INTRODUCTION
Mast cells (MCs) are multifunctional cells of the immune system, having been shown to participate in a wide variety of important biological processes such as allergy, immune defense against parasites, and tissue remodeling.1 More recently, MCs have been revealed as critical components of host defense against bacterial pathogens, exerting a variety of intra- and extracellular antimicrobial activities. Not unlike professional phagocytic cells such as neutropihls and macrophages, MCs have been shown to eliminate bacteria through phagocytosis,2 release of antimicrobial peptides3 and formation of MC extracellular traps (MCETs).4 However, the detailed signaling events including the involvement of specific transcriptional regulators to mediate MC antimicrobial activity during infection are still unknown.
Hypoxia-inducible factor-1 (HIF-1) plays a role in regulating the type and magnitude of inflammatory and innate immune responses produced by neutrophils and macrophages [5,6]. HIF-1 is an oxygen-sensitive heterodimeric helix-loop-helix transcription factor, composed of a constitutively expressed β-subunit and a tightly regulated α-subunit. HIF-1α-specific binding to hypoxia-resposive elements (HREs) regulates the transcription of target genes including those encoding erythropoietin, glucose transporters, glycolytic enzymes, antimicrobial factors and the angiogenic factor VEGF [7,8]. Here, we investigated the role of HIF-1α in the antimicrobial activities of MCs.
MATERIALS AND METHODS
Mast cells
In this study the human MC line HMC-1 was used as a model cell line [9] and cultured as previously described [4]. Bone marrow-derived mast cells (BMMCs) from C57BL/6 wild-type (WT) and myeloid HIF-1α-deficient mice [5] were isolated and cultured as reported [4]. To determine the purity and differentiation status of BMMCs, cells were stained with a PE-labeled anti-mouse CD117 antibody (0.2 μg/106 cells) (Southern Biotechnology) and analyzed by flow cytometry using a FACSCalibur™ flow cytometer (BD Biosciences). More than 95% of the cells were confirmed to be positive for CD117.
Co-incubation of MCs and bacteria
MC antimicrobial activity against S. aureus strain Newman was monitored in vitro at different time points of co-incubation. MCs were centrifuged for 10 min at 800 rpm, washed once with sterile 1 x PBS and resuspended in infection medium (IMDM including 25 mM Hepes, L-glutamine and sodium bicarbonate, supplemented with 2% nuclease-free heat-inactivated FCS [10]. In case of the BMMCs only, 0.1 mM MEM was also added. MCs were then seeded in 24-well-plates (HMC-1) or 48-well-plates (BMMCs) at a density of 4 x 106 cells/ml. Mid-log-phase bacteria grown in Todd Hewitt broth (THB) were harvested by centrifugation for 5 min at 4000 rpm and resuspended in infection medium. Bacteria were added in a volume of 50 μl to the seeded MCs at a multiplicity of infection (MOI) of 1 bacterium per cell. After co-incubation at 37 °C, 5 % CO2 for different time points, the MCs were lysed with a final concentration of 0.025 % (v/v) Triton X-100 and serial dilutions of the culture-suspension were plated on THB agar. The surviving CFU were enumerated and the percentage of surviving bacteria in comparison to bacterial growth control (100%), grown under the same conditions in the absence of MCs, was determined.
To analyze effector mechanisms underlying MC antimicrobial activity, MCs were treated with 10 μM diphenylene iodonium (DPI in DMSO; 1 h prior to infection) (Sigma) to block NADPH-oxidase-dependent ROS-synthesis. To differentiate an extracellular versus an intracellular (phagocytotic) process, MCs were treated for 10 min with 10 μg/ml cytochalasin D (in DMSO) (Sigma) to block phagocytosis prior to infection. To degrade MCETs, cells were treated with 500mU/ml micrococcal nuclease (Sigma) starting 1 h prior to infection. The results were compared to MCs that were treated with respective amounts of vehicle controls.
Blocking and boosting of HIF-1α-activity
To evaluate the impact of HIF-1α on the antimicrobial activity of MCs, cultures were preincubated with HIF-1α antagonist echinomycin (Alexis Biochemicals), prepared in DMSO and added at 0.32 μM for 4 h prior to infection, and/or HIF-1α agonist AKB-4924 [11] (Aerpio Therapeutics, Cincinnati, OH), prepared in DMSO at 10 μM at pH 4.3 and added 1 h prior to infection. The results were compared to MCs treated with respective amount of vehicle controls.
