (22) | 28 | 146 (17) | 22 | −0.59 (22) | 22 | −2 (27) | 0.81 |
| Diastolic BP (mm Hg) | 28 | 65 (11) | 27 | −3 (13) | 22 | −0.2 (9) | 28 | 67 (11) | 22 | −2 (12) | 22 | 2 (11) | 0.80 |
| Laboratory | |||||||||||||
| Hemoglobin (mg/dL) | 28 | 10.4 (0.9) | 26 | +0.7 (1.0) | 22 | 0.5 (1.0) | 28 | 10.6 (1.1) | 22 | 1.7 (1.4)† | 21 | 1.2 (1.2)† | 0.03 |
| Creatinine (mg/dL) | 28 | 1.5 (0.8) | 27 | 0.03 (0.4) | 24 | −0.03 (0.4) | 28 | 1.6 (0.7) | 22 | 0.1 (0.3) | 22 | 0.1 (0.4) | 0.61 |
| BUN (mg/dl) | 28 | 37 (16) | 27 | −1 (15) | 24 | −1 (17) | 28 | 33 (17) | 22 | 3 (16) | 22 | 3 (17) | 0.66 |
| eGFR (ml/min) | 28 | 48 (18) | 27 | 0.4 (18) | 24 | 1.0 (13) | 28 | 46 (19) | 22 | −3 (9) | 22 | −2 (9) | 0.83 |
| BNP (pg/mL) | 28 | 373 (336) | 27 | −60 (166) | 24 | −67 (256) | 28 | 488 (392) | 22 | 26 (273) | 20 | −13 (348) | 0.81 |
| Quality of Life | |||||||||||||
| KCCQ | 28 | 62 (22) | 27 | 13 (16)† | 24 | 16 (19)† | 27 | 58 (28) | 22 | 9 (19)† | 22 | 15 (24)† | 0.90 |
| MLWFQ | 28 | 32 (22) | 27 | −12 (16)† | 24 | −10 (22)† | 27 | 35 (25) | 22 | −7 (19)† | 22 | −11 (21)† | 0.80 |
| GDS | 28 | 7 (5) | 24 | −0.5 (4) | 24 | 0.7 (5) | 28 | 10 (7) | 22 | −1.0 (4) | 22 | −0.7 (4) | 0.95 |
| WOMAC | 28 | 41 (28) | 27 | −6 (19) | 24 | −7 (30) | 28 | 44 (29) | 22 | −7 (17) | 22 | −7 (21) | 0.95 |
| Three Dimensional Echocardiography | |||||||||||||
| LV ejection fraction (%) | 27 | 58 (9) | 27 | 3 (7)% | 23 | 4 (8) | 26 | 58 (10) | 21 | 1 (7)% | 20 | −2 (8) | 0.18 |
| LV end diastolic volume (ml) | 27 | 99 (28) | 27 | 0.03 (10) | 23 | −4 (16) | 26 | 98 (32) | 21 | −5 (9) | 20 | −6 (14) | 0.67 |
| Stroke Volume (ml) | 27 | 56 (13) | 27 | 3.9 (10) | 23 | 3 (9) | 26 | 56 (15) | 21 | −2 (7)† | 20 | −5 (8)† | 0.09 |
| LV end systolic volume (ml) | 27 | 43 (19) | 27 | −4 (9) | 23 | −7 (13) | 26 | 42 (22) | 21 | −3 (8) | 20 | −1 (12) | 0.61 |
| Mean e′ velocity (cm/s) | 27 | 6.5 (2.6) | 24 | −0.3 (1.9) | 21 | −1.1 (2.5) | 27 | 6.5 (3.2) | 20 | 0.5 (2.8) | 18 | −0.7 (3.5) | 0.61 |
| E-velocity/e′ | 27 | 19 (10) | 24 | 3 (8) | 21 | 3 (7) | 26 | 19 (15) | 20 | 0 (6) | 18 | −1 (11) | 0.61 |
