Abstract
To assess the impact of spleen status on engraftment and early morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT), we analyzed 9,683 myeloablative allograft recipients from 1990 to 2006; 472 had prior splenectomy (SP), 300 splenic irradiation (SI), 1,471 with splenomegaly (SM), and 7,440 with normal spleen (NS). Median times to neutrophil and platelet engraftment were 15 vs. 18 days and 22 vs. 24 days for the SP and NS groups, respectively (p<0.001). Hematopoietic recovery at day +100 was not different across all groups, however the odds of days +14 and +21 neutrophil and day +28 platelet engraftment were 3.26, 2.25, and 1.28 for splenectomy, and 0.56, 0.55, and 0.82 for splenomegaly groups compared to normal spleen (p<0.001), respectively. Among patients with splenomegaly, use of peripheral blood grafts improved neutrophil engraftment at day +21, and CD34+ cell dose >5.7x106/kg improved platelet engraftment at day+28. After adjusting variables by Cox regression, the incidence of graft-versus-host disease (GVHD) and overall survival were not different among groups. Splenomegaly is associated with delayed engraftment while splenectomy prior to HCT facilitates early engraftment without impact on survival.
Keywords: Engraftment, splenectomy, spleen, stem cell transplantation, myeloproliferative disease
INTRODUCTION
The spleen status prior to hematopoietic cell transplantation (HCT) may influence early outcomes. After myeloablative conditioning, time to hematologic recovery can be a key determinant of early morbidity and mortality, as prolonged cytopenias are associated with increased risk of severe infections and bleeding (1). The use of peripheral blood stem cells (PBSC) and myeloid colony stimulating factors may hasten hematopoietic recovery (2–4), but delayed engraftment remains a legitimate concern in patients with splenomegaly (SM) at the time of transplantation(1). Conversely, prior splenectomy (SP) may improve time to engraftment following HCT, but the relationship between SP and engraftment kinetics and other early transplant outcomes is largely unknown (5–7). More recent retrospective studies also suggest no significant advantage of SP on transplant outcome except for a modest improvement in transfusion requirement and neutrophil engraftment (8–12). Prior SP was also associated with increase risk of acute graft-versus host disease (GVHD) (13) and post-transplant lymphoproliferative disorder(14). As an alternative to SP, splenic irradiation (SI) is utilized in patients with massive splenomegaly to reduce symptoms and spleen size just before HCT(15).
Given the significant changes in HCT practice over the past two decades, especially with increasing use of PBSC and myeloid stimulating growth factors in allogeneic HCT, improvement in supportive care, the relevance of the spleen status on transplantation outcomes deserves re-evaluation. We hereby report an analysis of data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) focused on the effects of recipient spleen status on engraftment and other early transplant outcomes.
PATIENTS AND METHODS
Data Source
Data on patients who received HCT were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive HCT to a Statistical Center located at the Medical College of Wisconsin (MCW) in Milwaukee and at the National Marrow Program (NMDP) Coordinating Center in Minneapolis(16). The information on recipient spleen status is obtained from data reported to the CIBMTR.
Patients
The study population included all patients (age ≥ 18) with chronic myelogeneous leukemia (CML), other myeloproliferative disorders (MPD) including myelofibrosis (MF), and myelodysplastic syndrome (MDS), and who received myeloablative (MA) conditioning and allogeneic bone marrow (BM) or PBSC between 1990 and 2006. Patients with blast phase CML or transformed AML, those who received cord blood (CB) transplant, prior autologous HCT, or reduced intensity conditioning (RIC) HCT were excluded. MA conditioning regimen was classified according to the CIBMTR working definition (17).
Spleen status was categorized as normal (NS), splenomegaly (SM), prior splenic irradiation (SI), or splenectomy (SP). Data on size of the spleen by exam and/or imaging studies were not available in the database. Median follow-up of survivors was 99 months (range 1–234 mos.) for the entire cohort (SP-107, SI-116, NS-97, and SM-103 mos.).
Outcomes
The following outcomes were chosen for univariate and multivariate analyses.
Neutrophil engraftment (NE: Achievement of a sustained absolute neutrophil count (ANC) is greater than 500×106/L for 3 consecutive days. Death and second transplants for primary graft failure were considered competing risks for this endpoint.
Platelet engraftment (PE: Achievement of a continued platelet count of greater than 20,000×109/L without transfusions. Death and second transplants for primary graft failure were considered competing risks for this endpoint.
100-day transplant-related mortality: This is defined as death while in continuous complete remission on or before day 100 post-transplant; patients were censored at relapse or, for patients in continuous complete remission, at last follow-up. Patients alive at last observation with fewer than 100 days of follow-up were considered censored for this event.
Acute graft-versus-host disease (GVHD: The occurrence of grades II, III and/or IV acute GVHD19 was considered the event. Death was a competing risk, and patients alive without acute GVHD were censored at the time of last follow-up. Patients receiving a second transplant were censored at the time of second transplant.
Chronic GVHD: Occurrence of symptoms in any organ system fulfilling the criteria of chronic GVHD (limited or extensive)(18). Death was a competing risk, and patients alive without chronic GVHD were censored at time of last follow-up.
Overall survival: Time from transplant to death from any cause. Cases were analyzed at the time of last follow-up.