Immunofluorescence microscopy
For visualization and analyses of HIF-1α accumulation and MCET formation, MCs were seeded on poly-L-lysine (Sigma) coated glass-cover slides and treated as described above. MCETs were visualized without fixation using the Live/Dead viability/cytotoxicity kit for mammalian cells (Invitrogen) as recommended by the manufacturer. For immunostaining of HIF-1α, cells were fixed with 4% PFA, washed with PBS, and blocked for 45 min with 2% BSA, 2% goat-serum, 0.2% Triton X-100 in PBS. Next, samples were incubated with polyclonal rabbit-anti-HIF-1α (Novus Biologicals; 1:100 diluted) diluted in 1 x PBS including 2% BSA and 0.2% Triton X-100 for 60 min at room temperature. A universal rabbit IgG (Dako) served as negative control. After washing 3 times with 1 x PBS, samples were incubated with secondary Alexa 488-labelled goat-anti-rabbit-IgG (1:500 in 1 x PBS including 2% BSA and 0.2% Triton X-100) (Invitrogen) for 60 min at room temperature in the dark and mounted with ProlongGold with DAPI (Invitrogen).
The Live/dead BacLight TM Bacterial Viability Kit (Invitrogen) was used to determine viability of S. aureus entrapped in the MCETs by fluorescence microscopy. After staining as recommended by the manufacturer, cells were washed 3 times with PBS, fixed with 1% PFA for 5 min, washed, and mounted onto glass slides with ProlonGold with Dapi (Invitrogen).
Mounted samples were examined using an inverted confocal laser-scanning 2-photon microscope Olympus Fluoview FV1000 with Fluoview TM Spectral Scanning technology (Olympus). Confocal Z-stack-images were obtained using a 60x/1.42 PlanApo objective. Alternatively, fluorescence images were recorded using a Zeiss Axiolab microscope (Zeiss 20 x /0.5 Plan-Neofluor or 40x/0.65 Achroplan Zeiss objective) with an attached Sony Digital Photo Camera DKC-5000 at calibrated magnifications.
Statistical analysis
Data were analyzed using GraphPad Prism 4.0 (GraphPad Software). Each experiment was performed in duplicate at least 3 independent times. Mean and SEM were calculated from all independent experiments and compared to their respective controls. Differences between groups were analyzed using a paired Student's t-test.
RESULTS AND DISCUSSION
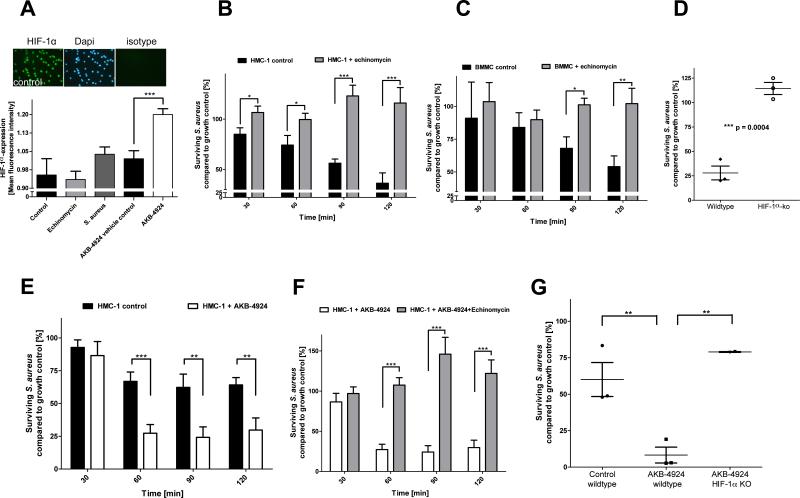
MCs have been reported to express HIF-1α constitutively [12], a finding we confirmed under our experimental conditions by immunofluorescence microscopy with HIF-1α-specific antibodies (Figure 1A). Infection of MCs with S. aureus slightly increased HIF-1α expression, but this effect was not statistically significant (Figure 1A, p = 0.1366, t-test). MCs were treated with echinomycin, which blocks HIF-1α activation of target genes by binding to HRE sequences [13]. Echinomycin did not influence HIF-1α expression of MCs, a potential secondary effect reported in certain epithelial cell cultures under normoxia (Figure 1A) [14]. Pre-treatment of HMC-1 cells (Figure 1B) or BMMCs (Figure 1C) with echinomycin completely abolished their antimicrobial activity against S. aureus Newman. To further corroborate the involvement of HIF-1α, BMMCs were isolated from mice lacking HIF-1α in the myeloid cell lineage [5] and tested for their antimicrobial activity. As shown in Figure 1D, HIF-1α-deficient BMMCs showed a significantly reduced antimicrobial effect against S. aureus compared to control BMMCs (Figure 1D).