| Pressure Volume Relations | |||||||||||||
| Estimated LVEDP (mm Hg) | 27 | 23 (6) | 24 | 1.75 (5) | 19 | 0.3 (4) | 26 | 24 (9) | 20 | 0.35 (4) | 18 | −0.4 (5) | 0.83 |
| Stroke Work | 27 | 5161 (1309) | 27 | 333 (839) | 23 | 211 (896) | 26 | 5374 (1610) | 21 | −120 (615) | 20 | −455 (675) | 0.09 |
| Stroke Work/LV mass | 27 | 53 (15) | 27 | 4 (8) | 23 | 2 (13) | 26 | 52 (14) | 21 | −3 (7)* | 20 | −4 (7)* | 0.32 |
| Functional | |||||||||||||
| 6-Minute walk (m) | 27 | 239 (102) | 26 | 9 (69) | 23 | 5 (59) | 27 | 242 (97) | 21 | 14 (51) | 21 | 16 (47) | 0.50 |
| VO2 (ml/kg/min) | 9 | 10.3 (2.9) | 7 | −0.9 (1.8) | 6 | −1.2 (1.4) | 10 | 9.0 (2.5) | 5 | 0.7 (2.1) | 4 | 1.0 (1) | 0.03 |
| RER | 9 | 1.1 (0.1) | 7 | −0.01 (0.1) | 6 | −0.04 (0.1) | 10 | 1.0 (0.2) | 5 | 0.04 (0.1) | 4 | 0.03 (0.08) | 0.95 |
| VE (ml) | 8 | 31 (8) | 6 | −0.9 (4) | 5 | −5 (8) | 8 | 24 (7) | 4 | −1.0 (3) | 3 | −2 (2) | 0.81 |
| VE/VCO2 | 9 | 34 (6) | 7 | 2.1 (7) | 6 | 0.2 (3) | 10 | 38 (16) | 5 | −0.6 (8) | 4 | −3 (7) | 0.61 |
| Blood Volume | |||||||||||||
| Red cell volume (ml) | 24 | 1323 (288) | N/A | N/A | 17 | 58 (237) | 20 | 1454 (433) | N/A | N/A | 15 | 173 (164) | 0.13 |
| Plasma Volume (ml) | 24 | 3264 (701) | N/A | N/A | 17 | 14 (287) | 20 | 3710 (975) | N/A | N/A | 15 | −206 (299)† | 0.04 |
| Total Blood Volume (ml) | 24 | 4586 (950) | N/A | N/A | 17 | 66 (433) | 20 | 5163 (1379) | N/A | N/A | 15 | −32 (318) | 0.40 |
| Red cell volume (%) | 24 | −18 (9) | N/A | N/A | 17 | 2 (13) | 20 | −18 (17) | N/A | N/A | 15 | 10 (10) | 0.07 |
| Plasma Volume (%) | 24 | 24 (22) | N/A | N/A | 17 | 0 (10) | 17 | 22 (20) | N/A | N/A | 15 | −6 (10) | 0.23 |
| Total Blood Volume (%) | 24 | 8 (15) | N/A | N/A | 17 | 1 (9) | 20 | 10 (18) | N/A | N/A | 15 | 0.3 (5) | 0.80 |
P value for between group comparisons from generalized estimating equation (GEE) at 6 months. For all p values except for those corresponding to primary and secondary endpoints, p values were adjusted for multiple hypothesis testing in the False Discovery Rate context.
