Abstract
The synthesis of a novel pharmacophore comprising a DNA-targeted platinum–acridine hybrid agent and estrogen receptor-targeted 4-hydroxy-N-desmethyltamoxifen (endoxifen) using carbamate coupling chemistry and its evaluation if breast cancer cell lines are described.
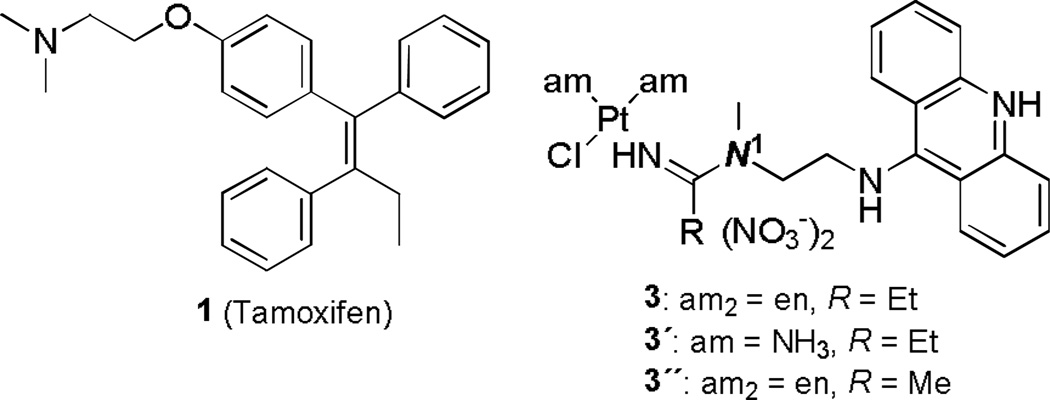
Breast cancer continues to be a major cause of cancer-related mortality in women.1 Anti-estrogens such as tamoxifen (Figure 1, compound 1) have become the mainstay in the management of the early-stage hormone-dependent form of the disease.1 Unfortunately, a significant population of patients does not respond to this drug or develops resistance to endocrine therapy during the course of treatment, and systemic chemotherapies often become the only armamentarium to combat recurrent disease.2 Current platinum-based drugs have shown limited utility as first-line or salvage therapies and no survival benefits have been reported for regimens based on these agents in combination with targeted therapies.3 The general lack of responsiveness of breast cancers to platinum has been attributed to unusually high levels of acquired resistance to this treatment including detoxification by elevated levels of cytosolic glutathione and repair of DNA adducts.3 One common strategy to overcome several of these problems and provide more effective drugs for hormone-dependent breast cancer is to combine an estrogen receptor (ER)-binding molecule with a classical cytotoxic agent. The rationale behind this approach is to improve the pharmacological properties of common cytotoxics by taking advantage of the targeting, antiestrogenic, and chemosensitizing effects of ER-interacting ligands.4 Various conjugates containing ER-affinic carriers have been designed with the goal of targeting ER-positive breast cancers more efficiently with the drug cisplatin (cis-diamminedichloridoplatinum(II)), 2)5–8 and other cytotoxic agents.9–12
Fig. 1.
Structures of tamoxifen and platinum–acridine hybrids.
While several of the cis-platinum-based compounds have shown promising activity in vitro and in mouse models,5 no attempts of extending this concept to promising nonclassical platinum anticancer agents have been reported. One such class of compounds are platinum–acridine agents (Figure 1), which, unlike cisplatin, do not induce cross-links but target DNA through a dual binding mode involving intercalation and monofunctional platination of nucleobase nitrogen.13 In non-small cell lung cancer (NSCLC), the hybrid agents produce more severe DNA damage than cisplatin and are significantly more cytotoxic than the clinical agent.14–17 Given the superior cell kill of these agents and their ability to circumvent resistance to cisplatin, we embarked on generating a breast cancer targeted agent containing our platinum–acridine hybrid as a cytotoxic component. Here, we report on the design of the first conjugates featuring a suitably functionalized platinum–acridine warhead coupled to an active metabolite of the ER modulator and anticancer drug tamoxifen. The synthetic methodology developed to generate these molecules is presented as well as screening results in breast cancer cells.