Figure 1. Role of HIF-1α in the antimicrobial activity of MCs.
(A) Relative HIF-1α-expression in HMC-1 cells. MCs were seeded on poly-L-lysine coated glass-cover slides, stimulated with respective reagents or bacteria, fixed with 4 % PFA and immunostained. HIF-1α-expression was visualized in green and compared to total cell (Dapi) and respective isotype background staining. Mean fluorescence intensities were measured at equal exposure times and quantified using Image J. (B) HIF-1α-antagonist echinomycin blocks the antimicrobial activity of HMC-1 cells against S. aureus. (C) HIF-1α-antagonist echinomycin blocks the antimicrobial activity of BMMCs against S. aureus. (D) Loss of HIF-1α results in significantly reduced anti-microbial activity of BMMCs against S. aureus. Each data point shows the mean value of one independent experiment, using murine BMMCs from different individuals. (E) HIF-1α-agonist AKB-4924 boosts the antimicrobial activity of HMC-1 against S. aureus. HMC-1 cells were treated with 10 μM AKB-4924 or respective amounts of vehicle control 1 h prior to infection to increase HIF-1α-activity. (F) Echinomycin abolishes the AKB-4924-induced antimicrobial activity of HMC-1 against S. aureus. HMC-1 cells were treated with 0.32 μM echinomycin 4 h prior to infection to block HIF-1α-signalling, and 10 μM AKB-4924 or respective amounts of vehicle control was added 1 h prior to infection to increase HIF-1α-activity. (G) AKB-4924 boosts the antimicrobial activity of BMMCs derived from WT, but not from myeloid HIF-1α-deficient mice. BMMCs were treated with 10 μM AKB-4924 or respective amounts of vehicle control 1 h prior to infection with S. aureus to increase HIF-1α-activity. Each data point shows the mean value of one independent experiment, using murine BMMCs from different individuals. All data are presented as percentage of surviving bacteria in comparison to bacterial growth control (100%). * p < 0.05; **p < 0.01; ***p < 0.005 by a paired t-test.
Pharmacological augmentation of HIF has been shown to boost the bactericidal activity of macrophages and neutrophils [11,15]. Here, the new pharmacological agent AKB-4924 [11] was used to inhibit prolyl hydroxylases involved in the HIF-1α degradation pathway and to increase HIF-1α protein levels in MCs, as confirmed by immunofluorescence microscopy (Figure 1A). As shown in Figure 1E, HMC1-cells pretreated with 10 μM AKB-4924 for 1h showed significantly increased antimicrobial activity against S. aureus compared to control cells treated with vehicle only. When echinomycin was used to block HIF-1α-signalling, the AKB-4924-induced antimicrobial effect was abolished (Figure 1F). AKB-4924 also increased the antimicrobial activity of WT BMMCs, but not that of HIF-1α-deficient BMMCs, further validating the HIF-1α-specific effect (Figure 1G).