Significant difference from baseline within group
NYHA – New York Heart Association, KCCQ – Kansas City Cardiomyopathy Questionnaire, GDS – Geriatric Depression Scale, WOMAC – Western Ontario and McMasters Arthritis Index
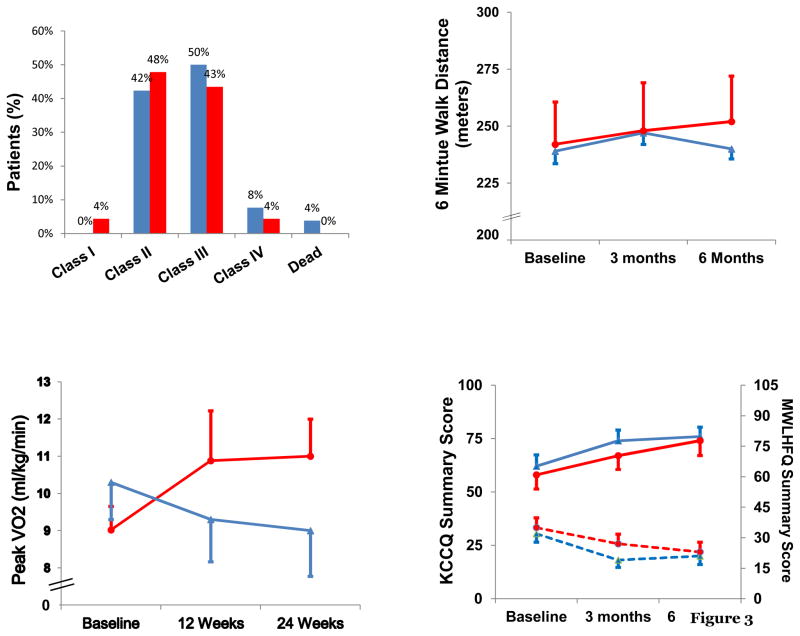
The effect of ESA on functional capacity was assessed by evaluating NYHA class, six minute walk distance and peak VO2. There were no significant changes in NYHA class during the course of the trial (Figure 4, upper left panel) nor did the changes in six-minute walk distance at 6 months (+16±11 vs. +5±12 meters, p=0.52, 95% CI: (−20.91, 42.91)) differ between the cohorts (Figure 4, upper right panel). Due to the frail nature of the study population, only a subset of patients (n=19) could perform cardiopulmonary exercise testing at baseline and at 6 months (n=10). The difference in peak oxygen consumption from baseline to 6-month follow-up was greater in subjects receiving ESA alfa as compared to placebo (+1.0±0.5 vs. −1.2±0.6 ml/kg/min, p<0.03; 95% CI: (0.67, 3.73)) (Figure 4, lower left panel).
Figure 4.
Distribution of NYHA class after 6 months in ESA (red bars) and placebo (blue bars) (upper left panel); six minute hall walk distance in ESA (red circles and placebo (blue triangles) (upper right panel); peak VO2 in ESA (red circles) and placebo (blue triangles) (lower left panel) and quality of life measures for KCCQ (solid lines) and MLWFQ (dashed lines) (lower right panel) at baseline and after 3 and 6 months of therapy.
Measures of quality of life improved as assessed by the KCCQ (+15±5 and +16±4 points, p<0.01 compared to baseline in those randomized to ESA and placebo, respectively) and MLHFQ (−11±4 and −10±5 points, p<0.05 compared to baseline in ESA and placebo, respectively) in both cohorts without significant differences between groups (Figure 4, lower right panel). An analysis of the subgroup of subjects with hemoglobin < 11 gm/dl at entry did not differ from the overall results.
During the course of the trial, there were 14 subjects with serious adverse events randomized to placebo and 11 subjects with a serious adverse event in subjects randomized to ESA, mainly hospitalizations. Among subjects randomized to placebo, one subject died suddenly and one subject was newly diagnosed with cancer. There were no thrombotic episodes (e.g. stroke, TIA, myocardial infarction, PE or venous thrombosis). The total number of hospitalizations did not differ in those randomized to ESA compared to placebo (19 vs. 16), nor did the number of hospitalizations for heart failure (3 vs. 2). Similarly, the number of individual subjects hospitalized during the course of the trial did not differ between those randomized to ESA compared to placebo (11 vs. 12).
Discussion
The principle findings of this study are that among older adults with HFPEF, the administration of ESA compared with placebo using the current dosing algorithm was safe with no differences in serious adverse events between those randomized to active therapy versus placebo but did not change left ventricular end diastolic volume. Additionally, treatment with ESA did not alter LV Mass nor improve sub-maximal exercise capacity as measured by six minute walk nor quality of life compared with placebo but was associated with increases in peak oxygen consumption in a subset of patients able to exercise.