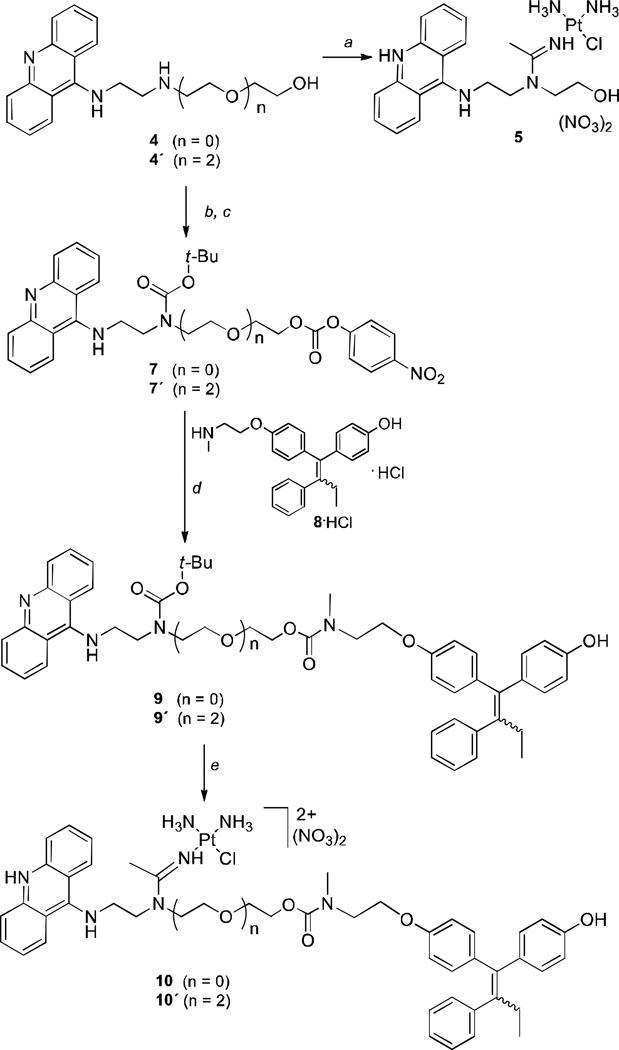
The strategy for generating the conjugates was to functionalize a suitable platinum–acridine at the N1 position (see Figure 1) and attach it to the secondary amino group of (E/Z)-4-hydroxy-N-desmethyltamoxifen [(E/Z)-endoxifen, 8, the monohydroxylated/N-demethylated metabolite of tamoxifen,18 Scheme 1]. Molecular models (not shown) were used to confirm that linking the two pharmacophores at this position does not interfere with the binding of the individual components with their pharmacological targets.19,20 The 4-hydroxo form of the pharmacophore was chosen because of its superior binding affinity for ER and exquisite potency as an inhibitor of hormone-dependent breast cancer cell proliferation18 (confirmed in this study). In addition, since the E- and Z-isomers of 4-hydroxytamoxifen (but not those of tamoxifen) interconvert under physiologically relevant conditions,21 it was not necessary to generate the stereochemically pure forms of the target compounds.
Scheme 1.
Synthesis of target molecules. Reagents and conditions: (a) 1. For precursor 4: [PtCl(NH3)2(MeCN)]NO3, DMF, −20 °C; 2. 1 M HNO3, MeOH. (b) Boc2O, DCM, rt. (c) 4-nitrophenyl chloroformate, TEA, DCM, rt. (d) 1. TEA, DCM, rt; 2. TFA, DCM, rt; 3. 1 M NaOH, DCM, rt. (e) 1. [PtCl(NH3)2(MeCN)]NO3, DMF, 4 °C; 2. 1 M HNO3, MeOH.