Statistical Analyses
Medians and ranges were tabulated for continuous demographic variables and percentages for categorical demographic variables. Patient-related (age, gender, Karnofsky score at transplant), disease-related (disease type and status at transplant) and transplant-related variables (year of transplant, graft type, donor type, conditioning regimen, GvHD prophylaxis, donor/recipient sex match, donor/recipient CMV status, HLA match status, postransplant growth factor use, total nucleated cell dose for BM transplant patients and CD34+ cell dose for PBSCT patients) were tested in the multivariable model.
Time to NE and PE was described using cumulative incidence estimates. Patients who died within 21 days after transplant due to other causes before the engraftment (event) were not evaluable for engraftment endpoint. The primary aim of the study was to compare NE and PE after HCT across transplant recipients based on their spleen status. Due to non-proportional hazards encountered with Cox modeling for engraftment, logistic regression was used instead to analyze engraftment outcome at predetermined time points (day 14, 21, and 28 for NE; day 28 and 60 for PE). Separate logistic regression models at each time point were used rather than generalized estimating equation (GEE) regression models across multiple time points for simplicity of interpretation because there were many interactions between various covariates including the main effect (spleen status) and time.
Secondary objectives included comparing the cumulative incidence of acute and chronic GVHD and overall survival at 1 year post transplant across all four groups. Probabilities for overall survival and 100-day mortality were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood's formula. Probability of acute and chronic GVHD was calculated using the cumulative incidence function. Ninety-five percent confidence intervals for each outcome at specified time points were calculated separately across the four groups.
The covariates that may influence acute and chronic GVHD, overall survival were adjusted for using Cox proportional hazards regression models. The proportional hazards assumption was assessed for each variable using time-dependent or graphical approach. Time-dependent covariates were used when non proportional hazards were detected, where the best-fitting model with time-varying risk coefficients are found by maximizing the partial likelihood. Forward stepwise regression with alpha=0.05 was used to build models, with the prior SP variable forced into the model. Two-way interactions were checked between the main effect and other variables in the model.
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. Of 9,683 patients, 1,471 had SM going into transplantation, 472 patients had SP and 300 received SI, and 7,440 had NS. SP was performed in 133 centers and SI in 53 centers. SI was given as part of the conditioning regimen as a boost in 96% of patients who received splenic radiation before HCT. The median radiation dose to the spleen was 900 cGY (8–5000).
Table 1.
Characteristics of patients (≥ 18), who received myeloablative conditioning allogeneic hematopoietic cell transplantation from 1990 to 2006 by spleen status.
| Characteristics of patients | Normal Spleen |
Splenomegaly | Splenic irradiation |
Splenectomy | P-valuee |
|---|---|---|---|---|---|
| Patient related | |||||
| Number of patients | 7440 | 1471 | 300 | 472 | |
| Number of centers | 317 | 227 | 53 | 133 | |
| Age at transplant, median (range), years | 39 (18 – 59) | 37 (18 – 59) | 39 (18 – 59) | 39 (18 – 60) | 0.1498 |
| 18–21 | 288 (4) | 58 (4) | 12 (4) | 12 (3) | 0.4484 |
| 21–40 | 3884 (52) | 815 (55) | 157 (52) | 242 (51) | |
| 41–60 | 3226 (43) | 590 (40) | 129 (43) | 216 (46) | |
| >60 | 42 (1) | 8 (1) | 2 (1) | 2 (<1) | |
| Male Sex | 4273 (57) | 989 (67) | 187 (62) | 272 (58) | <0.001 |
| Karnofsky score at transplant ≥90 | 5899 (79) | 1130 (77) | 259 (86) | 330 (70) | <0.001 |
| Disease related | |||||
| Disease | <0.001 | ||||
| Chronic myelogenous leukemia | 5745 (77) | 1229 (84) | 266 (89) | 310 (66) | |
| Myelodysplasia disorders (MDS) | 1530 (21) | 133 (9) | 25 (8) | 72 (15) | |
| Myeloproliferative disorders (MPD)1 | 95 (1) | 90 (6) | 8 (3) | 72 (15) | |
| Other MDS/MPD NOS | 70 (<1) | 19 (1) | 1 (<1) | 18 (4) | |
| Disease status at transplant | |||||
| MDS | 0.3397 | ||||
| Early | 509 (33) | 38 (29) | 9 (36) | 16 (22) | |
| Advanced | 983 (64) | 93 (70) | 16 (64) | 55 (76) | |
| Non Evaluable | 38 (2) | 2 (2) | 0 | 1 (1) | |
| FAB subtype | <0.001 | ||||
| RAEB-t | 362 (24) | 28 (21) | 4 (16) | 16(22) | |
| RAEB | 504 (33) | 33 (26) | 6 (24) | 22 (32) | |
| CMML | 84 (6) | 27 (20) | 4 (16) | 15(21) | |