Previous randomized clinical trials of subjects with heart failure and a low ejection fraction have demonstrated that ESAs are associated with improved exercise tolerance, reduction in symptoms and have benefits on clinical outcomes in anemic patients with heart failure including hospitalizations31, 32. These encouraging results coupled with the absence of effective therapies for subjects with heart failure and a preserved ejection fraction and a hypothesis that co-morbidities contribute significantly to the phenotype of HFPEF, resulted in the development of the current study. We are not aware of any randomized, prospective clinical trials that have specifically evaluated the treatment of anemia with ESAs in this population. Anemia has been shown to be more prevalent among community dwelling subjects with heart failure and a preserved ejection fraction than among those with a reduced ejection fraction33 and associated with increased risk of in hospital mortality34, early readmission35 and overall mortality36 irrespective of ejection fraction. Accordingly, we had hypothesized that treatment of anemia would have resulted in significant improvements in ventricular structure and function, functional capacity and quality of life.
However, the results of our study do not support this hypothesis. We were not able to demonstrate any significant effect of ESA on the primary endpoint of left ventricular end diastolic volume in comparison to placebo. Previous studies of ESA have shown statistical reductions in left ventricular mass and volumes and improvements in ejection fraction both in patient with chronic kidney disease not on dialysis 37–41 and in those on dialysis42, 43. Similar results have been shown in patients with systolic heart failure.44–46 However, not all trials have demonstrated a clinical effect47. Collectively these trials suggest that the more severe the anemia, greater the left ventricular mass, lower the ejection fraction and worse the renal function, the greater the benefit from correction of anemia with ESA therapy. Most of these studies enrolled subjects with more severe anemia than the population studied in this trial with more significant decrements in renal function and higher left ventricular mass. Most previous investigations did not have imaging performed by investigators blinded to the patients’ treatment assignment. Also unlike prior studies both groups received oral iron supplementation, which was employed to preserve the blind nature of the trial, but may have impacted on the outcomes. These differences may explain the discrepant results in this trial compared to previous investigations.
In the current trial, the improvements in quality of life were large (11 point declines in the MLWFQ and 15 point improvements in the KCCQ) and comparable in magnitude to improvement seen in trials of biventricular pacing for systolic heart failure48 and in a trial of treatment for acute decompensated heart failure49. However, there were no differences in the degree of improvement in subjects randomized to ESA or placebo. Additionally, the declines observed in plasma volume in subject receiving ESAs were not attributable to differences in the use of diuretics between the two cohorts and were similar to that observed in another trial of ESA in HFLEF in which plasma volume was measured by a similar technique50. Although the effects of ESAs on plasma volume are poorly understood, they have been observed in dialysis patients as well51.
This trial recruited an older adult cohort (mean age >75 years), predominately women (67%) with multiple co-morbidities (including chronic pain and depression) which mimics what is seen in community based studies of heart failure, but has, until now, not been replicated in a prospective clinical trial. We did not have formal measures of frailty (e.g. handgrip strength, weight loss, physical activity scores) except for gait speed which was 0.67 m/sec, well in the range to be considered frail. Given the frail nature of the subjects enrolled and the multiple comorbid conditions which resulted in significant functional limitations attributable to both their underlying heart failure as well as mobility limitations related to chronic arthritis, gait instability and sarcopenia, a majority were unable to perform exercise tests and “home visits” were considered an essential aspect of study design to ensure adherence. Such novel trial designs may be useful in addressing the knowledge gap that occurs when older adults are not recruited into clinical trials30.