First, we designed the conjugatable platinum–acridine (compound 5, Scheme 1) based on the most potent derivatives identified to date (Figure 1). The target cytotoxic warhead features simple ammine (NH3) nonleaving groups to maximize the aqueous solubility13 of the conjugate and an acetamidine donor group (R = Me), which has proven to produce a 3–4 times more cytotoxic hybrid than propionamidine (R = Et).17 The synthesis of compound 5 involved addition of the linker secondary amino group in 2-((2-(acridin-9-ylamino)ethyl)amino)ethanol (4) and its ethylene glycol extended derivative (4´) to the nitrile ligand in [PtCl(NH3)2(MeCN)]NO3 (Scheme 1). Attempts to directly attach the endoxifen moiety to the hydroxyl group in 5 using carbamate coupling chemistry failed because the reaction conditions were incompatible with the metal-containing fragment. Instead, it was necessary to introduce platinum as the last step of the reaction sequence after preassembling the entire organic scaffold. This was achieved by reacting N-Boc-protected and 4-nitrophenyl carbonate-activated acridine–amines 7 and 7´ with endoxifen (8) to generate the carbamate-coupled conjugates 9 and 9´, followed by addition of platinum nitrile complex to install the amidine-linked platinum moiety (Scheme 1). Conjugates 10 and 10´ were isolated as 1:1 mixtures of the E- and Z-isomers (for details of the synthetic procedures and product characterization, see the Supplementary Information).
The carbamate linkage in the conjugates was chosen for its chemical robustness to assure stability in circulation and delivery of the intact conjugate to the target site.22 To test the reactivity of the carbamate group in aqueous media, conjugate 10 was incubated at 37 °C for 48 h in buffers mimicking the environments in serum, cytosol, and the lysosomes. In-line high-performance liquid chromatography–mass spectrometry (LC–MS) analysis of the samples confirmed that the carbamate linkage in compound 10 resists hydrolytic cleavage in all cases (see Supplementary Information).
To assess the cytotoxic potency of the target conjugates, a colorimetric cell proliferation assay was performed in the human breast adenocarcinoma cell lines MCF-7, which overexpresses estrogen receptor (ER+), and the estrogen receptor-negative (ER-) cell line MDA-MB-231.23 Compounds 10 and 10´ were tested along with the two precursors, hydroxyl-modified platinum–acridine 5, and (E/Z)-4-hydroxy-N-desmethyltamoxifen (8). For comparison, the clinical drugs tamoxifen (1) and cisplatin (2) were also included in this assay (Table 1). The tamoxifen metabolite, 8, showed the strongest antiproliferative effect in MCF-7 with an IC50 value of 1.6±0.4 µM determined after exposure of cells to the drug for 72 h. Under the same conditions, a 2.4-fold higher concentration of compound 5 is required to produce the same cell kill effect in this cell line. Conjugate 10 shows virtually the same potency as its cytotoxic component, 5, whereas the extended conjugate 10´ was the least active compound in this assay. All compounds were significantly more cytotoxic in MCF-7 cells than in MDA-MB-231 cells. For compounds 5, 8, and 10, for example, the cell line-specific cytotoxic enhancements were 3-fold, 8-fold, and 6-fold, respectively. While compounds 5 and 10 are equipotent in MCF-7, the conjugate proves to be relatively less cytotoxic in MDA-MB-231. Tamoxifen, the mainstay of breast cancer treatment, and cisplatin were the least active compounds tested in the ER-positive cell line.
Table 1.
Summary of cytotoxicity data (IC50, µM)a in human breast cancer cell lines
| Compound | MCF-7 | MDA-MB-231 |
|---|---|---|
| 1 (tamoxifen) | 16.2 ± 1.2 | 20.1 ± 1.0 |
| 2 (cisplatin) | 12.1 ± 1.1 | 40.6 ± 4.2 |
| 5 | 3.8 ± 0.4 | 11.3 ± 0.2 |
| 8 (endoxifen) HCl | 1.6 ± 0.4 | 13.2 ± 0.1 |
| 10 | 3.5 ± 0.1 | 21.4 ± 1.0 |
| 10´ | 27.5 ± 1.4 | 46.5 ± 0.7 |
The IC50 values are reported as the mean ± standard deviation for two individual 72-h incubations performed in triplicate.