| RARS | 48 (3) | 4 (3) | 2 (8) | 1 (2) | |
| RA | 393 (26) | 15 (11) | 8 (32) | 9 (12) | |
| Other MDS | 139 (9) | 26 (20) | 1(4) | 9 (12) | |
| CML | <0.001 | ||||
| Chronic phase | 4999 (87) | 958 (78) | 226 (85) | 225 (72) | |
| Accelerated Phase | 746 (13) | 271 (22) | 40 (13) | 85 (28) | |
| Transplant related | |||||
| Donor type | <0.001 | ||||
| HLA identical sibling | 3539 (48) | 1119 (76) | 184 (61) | 219 (46) | |
| Unrelated Donor | 3901 (52) | 352 (24) | 116 (39) | 253 (54) | |
| Year of transplant | <0.001 | ||||
| 1990–1994 | 2461 (33) | 634 (43) | 208 (69) | 208 (44) | |
| 1995–1999 | 3002 (40) | 514 (35) | 74 (25) | 170 (36) | |
| 2000–2004 | 1487 (20) | 251 (17) | 16 (5) | 78 (17) | |
| 2005–2006 | 490 (7) | 72 (5) | 2 (<1) | 16 (3) | |
| Graft type | <0.001 | ||||
| Bone marrow | 6044 (81) | 1160 (79) | 286 (95) | 390 (83) | |
| Peripheral blood | 1396 (19) | 311 (21) | 14 (5) | 82 (17) | |
| Donor/recipient sex match | <0.001 | ||||
| Male-Male | 2616 (35) | 586 (40) | 109 (36) | 156 (33) | |
| Male-Female | 1632 (22) | 396 (27) | 78 (26) | 113 (24) | |
| Female-Male | 1668 (22) | 254 (17) | 59 (20) | 111 (24) | |
| Female-Female | 1482 (20) | 226 (15) | 53 (18) | 89 (19) | |
| Unknown | 42 (<1) | 9 (<1) | 1 (<1) | 3 (<1) | |
| Donor/recipient CMV match | <0.001 | ||||
| Negative/Negative | 2095 (28) | 347 (24) | 95 (32) | 141 (30) | |
| Positive/Positive | 2490 (33) | 656 (45) | 94 (31) | 128 (27) | |
| Positive/Negative | 965 (13) | 164 (11) | 41 (14) | 63 (13) | |
| Negative/Positive | 1596 (21) | 226 (15) | 51 (17) | 115 (24) | |
| Unknown | 294 (4) | 78 (5) | 19 (6) | 25 (5) | |
| HLA match status | <0.001 | ||||
| Well matched | 1315 (18) | 70 (5) | 14 (5) | 62 (13) | |
| Partially matched | 1516 (20) | 152 (10) | 32 (11) | 97 (21) | |
| Mismatched | 1064 (14) | 130 (9) | 70 (23) | 91 (19) | |
| HLA-Identical siblings | 3545 (48) | 1119 (76) | 184 (61) | 221 (46) | |
| Growth factor use | 2146 (29) | 353 (24) | 70 (23) | 165 (35) | <0.001 |
| Conditioning regimen | <0.001 | ||||
| CY + TBI | 4164 (56) | 527 (36) | 268 (89) | 311 (66) | |
| Bu + CY | 3242 (44) | 930 (63) | 32 (11) | 160 (34) | |
| Other2 | 34 (<1) | 14 (<1) | 0 | 1 (<1) | |
| ATG use | 659 (9) | 134 (9) | 19 (6) | 60 (13) | 0.0107 |
| Splenic radiation type | |||||
| Reported as part of conditioning | N/A | N/A | 289 (96) | N/A | |
| Reported as part of MDS treatment | N/A | N/A | 11 (4) | N/A | |
| Time from splenic RT to tx, median (range), days | N/A | N/A | 10 (<1 – 296) | N/A | |
| Median dose of splenic radiation, cGy | N/A | N/A | 900 (8–5000) | N/A | |
| GVHD prophylaxis | <0.001 | ||||
| T-cell depletion | 928 (12) | 96 (7) | 77 (26) | 143 (30) | |
| CSA + MTX ± other | 4999 (67) | 1146 (78) | 182 (61) | 251 (53) | |
| Tacro + MTX± other | 776 (10) | 48 (3) | 8 (3) | 34 (7) | |
| CSA ± Other | 514 (7) | 145 (10) | 24 (8) | 29(6) | |
| Other | 223(4) | 36 (2) | 9(2) | 15(4) | |
| BM TNC×108 cells/kg, median (range) | 3 (<1–11) | 3 (<1–13) | 2 (<1–7) | 3 (<1–9) | <0.001 |
| PB CD34×106 cells/kg, median (range) | 4 (<1 – 78) | 4 (<1 – 355) | 2 (<1 – 10) | 4 (<1 – 549) | 0.2745 |
| Median follow-up of survivors, range, months | 97 (2–234) | 103(1–230) | 116 (5–228) | 107(1–221) |
Abbreviations: CSA= cyclosporine; MTX=methotrexate; TBI= total body irradiation; Bu=busulfan; Cy=cyclophosphamide; GVHD= graft vs host disease; tacro= Tacrolimus; CML= chronic myelogenous leukemia; TNC=total nucleated cell dose; NOS=not otherwise specified.
MPD other disease subtype includes subtype: Myelofibrosis with myeloid metaplasia (n=20), polycythemia vera, Essential/primary thrombocythemia and Juvenile CML.)
Other conditioning regimen includes Cy± Other (n=48) Fludarabine + Atg ± other (n=1).\
There was no difference across all four spleen groups with respect to age distribution. More SP patients had poor performance status (KPS is less than 80%) compared with other groups. A majority of patients (78%) had CML. SP was proportionally more common in MDS/MPD groups combined as compared with CML patients.
There were significant differences in the following transplant variables among four groups: the SP group had relatively higher proportion of match unrelated donor (MUD) transplants (54%), partially match or mismatch transplants (40%). The majority of patients in the SP (80%) or SI (94%) groups were transplanted before 2000 and had more advanced stage disease compared to those with NS. While 66% of SP patients received CY-TBI, 63% of patients with SM received busulfan-based conditioning. A higher proportion of SP patients (35%) received post-transplant growth factors as compared with other groups. More patients (30%) in SP group had T-cell depletion for GVHD prophylaxis as compared to 12% in NS.