The dosing algorithm employed in this trial13 was designed to increase hemoglobin in a small, but safe manner with careful attention to the rate of rise52 and target hemoglobin53. Both of these factors have been shown to be associated with adverse outcomes in subjects receiving erythropoietin stimulating agents. Indeed, analysis of the TREAT trial suggested that current dosing algorithms for erythropoietin stimulating agents are of concern, because of observed increased risk for cardiovascular outcomes among subjects who are hypo-responsive to therapy54. While the targeted rise in hemoglobin was achieved (~1.5 gm/dl) with relatively low doses of ESA in this trial, the difference between active therapy and placebo averaged ~0.8 gm/dl for the duration of the trial. This was a result of an unanticipated rise in hemoglobin in the placebo arm. The latter may be attributable to several factors. First, the use of oral iron in both cohorts, employed to preserve the blinded nature of the study, could have affected increases in hemoglobin. Second, our previous analysis suggests that alterations in diuretic dosages were an independent contributor to hemoglobin values13. Accordingly, while the algorithm employed was safe and resulted in significant increases in hemoglobin that were associated with improvements in peak VO2 as predicted by the Fick equation, the study does not exclude that a different dosing algorithm could be associated with different outcomes. However, given the finding that unresponsiveness to ESAs is associated with an increased risk of adverse outcomes54 coupled with the fact that subjects with HFPEF share many of the same features of subjects who are hypo-responsive to ESA (female gender, obese, mild anemia), higher doses are not without their risks. Since we did not use different definitions of anemia for males and females, using a hemoglobin <=12 gm/dl, while at the higher end of the abnormal range for women, was a single hemoglobin value that met the definition of anemia and could increase the generalizability of our findings. Such a definition has been routinely used in previous trials of erythropoietin stimulating agents for systolic heart failure. Recruiting a population with more severe anemia may have enriched the population with subjects, who could have had a favorable response to erythropoietin, but this is not known and our current analysis of this subgroup does not suggest a difference in outcomes evaluated. Accordingly, alternative trial designs for the population may be warranted. Such designs could employ a run-in phase to assess for erythropoietin responsiveness prior to randomization and more carefully define the presence of a true red cell deficit in patients with volume overload states such as renal dysfunction and heart failure23 in whom a hemodilutional basis of their anemia could be present. Finally, use of iron supplementation may be a more appropriate means of addressing anemia in this population.
The study is limited by its small sample size and the inability to perform several secondary endpoints (e.g. CPET) in a large percentage of subjects. Additionally, as diabetes adversely affects left ventricular remodeling, the higher prevalence of diabetes in the ESA versus control population could have blunted beneficial effects of epoetin on the primary endpoint. By including subjects with an ejection fraction below 50%, some subjects admittedly had EF that were not ‘preserved’, but the final mean ejection fraction of the population was solidly “normal”. While the study subjects recruited were only a small percentage of the total heart failure population seen at our institution, the clinical and demographic characteristics of these subjects suggest that they are quite representative of the majority of patients with HFPEF who are older adult women with long standing hypertension and multiple co-morbidities. Indeed such patients have been traditionally excluded from randomized clinical trials 30, 55, resulting in limited data from which to derive appropriate therapies. Additionally, the trial was of a relatively short duration of six months. However, we included multiple endpoints focusing on both ventricular function measured with tomographic reconstruction, functional capacity measured with multiple modalities and quality of life evaluated in distinct domains.
In conclusion, administration of epoetin alfa to older adult patients with HFPEF compared with placebo did not change cardiac structure nor improve submaximal exercise capacity or quality of life.
Acknowledgments
The authors would like to thank Drs. Stuart Katz, Rachel Bijou, Marrick Kukin, Eva Petkova and Jeanne Teresi who served as members of the DSMB for this trial. We would also like to acknowledge the help in data analysis by Mr. Wei-Ti Huang and Ms. Elizabeth Wang.
Sources of Funding
This research was supported by a grant from the National Institute on Aging (RO1 AG027518). Dr. Maurer is supported by a grant from NIA (K24 AG036778).