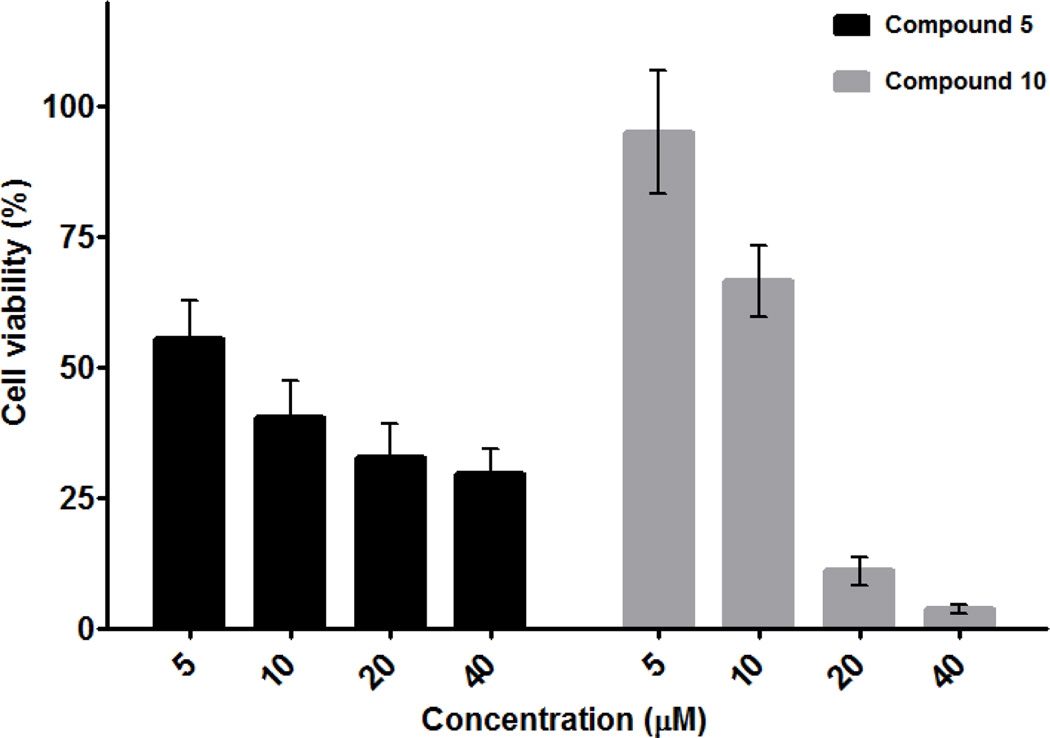
Modification of compound 5 with endoxifen (8) produces a conjugate (10) that shows essentially the same cytotoxic response (based on IC50 values) as the unmodified platinum–acridine hybrid in MCF-7 cells. While equipotent, both compounds can be expected to induce cancer cell kill by different mechanisms. A comparison of the drug–response profiles for compounds 5 and 10 after 48 h of incubation with MCF-7 cells confirms this supposition. While the platinum–acridine agent 5 clearly shows an antiproliferative advantage over compound 10 at low concentrations, it is unable to kill a major fraction of the cells at the highest concentrations tested (Figure 2). These findings suggest that a subpopulation of cells may exist that are relatively insensitive to this agent, as has been observed previously for MCF-7 cells exposed to cytotoxics.24 Importantly, compound 10 appears to overcome this form of resistance, based on the considerably lower percentage of cells that remain viable after incubation with 20 and 40 µM conjugate for 48 h.
Fig. 2.
Differential response of MCF-7 cancer cells to compounds 5 and 10.
Here, we have presented the first conjugate that combines a DNA-platinating moiety, a DNA intercalator, and a ER-binding moiety in a single molecular entity. The synthetic approach provides a versatile platform for incorporating a wide range of small-molecule receptor- and cancer cell-affinic ligands, as well as enzymatically cleavable and self-immolative linkers.25 Such modifications will help target cancers more selectively and reduce the undesired high systemic toxicity13 observed for the simple platinum–acridines. Using a hormone-dependent cancer model, we demonstrated that the ER-targeted form of platinum–acridine maintains the same cytotoxicity as compound 5. A comparison of the relative cytotoxic enhancements observed for 5 and 10 in ER+ MCF-7 vs. ER- MDA-MB-231 (Table 1) and the individual drug responses for each derivative suggest that conjugate 10 acts by a distinct mechanism potentially involving interactions with ER. Further investigation into the molecular mechanism of the conjugate is necessary to substantiate this notion. Likewise, it will be important to determine why introduction of an extended linker in compound 10´ results in a greatly diminished cytotoxic response and if the activity of the components can be improved by introducing a cleavable linker.
