Abstract
Several genetic risk factors have been identified for Parkinson disease (PD), including mutations in glucocerebrosidase (GBA1). Recently, two single nucleotide polymorphisms (SNPs) described as SCARB2 SNPs were reported to be associated with PD. SCARB2 is an attractive candidate gene for PD as it encodes for lysosomal integral membrane protein type 2 (LIMP-2), a protein involved in transporting glucocerebrosidase from the ER to the lysosome. The first SNP, rs6812193, located 64 kb upstream of SCARB2, was identified in a Parkinson disease Genome Wide Association study of Americans with European ancestry (p = 7.6 × 10−10, OR = 0.84), but was not replicated in a study in the Han Chinese. The second SNP, rs6825004, located within intron 2 of SCARB2 was reported in an association study of Parkinson disease in Greece (p = 0.02, OR=0.68). We explored whether the two SNPs impact SCARB2 expression or LIMP-2 protein levels, testing fifteen control samples. First, the genotypes for each subject were determined for both SNPs using a Taqman assay. Then, RNA and protein were extracted from the corresponding cell pellets. Neither the relative RNA expression by real-time PCR, nor LIMP-2 levels on Western blots correlated with SNP genotype. Thus, these two reported SNPs may not be related to SCARB2 and demonstrate the challenges in interpreting some association studies. While LIMP-2 could still play a role in PD pathogenesis, this study does not provide evidence that the SNPs identified are in fact related to LIMP-2.
Keywords: association, expression, genotyping, single nucleotide polymorphism, Parkinson disease, SCARB2, glucocerebrosidase, lysosomal integral membrane protein type 2 (LIMP-2)
1. Introduction
Several genetic susceptibility alleles have been identified for Parkinson disease (PD) including mutations in GBA1 (glucocerebrosidase) [1, 2]. Recently, two studies reported an association between different single nucleotide polymorphisms (SNPs) near and within intron 2 of the scavenger receptor class B, member 2 (SCARB2) gene and PD. The first, a genome-wide association study (GWAS) of 3,426 American subjects of European descent with PD and 29,624 controls, revealed a significant association with SNP rs6812193, located 64 kb upstream of SCARB2, within an intron of FAM47E ( odds ratio (OR) = 0.84 (p = 7.6 × 10−10)) [3]. A candidate-gene study from Greece showed an association between PD and one of five selected SNPs in SCARB2, rs6825004, located in intron 2 (OR= 0.68 –95% confidence interval, 0.51–0.90) [4].
SCARB2 encodes for lysosomal integral membrane protein type 2 (LIMP-2), involved in the mannose-6-phosphate independent pathway trafficking of glucocerebrosidase(GCase), the enzyme defective in patients with Gaucher disease (GD), to the lysosome [5, 6]. LIMP-2, a member of the CD36 family of scavenger receptors containing 478 amino acids, is among the most abundant lysosomal membrane proteins [7]. Mutations in SCARB2 are responsible for action myoclonus-renal failure syndrome [8], and a SCARB2 mutation was shown to be a disease modifier in GD [9]. The location of rs6812193 and rs6825004 near or in SCARB2, with PD prompted this investigation of their effects on SCARB2 expression prior to investing further in studies of these specific SNPs.
2. Materials and methods
2.1 Genotyping
Lymphocytes and leukocytes from 15 control individuals of Caucasian descent, collected under NIH Institute Review Board approved clinical protocols, were studied. All 15 subjects had no clinical symptoms of PD. DNA was extracted from each sample and screened for GBA mutations by Sanger sequencing as previously described [10]. Genotyping for the rs6812193 and rs6825004 SNPs were performed using tag SNP and the TaqMan SNP Genotyping Assay (Applied Biosystems) following the manufacturer’s protocol.
2.2 Quantitative Real-Time PCR Analysis
RNA samples were extracted using an RNeasy kit (Qiagen), converted to cDNA by the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and relative expression was quantified using the StepOnePlus Real-Time PCR System (Applied Biosystems). Each 20-μl reaction mixture was composed of the following: 10 μl of TaqMan Universal PCR master mix, 1 μl of 20X SCARB2 or 20X HPRT probe (primers included), 100 ng cDNA, and 8 μl nuclease-free water. Three replicates of each reaction were ran for one cycle at 50°C for 2 min then at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec an d 60°C for 1 min.