Footnotes
Disclosures
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Chs research group. Cardiovascular health study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: The cardiovascular health study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Abramov D, He KL, Wang J, Burkhoff D, Maurer MS. The impact of extra cardiac comorbidities on pressure volume relations in heart failure and preserved ejection fraction. J Card Fail. 2011;17:547–555. doi: 10.1016/j.cardfail.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 6.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: A 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (pep-chf) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 10.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: Treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 11.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the united states. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 12.Patwardhan VN. Nutritional anemias--who research program. Early developments and progress report of collaborative studies. Am J Clin Nutr. 1966;19:63–71. doi: 10.1093/ajcn/19.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Altincatal A, Macarthur RB, Teruya S, Helmke S, Maurer MS. A dosing algorithm for erythropoietin alpha in older adults with heart failure and a preserved ejection fraction. Cardiovasc Ther. 2011 Aug 26; doi: 10.1111/j.1755-5922.2011.00295.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teruya SL, Gil HR, Teresi JA, Kong J, Eimicke J, Helmke S, Maurer MS. Facilitating clinical trials of anemia in older adults: A point-of-care system to measure hemoglobin in the home and its agreement with a hospital core laboratory. J Am Geriatr Soc. 2009;57:2362–2364. doi: 10.1111/j.1532-5415.2009.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 16.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal AS, Keller AM, Shen Z, Sapin PM, Schroeder KM, King DL, Jr, King DL. Three-dimensional echocardiography: In vitro and in vivo validation of left ventricular mass and comparison with conventional echocardiographic methods. J Am Coll Cardiol. 1994;24:504–513. doi: 10.1016/0735-1097(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 18.King DL, Coffin Lel K, Maurer MS. Noncompressibility of myocardium during systole with freehand three-dimensional echocardiography. J Am Soc Echocardiogr. 2002;15:1503–1506. doi: 10.1067/mje.2002.126418. [DOI] [PubMed] [Google Scholar]
- 19.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Cohn JN. Assessment of patient outcome with the minnesota living with heart failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan multicenter research group. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Hawker G, Melfi C, Paul J, Green R, Bombardier C. Comparison of a generic (sf-36) and a disease specific (womac) (western ontario and mcmaster universities osteoarthritis index) instrument in the measurement of outcomes after knee replacement surgery. J Rheumatol. 1995;22:1193–1196. [PubMed] [Google Scholar]
- 23.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008;102:1069–1072. doi: 10.1016/j.amjcard.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldschuh J, Katz S. The importance of correct norms in blood volume measurement. Am J Med Sci. 2007;334:41–46. doi: 10.1097/MAJ.0b013e318063c707. [DOI] [PubMed] [Google Scholar]
- 25.Gerry JL, Baird MG, Fortuin NJ. Evaluation of left ventricular function in patients with sickle cell anemia. Am J Med. 1976;60:968–972. doi: 10.1016/0002-9343(76)90568-4. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RS, Karlin P, Yushak M, Mancini D, Maurer MS. The effect of erythropoietin on exercise capacity, left ventricular remodeling, pressure-volume relationships, and quality of life in older patients with anemia and heart failure with preserved ejection fraction. Congest Heart Fail. 2010;16:96–103. doi: 10.1111/j.1751-7133.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diggle PJHP, Liang K, Zeger SL. Analysis of longitudinal data. 2002. [Google Scholar]
- 28.Zeger, KYLaS Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Storey JD. A direct approach to false discovery rates. J Royal Stat Soc, Series B. 2002;64:479–498. [Google Scholar]