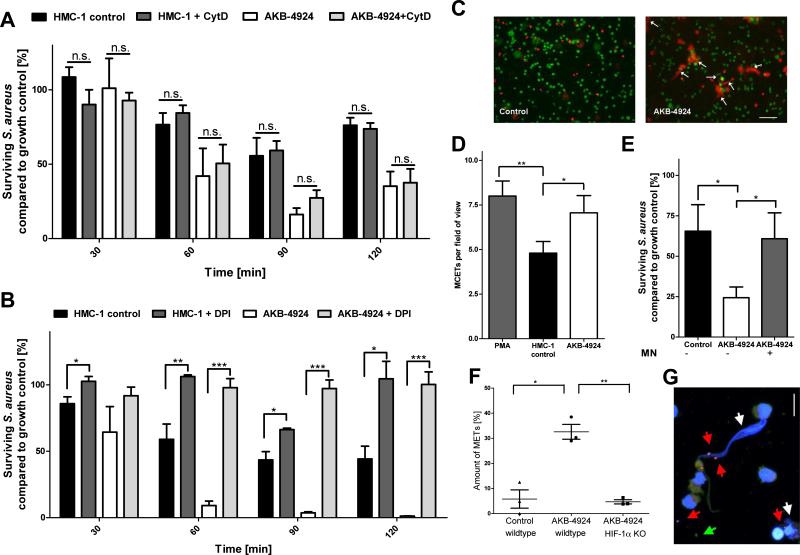
To differentiate whether the HIF-1α effect in boosting MC antimicrobial activity was operating through intra- vs. extracellular mechanisms, AKB-4924 treated and control cells were treated with cytochalasin D to block phagocytosis. As shown in Figure 2A, inhibition of phagocytosis did not alter the antimicrobial activity of MCs, whether or not HIF-1α activity was boosted with AKB-4924. These data suggest that HIF-1α primarily mediates an extracellular bactericidal activity of the MCs. Recently, ROS-dependent formation of MCETs has been identified as a significant contributor to the antimicrobial activity of MCs [4]. MCETs are the result of a specialized cell death process [16] in which extracellular fibres consisting of a DNA backbone and embedded antimicrobial peptides, histones and proteases locally entrap and kill bacterial pathogens such as S. aureus [4]. Interestingly, when MCs were treated with DPI to block the NADPH-dependent formation of ROS, baseline as well as AKB-4924-boosted antimicrobial activity was blocked (Figure 2B), supporting a role for MCETs in this process. As directly visualized and quantified by fluorescence microscopy, significantly more MCETs were detected after treating HMC-1 cells and BMMCs with AKB-4924 to boost HIF-1α levels (Figures 2C, D and F). AKB-4924-induced MCET-formation was absent in HIF-1α-deficient BMMCs (Figure 2F). Supporting the antimicrobial role of MCETs, treatment of MCs with micrococcal nuclease to degrade MCETs resulted in a significant loss of MC-antimicrobial activity, even with prior boosting of HIF-1α with AKB 4924 (Figure 2E). Additionally, visualization of bacterial viability after boosting of HIF-1α with AKB 4924 revealed dead bacteria entrapped in MCETs (Figure 2G).
Figure 2. HIF-1α is mediating the extracellular antimicrobial activity of MCs.
(A) HIF-1α-mediated antimicrobial activity of HMC-1 cells against S. aureus via extracellular mechanism. HMC-1 cells were treated with 10 μM AKB-4924 or vehicle control to boost HIF-1α-signalling (1 h prior to infection) and 10 μg/ml cytochalasin D to block phagocytosis (10 min prior to infection). (B) HIF-1α-mediated antimicrobial activity of HMC-1 cells against S. aureus is ROS-dependent. HMC-1 cells were treated with 10 μM DPI to block NADPH-oxidase-dependent formation of ROS simultaneously with 10 μM AKB04924 to boost HIF-1α-activity. (C) Boosting of HIF-1α with AKB-4924 results in the induction of MCETs. Representative fluorescent images of MCETs, stained with Live/Dead viability/cytotoxicity kit for mammalian cells (staining cytoplasm of viable cells in green and DNA released by dead cells in red). The extracellular deposition of DNA is indicated by white arrows. Bar 25 μm. (D) MCETs were counted per field of view with approximately 125 MCs after stimulation for 2.5 h with 10 μM AKB04924, vehicle control or 1.5 h with 25 nM PMA as positive control. (E) HMC-1 cells were treated with 10 μM A4924 or vehicle control to boost HIF-1α-activity and 500 mU/ml micrococcal nuclease (1 h prior to infection) to degrade MCETs. Then, HMC-1 cells were infected with S. aureus (MOI=1) for 90 minutes and the antimicrobial activity was measured. (F) AKB-4924 boosts the formation of MCETs of BMMCs derived from WT, but not from myeloid HIF-1α-deficient mice. Each data point shows the mean value of an independent experiment, using BMMCs from different individual mice. (G) Representative high resolution confocal fluorescent z-stack images of MCETs boosted with AKB-4924 and infected with S. aureus. Analysis of viable (green, green arrows) versus dead (red, red arrows) bacteria entrapped in MCETs (white arrows) as determined by the Live/dead BacLight viability assay. Bar 8 μm. All data in (A, B, D-F) are presented as percentage of surviving bacteria in comparison to bacterial growth control (100%). *p < 0.05; **p < 0.01; ***p < 0.005 by a paired t-test.
