Abstract
Objective
Quantitative trait loci identified in animal models provide potential candidate susceptibility loci for human disorders. In this study, we investigated whether internalizing disorders (anxiety disorders, major depression, and neuroticism) were associated with a region on human chromosome 1 syntenic with a quantitative trait locus for rodent emotionality.
Methods
We genotyped 31 single-nucleotide polymorphisms in genes located on chromosome 1q31.2 in a two-stage association study of 1128 individuals chosen for a high or a low genetic risk for internalizing disorders from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.
Results
None of the individual single-nucleotide polymorphisms showed consistent association across stages. A four-marker haplotype in the regulator of G-protein signaling 1 gene (RGS1) was significantly associated with decreased internalizing risk in both stages, whereas another showed a nominal association with a higher risk.
Conclusion
Our data suggest that markers in the RGS1 gene might be in linkage disequilibrium with a protective allele that reduces the risk of anxiety and depressive disorders.
Keywords: anxiety, association study, depression, genetics, personality
Introduction
Internalizing disorders, that is, anxiety and depressive disorders, are quite common in the general population and account for a substantial clinical burden worldwide (Andrews et al., 1998). They are complex genetic syndromes, showing shared familial aggregation (Weissman et al., 1997) and overlapping genetic risk factors (Kendler et al., 2003). The personality trait of neuroticism has considerable genetic correlation with internalizing symptoms and disorders (Jardine et al., 1984; Kendler et al., 2006; Hettema et al., 2006b). Study of these internalizing phenotypes in a coordinated manner may prove effective for identification of the primary susceptibility genes for these conditions.
Gene discovery efforts remain challenging for most polygenic disorders because of small effect sizes; thus, animal models continue to play an important role in the identification of genes involved in human diseases. Rodent ‘emotionality’ has been proposed as a model for human internalizing phenotypes (Flint et al., 1995). A behavioral quantitative trait locus (QTL) for emotionality has been reliably localized to a 4.8Mb region on mouse chromosome 1 (Yalcin et al., 2004). Interestingly, the syntenic region on human chromosome 1 coincides with linkage peaks reported for several human internalizing phenotypes (reviewed in Fullerton, 2006). Further dissection of this region provided support for three separate murine QTLs, including effects attributable to the gene, regulator of the G-protein signaling 1 gene (RGS2). The RGS2 gene has since been reportedly associated with several anxiety-related phenotypes in humans (Leygraf et al., 2006; Smoller et al., 2008; Amstadter et al., 2009; Koenen et al., 2009; Mouri et al., 2010; Otowa et al., 2011). Another study examined the potential relevance, for neuroticism, of human polymorphisms that correspond to functional murine sequence variants in that syntenic region, finding significant evidence for a single-nucleotide polymorphism (SNP) near RGS18 (Fullerton et al., 2008). However, there are many genes in that region that have not been explored adequately for their potential contribution to these linkage signals. To further examine the relevance of the mouse chromosome 1 emotionality QTLs for human internalizing disorders, in this study, we tested genes in the syntenic human region chr1q31.2 for an association with genetic risk for these phenotypes.
Methods
Participants
The Caucasian participants in this study were from the population-based Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (Kendler and Prescott, 1999; Kendler and Prescott, 2006). Approval of the local Institutional Review Board was obtained before the study and informed consent was obtained from all participants before data collection.
Diagnostic measures
We obtained lifetime psychiatric diagnoses on the basis of DSM-III-R (American Psychiatric Association, 1987) through a face-to-face or a telephone structured psychiatric interview on the basis of the SCID (Spitzer and Williams, 1985). Neuroticism and extroversion were assessed using the short form of the Eysenck Personality Questionnaire (Eysenck and Eysenck, 1975).
Sample selection
As described previously (Hettema et al., 2006a), we used a two-stage association design in which candidate loci were screened in stage 1, the positive results of which were tested for replication in stage 2. The parameters for this design were calculated using the LGA program (Robles and Van den Oord, 2004) to achieve 80% power to detect markers that explained 1–2% of the variance of the liability distribution while controlling the false discovery rate at 0.1 (Van den Oord and Sullivan, 2003). If any of the markers genotyped in stage 1 met the estimated threshold P-value of 0.1 or less, they were then also tested in the stage 2 sample.
We have incorporated two novel strategies to optimize participant selection. First, we have taken advantage of research suggesting shared genetic susceptibility across internalizing disorders (Scherrer et al., 2000; Middeldorp et al., 2005; Hettema et al., 2006b). Starting with a total of 9270 twin individuals, we used multivariate structural equation modeling to estimate a latent additive genetic risk factor (A1) for neuroticism that is highly correlated with genetic susceptibility to internalizing disorders (see Hettema et al., 2006b for details). Second, we used an extreme selection strategy (Van den Oord, 1999; Schork et al., 2000; Van Gestel et al., 2000) to choose one member from each twin pair for whom DNA was available as a ‘case’ (high genetic risk) or ‘control’ (low genetic risk) for genotyping on the basis of scoring above the 80th or below the 20th percentile, respectively, of the genetic factor A1. This led to a total sample size of N = 1128 that included 589 cases and 539 controls: 188 cases and controls in stage 1 and 401 cases and 351 controls in stage 2. Overall, the cases had a mean raw neuroticism score of 6.3 (z-score = 1.04) and had the following frequencies of the target internalizing disorders: major depressive disorder (80.1%), generalized anxiety disorder (53.8%), panic disorder (20.5%), agoraphobia (14.1%), and social phobia (17.5%). The controls were free of these five disorders and had a mean raw neuroticism score of 0.55 (z-score= − 0.89). These phenotypic distributions did not differ significantly between the stage 1 and stage 2 samples.