While there was no significant difference between BM total nucleated cell dose and peripheral blood CD34+ cell dose given between SP and NS groups, the SI group received the lowest BM TNC and PBSC CD34+ cell dose.
Engraftment
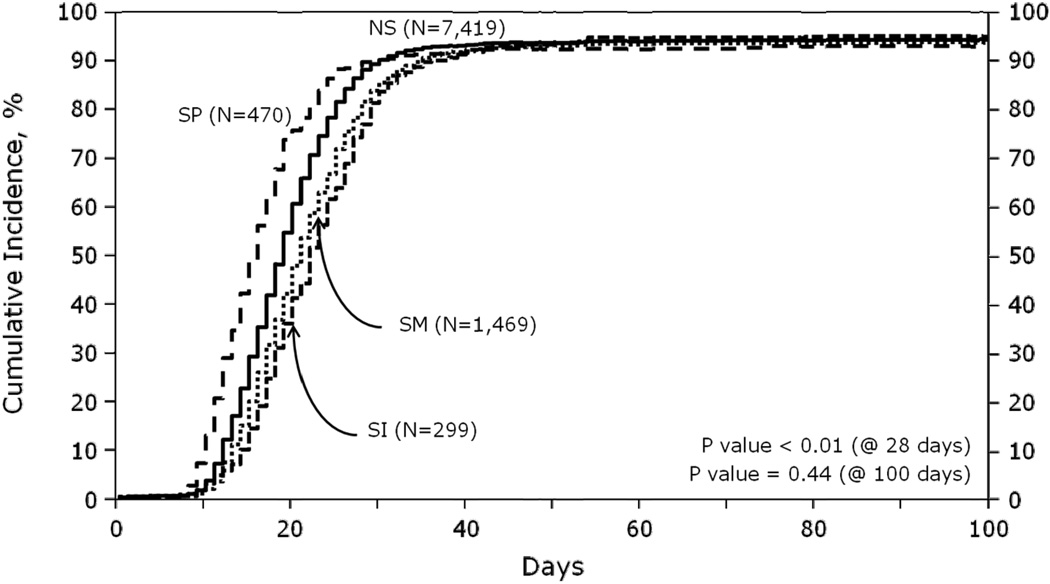
Median times of neutrophil engraftment (NE) were 20 and 18 days (p<0.001), and platelet engraftment (PE) were 25 and 24 days (p=0.088) for the SM and NS groups, respectively. SM was also associated with decreased probability of NE at day+28. Conversely, patient with SP had earlier NE and PE compared to patients with NS. Median times of NE were 15 and 18 days (p<0.001), and PE were 22 and 24 days (p<0.001) for the SP and NS groups, respectively. In univariate analysis, the percentage of patients who achieved NE at day+28 was the lowest in among patients who received prior SI (77%), and the highest (90%) in the SP group (Figure 1).The percentage of patients achieving NE and PE by day+100 was not different (93–96%) across all groups.
Figure 1.
Cumulative incidence of neutrophil engraftment by spleen status
Abbreviations: NS, normal spleen; SI, splenic irradiation; SM, splenomegaly; SP, splenectomy.
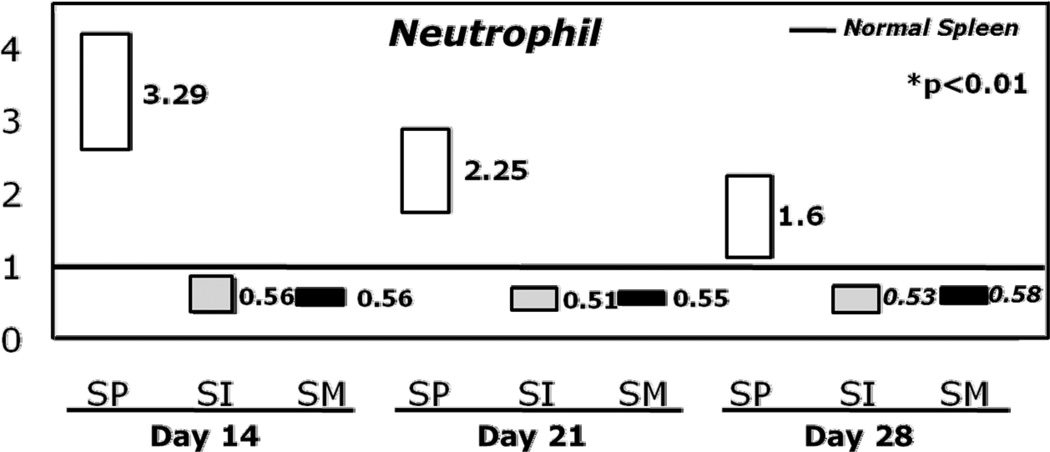
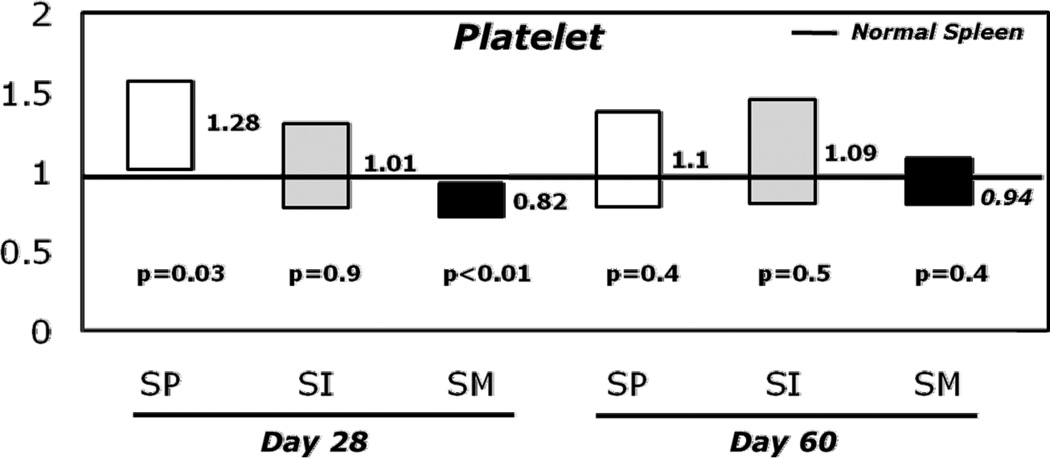
After adjusting for other variables including HLA-matching, growth factor use, type of GVHD prophylaxis, T-cell depletion, ATG use, stem cell source (PB vs. BM), TNC and CD34+ cell doses by multivariate logistic regression, the odds ratio of NE and PE by day+28 were significantly higher for SP patients and lower for patients with SM (Figures 2A and 2B, Table 3). Compared to NS, prior SP significantly increased the odds of NE by 3.29 fold (p<0.001), 2.25 fold (p<0.001), and 1.6 fold (p<0.006) on days +14, +21 and +28, respectively. The odds of PE also increased by 1.28 fold (p= 0.02) on day +28 in patients with prior SP compared to NS.