In summary, the results of our study show for the first time that the transcription factor HIF-1α is a key regulator of the extracellular antimicrobial activity of MCs. An augmentation of HIF-1α-activity resulted in a boosting of the antimicrobial activity of human and murine MCs by inducing the formation of MCETs. This new knowledge has significant implications for understanding the role of MCs in host defense against bacterial infections.
SYNOPSIS.
Mast cells (MCs) are critical components of the host innate immune defense against bacterial pathogens, providing a variety of intra- and extracellular antimicrobial activities. Here we show for the first time that the transcriptional regulator hypoxia-inducible factor-1α (HIF-1α) mediates the extracellular antimicrobial activity of human and murine MCs by increasing the formation of mast cell extracellular traps.
ACKNOWLEDGEMENTS
We thank Drs. Robert Shalwitz and Anna Kotsakis of Aerpio Therapeutics, Cincinnati, OH, for providing AKB-4924. Confocal imaging used in the present study was performed at the UCSD Neurosciences Light Microscopy Facility, La Jolla, California, USA.
FUNDING
M. v. K.-B. was supported by a fellowship from the Deutsche Akademie der Naturforscher Leopoldina (BMBF-LPD 9901/8-187) and K. B.-H. by a fellowship from the German Academic Exchange Service (DAAD). C. Y. O. was supported through the UCSD/SDSU IRACDA Postdoctoral Fellowship Program (GM06852). Support for this work was provided by NIH grants AI090863 and (V. N.).
Footnotes
AUTHOR CONTRIBUTIONS
K. B.-H. performed research, analysed and interpreted data and wrote the manuscript; C.O. and L. V. performed research, analysed and interpreted data, and provided critical reading of the manuscript; T. K., Y. K. provided isolated and differentiated BMMCs and critical proof-reading of the manuscript; H. Y. N., provided interpretation of data and critical proof-reading of the manuscript; V.N. designed research, interpreted data and wrote the manuscript; M.v.K.-B. designed and performed research, analysed and interpreted data and wrote the manuscript.
REFERENCES
- 1.Henz BM. Exploring the mast cell enigma: a personal reflection of what remains to be done. Exp. Dermatol. 2008;17:91–99. doi: 10.1111/j.1600-0625.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 2.Malaviya R, Ross EA, MacGregor JI, Ikeda T, Little JR, Jakschik BA, Abraham SN. Mast cell phagocytosis of FimH-expressing enterobacteria. J. Immunol. 1994;152:1907–1914. [PubMed] [Google Scholar]
- 3.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 4.von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 5.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackmann N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber H-P, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk. Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 10.von Köckritz-Blickwede M, Chow O, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Köckritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, Shalwitz RA, Johnson RS, Nizet V. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infections. J. Mol. Med. 2012 doi: 10.1007/s00109-012-0882-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong HJ, Chung HS, Lee BR, Kim SJ, Yoo SJ, Hong SH, Kim HM. Expression of proinflammatory cytokines via HIF-1α and NF-κB activation on desferrioxamine-stimulated HMC-1 cells. Biochem. Biophys. Res. Commun. 2003;306:805–811. doi: 10.1016/s0006-291x(03)01073-8. [DOI] [PubMed] [Google Scholar]
- 13.Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 14.Vlaminck B, Toffoli S, Ghislain B, Demazy C, Raes M, Michiels C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under mormoxic and hypoxic conditions. FEBS J. 2007;274:5533–5542. doi: 10.1111/j.1742-4658.2007.06072.x. [DOI] [PubMed] [Google Scholar]
- 15.Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor–1α with mimosine boosts the bactericidal capacity of phagocytes. J. Infec. Dis. 2008;197:214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]
- 16.von Köckritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009;87:775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]