2.3 SDS-PAGE and Western Blotting
Total protein was isolated from six cell pellets by centrifugation and sonication at room temperature in RIPA buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 25 mM Tris-HCl, pH 7.5). 20 ug of each sample was separated by SDS-PAGE and transferred to iBlot PVDF nitrocellulose membranes (Invitrogen) after quantification with a bicinchoninic acid assay (BCA) (Thermo Scientific). Blots were blocked at room temperature for 1 hr in 1X phosphate buffered saline (PBS) containing 0.5% Tween-20 (Sigma) and 4% fat-free milk. After blocking, the membrane was incubated in 1:1000 of LIMP-2 primary antibody (OriGene Technologies) and blocking buffer at 4°C overnight. The blots were then washed three times for 10 min each, and incubated in 1:2000 horseradish peroxidase (HRP)-conjugated secondary antibody (KPL) and blocking buffer at room temperature for 2 hr. HRP immunoblots were developed using enhanced chemiluminescence (ECL Prime, GE Healthcare). The amount of LIMP-2 was quantified using primary and HRP-conjugated antibodies, and normalized with β-actin.
3. Results
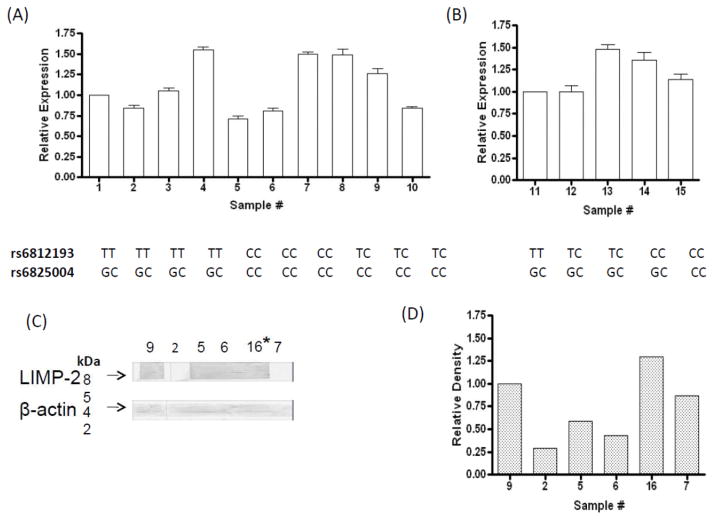
While all three rs6812193 genotypes were identified, none of the samples were homozygous for the minor G allele in rs6825004 (Fig. 1). The relative levels of RNA expression, performed in triplicate and analyzed using the 2−ΔΔCt method with HPRT as an endogenous control, did not correlate with the rs6812193 or rs6825004 genotype (Fig. 1). On Western blots, LIMP-2 was quantified using primary and HRP-conjugated antibodies, and normalized with β-actin. The relative amounts of LIMP2, quantified from the Western blots, showed no association with SNP genotype.
Figure 1.
SCARB2 expression and LIMP-2 levels in lymphocytes. (A) Relative expression of the minor allele for rs6812193 (T) and for rs6825004 (G). (B) Relative expression of LIMP-2 RNA from 5 samples directly extracted from leukocytes. No significant difference was observed. (C- D) Western blot analysis and quantification of LIMP-2 in selected samples. β-actin=loading control. * RNA was not obtained for sample #16, although its genotype was CC for both rs6812193 and rs6825004.