In conclusion, the novel conjugate 10 was generated in an attempt to marry the cytotoxic, DNA-damaging properties of a nonclassical platinum–acridine agent with the targeting potential of an antiestrogen. Initial tests show promising cell kill effects superior to the responses elicited by either cisplatin or tamoxifen. This observation warrants further studies into structure–activity relationships in derivatives of the prototypical agent and their in vivo efficacy against hormone-dependent breast cancer. Multifunctional agents like compound 10 may have applications in patients not responding to tamoxifen due to altered expression levels of ER or an intrinsic inability to metabolize tamoxifen.26
Supplementary Material
Acknowledgments
This work was supported by the US National Institutes of Health (grant CA101880) and a scholarship to X. Q. from the China Scholarship Council (grant #2011694010).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures, details of product characterization and purity. S e e DOI: 10.1039/b000000x/
Notes and references
- 1.Cancer Facts & Figures 2012. American Cancer Society: Atlanta, GA; 2012. [Google Scholar]
- 2.Higgins MJ, Stearns V. Clin. Chem. 2009;55:1453–1455. doi: 10.1373/clinchem.2009.125377. [DOI] [PubMed] [Google Scholar]
- 3.Decatris MP, Sundar S, O'Byrne KJ. Cancer Treat. Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 4.Smith CL, O'Malley BW. Endocr. Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 5.Gust R, Beck W, Jaouen G, Schonenberger H. Coord. Chem. Rev. 2009;253:2760–2779. [Google Scholar]
- 6.Gust R, Beck W, Jaouen G, Schonenberger H. Coord. Chem. Rev. 2009;253:2742–2759. [Google Scholar]
- 7.Barnes KR, Kutikov A, Lippard SJ. Chem. Biol. 2004;11:557–564. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Rye PT, Essigmann JM, Croy RG. J. Inorg Biochem. 2009;103:256–261. doi: 10.1016/j.jinorgbio.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra K, Marquis JC, Hillier SM, Rye PT, Zayas B, Lee AS, Essigmann JM, Croy RG. J Am. Chem. Soc. 2002;124:1862–1863. doi: 10.1021/ja017344p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke PJ, Koch TH. J Med Chem. 2004;47:1193–1206. doi: 10.1021/jm030352r. [DOI] [PubMed] [Google Scholar]
- 11.Peng K-W, Wang H, Qin Z, Wijewickrama GT, Lu M, Wang Z, Bolton JL, Thatcher GRJ. ACS Chem. Biol. 2009;4:1039–1049. doi: 10.1021/cb9001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dao K-L, Sawant RR, Hendricks JA, Ronga V, Torchilin VP, Hanson RN. Bioconj. Chem. 2012;23:785–795. doi: 10.1021/bc200645n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Z, Choudhury JR, Wright MW, Day CS, Saluta G, Kucera GL, Bierbach U. J. Med Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao X, Zeitany AE, Wright MW, Essader AS, Levine KE, Kucera GL, Bierbach U. Metallomics. 2012;4:645–652. doi: 10.1039/c2mt20031g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyre CL, Saluta G, Kute TE, Kucera GL, Bierbach U. ACS Med. Chem. Lett. 2011;2:870–874. doi: 10.1021/ml2001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostrhunova H, Malina J, Pickard AJ, Stepankova J, Vojtiskova M, Kasparkova J, Muchova T, Rohlfing ML, Bierbach U, Brabec V. Mol. Pharmaceut. 2011;8:1941–1954. doi: 10.1021/mp200309x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham LA, Wilson GM, West TK, Day CS, Kucera GL, Bierbach U. ACS Med. Chem. Lett. 2011;2:687–691. doi: 10.1021/ml200104h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Breast Cancer Res. Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 19.Baruah H, Wright MW, Bierbach U. Biochemistry. 2005;44:6059–6070. doi: 10.1021/bi050021b. [DOI] [PubMed] [Google Scholar]
- 20.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 21.Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF. Cancer Res. 1984;44:112–119. [PubMed] [Google Scholar]
- 22.Alley SC, Okeley NM, Senter PD. Current Opin. Chem. Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix M, Leclercq G. Breast Cancer Res. Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 24.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Cell Death Differ. 2007;14:500–510. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 25.Wang RE, Costanza F, Niu Y, Wu H, Hu Y, Hang W, Sun Y, Cai J. J. Control. Release. 2012;159:154–163. doi: 10.1016/j.jconrel.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Musgrove EA, Sutherland RL. Nature Rev. Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.