Figure 2.
A: Box plots of odds for neutrophil engraftment at 14, 21 and 28 days post-transplant by spleen status compared to normal spleen.
B: Box plots of odds for platelet engraftment at 28 and 60 days post-transplant by spleen status compared to normal spleen.
Abbreviations: NS, normal spleen; SI, splenic irradiation; SM, splenomegaly; SP, splenectomy.
Table 3.
Multivariate analysis of day 21 neutrophil engraftment, day 28 platelet engraftment and overall survival according to spleen status
| OR (95% CI2) D21 NE1 |
p-value | OR (95% CI) d28 PE3 |
p-value | RR4 (95% CI) Overall Survival |
p-value | |
|---|---|---|---|---|---|---|
| Normal spleen (NS) | 1 | - | 1 | - | 1 | - |
| Splenomegaly (SM) | 0.55 (0.48–0.63) | <0.01 | 0.82 (0.72–0.93) | <0.01 | 1.01 (0.93–1.09) | 0.85 |
| Splenic irradiation (SI) | 0.51 (0.40–0.66) | <0.01 | 1.01 (0.78–1.31) | 0.92 | 1.05 (0.90–1.22) | 0.53 |
| Splenectomy (SP) | 2.25 (1.76–2.89) | <0.01 | 1.28 (1.03–1.58) | 0.03 | 1.01 (0.89-1-14) | 0.85 |
Abbeviation: CI, confidence interval; OR odds ratio; RR, relative risk.
Conversely, patients with SM at the time of transplant and those who received SI had significantly decreased odds of NE on days +14, +21, and +28, respectively (Figure 2A, p<0.001). The odds of PE at day+28 was also significantly decreased in the SM group (Figure 2B, p=0.002, Table 3). Neither SP nor SM had a significant influence on day+60 PE. SI had no effect PE.
Among patients with SM who received PBSC, the odds of neutrophil engraftment at day +21 was better than recipients of bone marrow grafts. Recipients of PBSC with cell dose greater than 5.7 X106 CD34+cells/kg had higher odds of platelet engraftment at day +28 than recipients of lower cell dose.
Acute GVHD
Probabilities of grades II to IV acute GVHD at day+100 were 25% (95% CI, 24%–26%), 21% (95% CI, 18%–25%), 20% (95% CI, 18%–22%), and 22% (95% CI, 18%–27%) in NS, SP, SM and SI groups, respectively. After stratifying on graft type and cell dose, the relative risk (RR) of grade II-IV acute GVHD was not different across all 4 groups.
Chronic GVHD
The probability of chronic GVHD at 1 year after transplantation was 33% (95% CI, 29%–38%) in SP group, 42% (95% CI, 41%–44%) in NS group, 42% (95% CI, 40%–45%) in SM group and 40% (95% CI, 34%–45%) in SI group. After adjusting for graft type and cell dose, the spleen status did not influenced the development of chronic GVHD. However, there was a significant interaction between HLA matching and spleen status when matching status was included in the model. SP increased the relative risk (RR) of chronic GVHD only in HLA-sibling match transplants, by 29% (1.02 – 1.48, p<0.03). On the other hand, SM was significantly associated with increased risk of chronic GVHD in HLA-mismatched transplant recipients with RR of 2 (1.4 to 2.86), p<0.001.