4. Discussion
Despite the attractiveness of SCARB2 as a candidate gene, our results fail to demonstrate any appreciable difference in SCARB2 expression and LIMP-2 levels among samples after grouping by rs6812193 or rs6825004 genotypes. Thus, this work does not provide support that these SNPs actually impact the RNA expression or protein translation of LIMP2. There are several reasons why we are troubled by the previous reports of an association between SCARB2 and PD. The GWAS conducted by Do et al. [3], allowed for the identification of common variations influencing susceptibility to PD using a “case-control” design. Such studies, where allele frequencies in patients are compared to that of a control group, [3, 4, 11, 12] are prone to a number of biases leading to spurious associations, including population stratification. This is supported by the lack of association of rs6812193 with PD in the Han Chinese, a more homogenous ethnic group [13]. The candidate-gene study performed by Michelakakis et al. [4], while less vulnerable to population structure bias because it was conducted solely in central Greece, was the first candidate-gene study used to determine an association between PD and SCARB2. However, rs6825004 was not reported among the most significant SNPs in larger association studies, although this could be because rs6825004 has a small effect size. SNP rs6812193 was not identified in a large PD GWAS in Caucasians [14]. Furthermore, this SNP is in the intronic region of FAM47E, and is closer to the promoter region of the STBD1 gene than SCARB2 [14]. A two-stage meta-analysis also revealed an association between rs6812193 and PD, however, its relationship to SCARB2 is still questionable as it was reported to be near STBD1[15].
5. Conclusions
Gene expression data can substantiate an association signal and further implicate the gene as disease causing. However, it is still quite difficult to definitively conclude that a given SNP is not a risk allele for a given phenotype. Studies of expression quantitative trait loci (eQTLs) are proving to be very complicated. EQTLs can be tissue specific, have small effects, or only manifest when stimulated or activated. In this case, our data is clearly limited by the cell type studied and assays available to us. Furthermore, while our data does not provide any indication that LIMP-2 expression is directly modulated by either of the SNPs identified, it remains possible that other SNPs nearby are true susceptibility SNPs, and that rs6812193 could impact the expression of FAM47E or STBD1.
Acknowledgments
This research was supported by the Intramural Research Program of the National Human Genome Research Institute, NIH.
Footnotes
Chief, Section on Molecular Neurogenetics, Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Building 35, Room 1A213, 35 Convent Dr., MSC 3708, Bethesda, MD 20892-3708m, SCARB2, the gene encoding LIMP-2 is an attractive candidate gene for PD.
Two single nucleotide polymorphisms near SCARB2 were reported as associated with PD. These SNPs did not impact SCARB2 expression or LIMP-2 protein levels in 15 control samples. This study does not provide evidence that the SNPs identified are in fact related to LIMP-2.
Disclosures:
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, Rudolph A, Wojcieszek J, Pfeiffer RF, Foroud T. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72:310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelakakis H, Xiromerisiou G, Dardiotis E, Bozi M, Vassilatis D, Kountra PM, Patramani G, Moraitou M, Papadimitriou D, Stamboulis E, Stefanis L, Zintzaras E, Hadjigeorgiou GM. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson’s disease. Mov Disord. 2012;27:400–405. doi: 10.1002/mds.24886. [DOI] [PubMed] [Google Scholar]
- 5.Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin XY, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-Glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/s0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 8.Berkovic SF, Dibbens LM, Oshlack A, Silver JD, Katerelos M, Vears DF, Lullmann-Rauch R, Blanz J, Zhang KW, Stankovich J, Kalnins RM, Dowling JP, Andermann E, Andermann F, Faldini E, D’Hooge R, Vadlamudi L, Macdonell RA, Hodgson BL, Bayly MA, Savige J, Mulley JC, Smyth GK, Power DA, Saftig P, Bahlo M. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:673–684. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velayati A, DePaolo J, Gupta N, Choi JH, Moaven N, Westbroek W, Goker-Alpan O, Goldin E, Stubblefield BK, Kolodny E, Tayebi N, Sidransky E. A mutation in SCARB2 is a modifier in Gaucher disease. Hum Mutat. 2011;32:1232–1238. doi: 10.1002/humu.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TA, Manolio TA. How to interpret a genome-wide association study. Jama-Journal of the American Medical Association. 2008;299:1335–1344. doi: 10.1001/jama.299.11.1335. [DOI] [PubMed] [Google Scholar]
- 12.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhang Y, Chen W, Wang Y, Liu J, Rong TY, Ma JF, Wang G, Zhang J, Pan J, Xiao Q, Chen SD. Association study of SCARB2 rs6812193 polymorphism with Parkinson’s disease in Han Chinese. Neurosci Lett. 2012;516:21–23. doi: 10.1016/j.neulet.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Parkinson’s Disease Genomics Consortium (IPDGC), Wellcome Trust Case Control Consortium 2 (WTCCC2) A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011;7:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]