- 30.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo K, Kotecha D, Walters JA, Manzano L, Palazzuoli A, van Veldhuisen DJ, Flather M. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. Cochrane Database Syst Rev. 2010:CD007613. doi: 10.1002/14651858.CD007613.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Kotecha D, Ngo K, Walters JA, Manzano L, Palazzuoli A, Flather MD. Erythropoietin as a treatment of anemia in heart failure: Systematic review of randomized trials. Am Heart J. 2011;161:822–831. e822. doi: 10.1016/j.ahj.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: A community study. Am J Med. 2008;121:726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latado AL, Passos LC, Darze ES, Lopes AA. Comparison of the effect of anemia on in-hospital mortality in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol. 2006;98:1631–1634. doi: 10.1016/j.amjcard.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Muzzarelli S, Leibundgut G, Maeder MT, Rickli H, Handschin R, Gutmann M, Jeker U, Buser P, Pfisterer M, Brunner-La Rocca HP. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010;160:308–314. doi: 10.1016/j.ahj.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 36.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: Results of the candesartan in heart failure: Assessment of reduction in mortality and morbidity (charm) program. Circulation. 2006;113:986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 37.Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: Effects on ventricular hypertrophy in patients with chronic kidney disease. J Nephrol. 2008;21:543–549. [PubMed] [Google Scholar]
- 38.Pappas KD, Gouva CD, Katopodis KP, Nikolopoulos PM, Korantzopoulos PG, Michalis LK, Goudevenos JA, Siamopoulos KC. Correction of anemia with erythropoietin in chronic kidney disease (stage 3 or 4): Effects on cardiac performance. Cardiovasc Drugs Ther. 2008;22:37–44. doi: 10.1007/s10557-007-6075-6. [DOI] [PubMed] [Google Scholar]
- 39.Portoles J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis. 1997;29:541–548. doi: 10.1016/s0272-6386(97)90335-8. [DOI] [PubMed] [Google Scholar]
- 40.Ayus JC, Go AS, Valderrabano F, Verde E, de Vinuesa SG, Achinger SG, Lorenzo V, Arieff AI, Luno J. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dl. Kidney Int. 2005;68:788–795. doi: 10.1111/j.1523-1755.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi T, Suzuki A, Shoji T, Togawa M, Okada N, Tsubakihara Y, Imai E, Hori M. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;35:250–256. doi: 10.1016/s0272-6386(00)70334-9. [DOI] [PubMed] [Google Scholar]
- 42.Hampl H, Hennig L, Rosenberger C, Amirkhalily M, Gogoll L, Riedel E, Scherhag A. Effects of optimized heart failure therapy and anemia correction with epoetin beta on left ventricular mass in hemodialysis patients. Am J Nephrol. 2005;25:211–220. doi: 10.1159/000085881. [DOI] [PubMed] [Google Scholar]
- 43.Low I, Grutzmacher P, Bergmann M, Schoeppe W. Echocardiographic findings in patients on maintenance hemodialysis substituted with recombinant human erythropoietin. Clin Nephrol. 1989;31:26–30. [PubMed] [Google Scholar]
- 44.Palazzuoli A, Silverberg D, Iovine F, Capobianco S, Giannotti G, Calabro A, Campagna SM, Nuti R. Erythropoietin improves anemia exercise tolerance and renal function and reduces b-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152:1096 e1099–1015. doi: 10.1016/j.ahj.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Palazzuoli A, Silverberg DS, Iovine F, Calabro A, Campagna MS, Gallotta M, Nuti R. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and b-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007;154:645 e649–615. doi: 10.1016/j.ahj.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Parissis JT, Kourea K, Panou F, Farmakis D, Paraskevaidis I, Ikonomidis I, Filippatos G, Kremastinos DT. Effects of darbepoetin alpha on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J. 2008;155:751, e751–757. doi: 10.1016/j.ahj.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Roger SD, McMahon LP, Clarkson A, Disney A, Harris D, Hawley C, Healy H, Kerr P, Lynn K, Parnham A, Pascoe R, Voss D, Walker R, Levin A. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): Results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148–156. doi: 10.1097/01.asn.0000102471.89084.8b. [DOI] [PubMed] [Google Scholar]
- 48.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 49.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 50.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 51.Macdougall IC, Davies ME, Hutton RD, Cavill I, Lewis NP, Coles GA, Williams JD. The treatment of renal anaemia in capd patients with recombinant human erythropoietin. Nephrol Dial Transplant. 1990;5:950–955. doi: 10.1093/ndt/5.11.950. [DOI] [PubMed] [Google Scholar]
- 52.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents--time for a reevaluation. N Engl J Med. 2010;362:189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 53.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 54.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363:1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 55.Kitzman DW. Outcomes in patients with heart failure with preserved ejection fraction: It is more than the heart. J Am Coll Cardiol. 2012;59:1006–1007. doi: 10.1016/j.jacc.2011.12.011. [DOI] [PubMed] [Google Scholar]