Survival
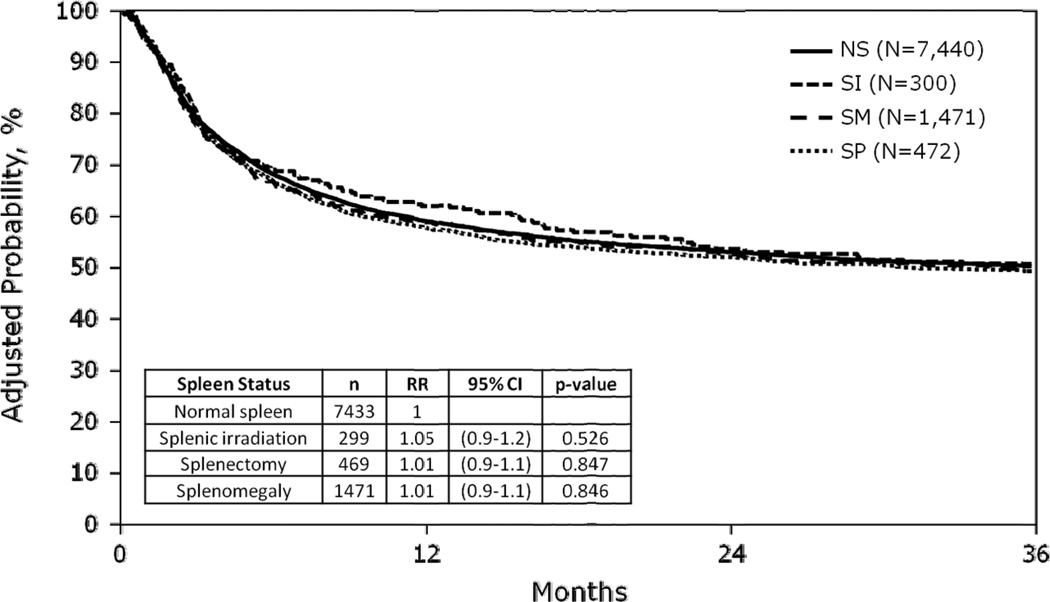
Day +100 mortality, and 3-year adjusted overall survival, and causes of death by spleen status groups are summarized in Table 2. One hundred day adjusted probabilities of mortality were 24%, 22%, 23%, 23% and 3-year adjusted probabilities of survival were 50%, 50%, 50%, 51% in SP, NS, SM, and SI groups, respectively. In multivariate analysis, there was no statistically significant difference in overall mortality among the groups based on spleen status (Table 3 and Figure 3). Table 2 also shows the causes of death according to spleen status.
Table 2.
Adjusted probabilities of mortality, survival and primary causes of death among patients, who received allogeneic hematopoietic cell transplantation with myeloablative conditioning for malignant disease according to spleen status.
| Normal Spleen |
Splenomegaly | Splenic irradiation |
Splenectomy | p-value | |
|---|---|---|---|---|---|
|
100-day adjusted mortality (95% confidence interval) |
22% (21–23) | 23% (21–25) | 23% (19–28) | 24% (21–28) | n.s. |
|
3-year adjusted survival (95% confidence interval) |
50% (49–52) | 50% (47–52) | 51% (45–56) | 50% (45–54) | n.s. |
| Primary causes of death | <0.001 | ||||
| Infection | 1050 (24%) | 158 (20%) | 33 (18%) | 56 (17%) | |
| Bacterial | 277 (26%) | 37 (23%) | 8 (24%) | 15 (27%) | |
| Fungal | 360 (34%) | 49 (31%) | 10 (30%) | 22 (39%) | |
| Viral | 172 (16%) | 24 (15%) | 3 (9%) | 10 (18%) | |
| NOS/Other | 241 (23%) | 48 (30%) | 11 (33%) | 9 (16%) | |
| Organ Failure | 967 (22%) | 159 (24%) | 39 (29%) | 65 (22%) | |
| Pulmonary Failure | 553 (58%) | 86 (54%) | 22 (56%) | 34 (52%) | |
| Liver | 139 (14%) | 31 (19%) | 5 (13%) | 9 (14%) | |
| Other | 275 (28%) | 42 (26%) | 12 (31%) | 22 (34%) | |
| GVHD | 789 (19%) | 141(19%) | 30 (17%) | 49 (16%) | |
| Primary disease | 549 (13%) | 161 (21%) | 30 (17%) | 62 (20%) | |
| Hemorrhage/Vascular | 238 (6%) | 41 (5%) | 10 (6%) | 22 (7%) | |
| Graft Failure | 110 (3%) | 17 (2%) | 5 (3%) | 9 (3%) | |
| Second Malignancy | 63 (1%) | 7 (<1%) | 4 (<1%) | 12 (<1%) | |
| Other | 154 (4%) | 20 (3%) | 6 (3%) | 11 (4%) | |
| Unknown | 115 (3%) | 20 (3%) | 7 (4%) | 6 (2%) |
Abreviation: n.s., p value > 0.05.
Figure 3.
Adjusted overall survival by spleen status
Abbreviations: NS, normal spleen; SI, splenic irradiation; SM, splenomegaly; SP, splenectomy.
DISCUSSION
This large series demonstrates the spleen status at time of transplantation impacts the speed of neutrophil and platelet engraftment without significant impact on survival or GVHD incidence. Additionally, engraftment delay observed in patients with SM at time of transplant can be counterbalanced by the use of PBSC, especially when the cell dose exceeds 5.7 X106 CD34+cells/kg.
These observations may reflect the role of the spleen and stem cell homing/trafficking prior to engraftment (19–22). Plett et al. reported that high quality stem cells tend to home BM as opposed to spleen(23). As such, it is possible that splenic sequestration of transplanted committed progenitor cells rather than pluripotent stem cells could account for our observation that initial NE and PE were retarded by SM (and hastened with SP), but there was no difference in engraftment by day+100 among all 4 groups. Splenomegaly could also delay count recovery by splenic sequestration of newly formed donor derived blood cells. Animal models have demonstrated that BM recovery after sub-ablative radiation is faster in splenectomized mice compared to mice with intact spleen (24, 25). Consistent with this notion, there are case reports that prolonged severe cytopenia after MA allogeneic or autologous HCT can be ameliorated by splenectomy in the post-transplant setting (26–28).
Despite the effect on time to engraftment, spleen status at time of transplant did not affect survival at any time. Although no apparent increase in infectious related deaths was seen in the patients with SM, there was about 5-fold difference in the odds of day+21 neutrophil engraftment between SM and SP, which may justify SP in selected patients with SM. However, the risk of procedure-related mortality should be considered prior to recommending SP in patients with hematologic malignancies (8–10, 20). It should be noted that the morbidity and mortality figures associated with SP have declined with the advent of laparoscopic surgical techniques(29). Our study cohort includes only patients who survived SP and who received transplant, thus it is difficult to ascertain the impact on survival from the time of SP.
One of the limitations of the current study is the absence of detailed information regarding spleen size. Bacigalupo et al (30) analyzed transplant outcomes of 46 patients with MF and identified that spleen size greater than 22 cm was an unfavorable prognostic factor for survival. As such, it is possible that the impact of splenomegaly on engraftment and early transplant mortality could be more discernible when “massive” SM is present. As a surrogate to address this question, we performed a subset analysis restricted to patient who had non-CML MPD, since these are patients most likely to have massive spleens. Another observation among patients with SM was an increase in cGVHD among recipients of mismatched grafts. It is unclear how to interpret this interaction between spleen status and HLA-matching, which will need to be confirmed in other datasets.
We found that among MPD patients, the odds ratio for neutrophil and platelet engraftment, and survival probabilities were not different according to spleen status (data not shown). However, the power of this subset analysis was limited because the smaller number of MPD patients in this cohort. Although initial observations in early 1980’s also suggested a faster engraftment with pre-transplant SP in patients with MPD(5, 6), neither survival benefit nor decrease in relapse was observed in subsequent studies(7). More recent retrospective studies also suggest no significant advantage of ST on transplant outcome except for a modest improvement in transfusion requirement and neutrophil engraftment.(8–11)
Another notable finding is that the use of PBSC or higher cell doses was associated with improved day 21 and 28 neutrophil and platelet engraftment. Among patients with SM, use of PBSC with CD34+cell dose of > 5.7x106/kg abrogated the delay in platelet and neutrophil recovery. These results are particularly relevant today given the increasing use of PBSC in allogeneic HCT, and suggest that in patients with splenomegaly, use of of PBSC with higher cell dose result in faster engraftment.
We did not observe any benefit of SI on engraftment endpoints. It was actually associated with delayed neutrophil engraftment in all time points. SI in this patient population is likely a surrogate for massive SM since this treatment is often used in patients with CML or MPD with symptomatic SM. It was more frequently administered before 1994 and patients in this group were more likely to receive BM grafts from HLA-matched sibling donors. The intent of irradiation was likely a boost during the conditioning and therefore the time between SI and stem cell infusion was not long enough to alter the degree of splenic migration. The dose, indication and timing of SI were heterogeneous, limiting interpretability. Our finding of no benefit in engraftment or survival with SI suggests that splenic irradiation should be used with caution, especially since at higher doses, abdominal irradiation it may increase the risk of hepatic veno-occlusive disease, radiation nephritis and/or pneumonitis.
In conclusion, this large series demonstrates that spleen status affects engraftment kinetics after allogeneic HCT with myeloablative conditioning. SM retards neutrophil and platelet engraftment, while prior SP hastens neutrophil and platelet recovery relative to patients with normal intact spleens. Our data also suggest that among patients with SM, the delay in engraftment can be mitigated with the use of PBSC over bone marrow, especially at a higher CD34 dose (>5.7×106/CD34+ cells/kg). These findings suggest that in the absence of symptoms, spleen directed therapy prior to HCT is not necessary as there is no impact on survival outcomes. Transplant candidates with symptomatic SM, should be considered for SP, if surgical risks are low.
ACKNOWLEDGEMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Authorship and Disclosures:
GA designed the study, interpreted results, and drafted the manuscript; BL, MAA. analyzed data and interpreted results; MCP, HML, DIM, MB, OR, RTM, VG, UP, DM, BJB, JDR, KKB, KRC, PLM, and VTH critically reviewed and revised the manuscript; and all authors approved the final version.
No conflict of interest relevant to this manuscript to report.
REFERENCES
- 1.Helenglass G, Treleaven J, Parikh P, Aboud H, Smith C, Powles R. Delayed engraftment associated with splenomegaly in patients undergoing bone marrow transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 1990;5(4):247–251. [PubMed] [Google Scholar]
- 2.Battiwalla M, McCarthy PL. Filgrastim support in allogeneic HSCT for myeloid malignancies: a review of the role of G-CSF and the implications for current practice. Bone Marrow Transplant. 2009;43(5):351–356. doi: 10.1038/bmt.2008.443. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 4.Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18(20):3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 5.Baughan AS, Worsley AM, McCarthy DM, Hows JM, Catovsky D, Gordon-Smith EC, et al. Haematological reconstitution and severity of graft-versus-host disease after bone marrow transplantation for chronic granulocytic leukaemia: the influence of previous splenectomy. Br J Haematol. 1984;56(3):445–454. doi: 10.1111/j.1365-2141.1984.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Devergie A, Bernheim A, Berger R. Splenectomy and bone marrow transplantation in chronic granulocytic leukaemia. Lancet. 1983;1(8338):1392–1393. doi: 10.1016/s0140-6736(83)92182-7. [DOI] [PubMed] [Google Scholar]
- 7.Gratwohl A, Goldman J, Gluckman E, Zwaan F. Effect of splenectomy before bone-marrow transplantation on survival in chronic granulocytic leukaemia. Lancet. 1985;2(8467):1290–1291. doi: 10.1016/s0140-6736(85)91566-1. [DOI] [PubMed] [Google Scholar]
- 8.Deeg HJ, Gooley TA, Flowers ME, Sale GE, Slattery JT, Anasetti C, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003;102(12):3912–3918. doi: 10.1182/blood-2003-06-1856. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Deeg HJ. Pros and cons of splenectomy in patients with myelofibrosis undergoing stem cell transplantation. Leukemia. 2001;15(3):465–467. doi: 10.1038/sj.leu.2402043. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Gooley T, Applebaum FR, Deeg HJ. Splenectomy and hemopoietic stem cell transplantation for myelofibrosis. Blood. 2001;97(7):2180–2181. doi: 10.1182/blood.v97.7.2180. [DOI] [PubMed] [Google Scholar]
- 11.Martino R, Altes A, Muniz-Diaz E, Brunet S, Sureda A, Domingo-Albos A, et al. Reduced transfusion requirements in a splenectomized patient undergoing bone marrow transplantation. Acta Haematol. 1994;92(3):167–168. doi: 10.1159/000204213. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WA, Pearce R, Kirkland KE, Bloor A, Thomson K, Apperley J, et al. The role of allogeneic SCT in primary myelofibrosis: a British Society for Blood and Marrow Transplantation study. Bone Marrow Transplant. 2010;45(11):1587–1593. doi: 10.1038/bmt.2010.14. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Nilsson B. Death by graft-versus-host disease associated with HLA mismatch, high recipient age, low marrow cell dose, and splenectomy. Transplantation. 1985;40(1):39–44. doi: 10.1097/00007890-198507000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Sundin M, Le Blanc K, Ringden O, Barkholt L, Omazic B, Lergin C, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91(8):1059–1067. [PubMed] [Google Scholar]
- 15.Elliott MA, Tefferi A. Splenic irradiation in myelofibrosis with myeloid metaplasia: a review. Blood Rev. 1999;13(3):163–170. doi: 10.1054/blre.1999.0110. [DOI] [PubMed] [Google Scholar]
- 16.Pasquini MC, Wang Z, Horowitz M, Gale RP. 2010 Report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current Uses and Outcomes of Hematopoietic Cell Transplant for Blood and Bone Marrow Disorders. In: Cecka JM, Terazaki PI, editors. Clinical Transplants 2010. Los Angeles: The Terasaki Foundation Laboratory; 2011. [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Jetmore A, Plett PA, Tong X, Wolber FM, Breese R, Abonour R, et al. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99(5):1585–1593. doi: 10.1182/blood.v99.5.1585. [DOI] [PubMed] [Google Scholar]
- 20.Szilvassy SJ, Bass MJ, Van Zant G, Grimes B. Organ-selective homing defines engraftment kinetics of murine hematopoietic stem cells and is compromised by Ex vivo expansion. Blood. 1999;93(5):1557–1566. [PubMed] [Google Scholar]
- 21.van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94(9):3055–3061. [PubMed] [Google Scholar]
- 22.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 23.Plett PA, Frankovitz SM, Orschell CM. Distribution of marrow repopulating cells between bone marrow and spleen early after transplantation. Blood. 2003;102(6):2285–2291. doi: 10.1182/blood-2002-12-3742. [DOI] [PubMed] [Google Scholar]
- 24.Smith LH, McKinley TW., Jr Recovery from radiation injury with and without bone marrow transplantation: effects of splenectomy. Radiat Res. 1970;44(1):248–261. [PubMed] [Google Scholar]
- 25.Tanaka N. Experimental studies on role of spleen in recovery from radiation injury in mice. 3. Effect of splenectomy on survival of mice with spleen and bone marrow transplantation following lethal x-irradiation. Hiroshima J Med Sci. 1966;15(4):347–378. [PubMed] [Google Scholar]
- 26.Richard C, Romon I, Perez-Encinas M, Baro J, Rabunal MJ, Mazorra F, et al. Splenectomy for poor graft function after allogeneic bone marrow transplantation in patients with chronic myeloid leukemia. Leukemia. 1996;10(10):1615–1618. [PubMed] [Google Scholar]
- 27.Robin M, Esperou H, de Latour RP, Petropoulou AD, Xhaard A, Ribaud P, et al. Splenectomy after allogeneic haematopoietic stem cell transplantation in patients with primary myelofibrosis. Br J Haematol. 150(6):721–724. doi: 10.1111/j.1365-2141.2010.08276.x. [DOI] [PubMed] [Google Scholar]
- 28.von Bueltzingsloewen A, Bordigoni P, Dorvaux Y, Witz F, Schmitt C, Chastagner P, et al. Splenectomy may reverse pancytopenia occurring after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;14(2):339–340. [PubMed] [Google Scholar]
- 29.Park AE, Birgisson G, Mastrangelo MJ, Marcaccio MJ, Witzke DB. Laparoscopic splenectomy: outcomes and lessons learned from over 200 cases. Surgery. 2000;128(4):660–667. doi: 10.1067/msy.2000.109065. [DOI] [PubMed] [Google Scholar]
- 30.Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45(3):458–463. doi: 10.1038/bmt.2009.188. [DOI] [PubMed] [Google Scholar]