Genotyping
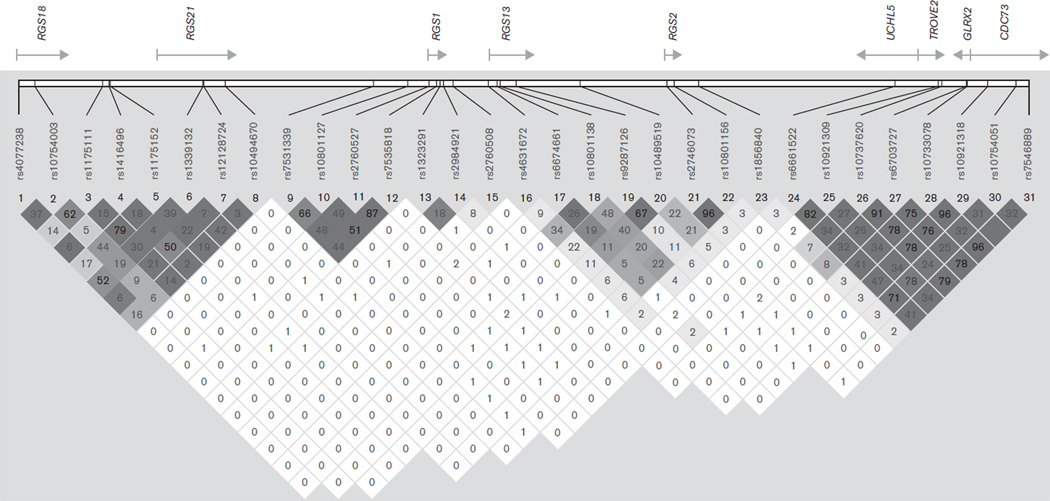
DNA was extracted from buccal epithelial cells obtained using cytology brushes. SNPs were genotyped by the 5′ nuclease cleavage assay (TaqMan method) (Livak, 1999). We selected SNP markers with minor allele frequency greater than 0.10 in the human chr1q31.2 region syntenic with the murine emotionality locus (Fig. 1). The region of interest was large, about 1 Mb in length (start position: 190 387 326; end position: 191 399 900 in the March 2006 assembly, NCBI build 36); thus, we focused our analyses on the known genes in the regions. We used the Tagger module of Haploview 4.1 (Barrett et al., 2005) with HapMap (The International HapMap Consortium, 2003) phase II data (release 22) and pairwise tagging to capture the allelic variation (with r2 > 0.7) in and near all of the known genes. We used quality control genotyping thresholds to exclude poorly performing assays: genotyping rate less than 90% and missingness greater than 10%.
Fig. 1.
Relative gene locations and linkage disequilibrium (r2) for all single-nucleotide polymorphisms genotyped in the stage 1 sample.
Statistical analysis
Pearson’s χ2-tests were used to test for allelic or genotypic differences by marker between cases and controls in the two stages. We used Haploview 4.1 to find the regions of high linkage disequilibrium (LD) (i.e. haplotype blocks) using the default confidence interval algorithm (Gabriel et al., 2002). Haplotype association analyses were carried out using the Cocaphase module of the UNPHASED program (Dudbridge, 2003).
Results
The genotype and allele frequencies and the results of χ2-association tests for the 31 markers genotyped in stage 1 are listed in Table 1. None of the SNPs showed deviations from Hardy–Weinberg equilibrium. Five markers in and around the RGS1 gene locus (markers 9–13) met the threshold criteria of allelic P-value less than 0.1 in our stage 1 sample (Table 1). However, none of these individual markers were associated in stage 2.
Table 1.
Individual marker association results for stage 1 (N = 188 cases, 188 controls)
| P-value | |||||
|---|---|---|---|---|---|
| Marker # | Marker ID | Locus | MAF | Genotypic | Allelic |
| 1 | rs4077238 | RGS18 | 0.286 | 0.63 | 0.79 |
| 2 | rs10754003 | RGS18 | 0.497 | 0.71 | 0.46 |
| 3 | rs1175111 | RGS18 | 0.490 | 0.40 | 0.50 |
| 4 | rs1416496 | Intergenic | 0.143 | 0.59 | 0.45 |
| 5 | rs1175152 | Intergenic | 0.440 | 0.12 | 0.74 |
| 6 | rs1339132 | RGS21 | 0.254 | 0.91 | 0.70 |
| 7 | rs12128724 | RGS21 | 0.171 | 0.92 | 0.71 |
| 8 | rs10494670 | RGS21 | 0.130 | 1.00 | 0.98 |
| 9 | rs7531339 | Intergenic | 0.273 | 0.05 | 0.067 |
| 10 | rs10801127 | RGS1 | 0.276 | 0.05 | 0.041 |
| 11 | rs2760527 | RGS1 | 0.170 | 0.06 | 0.019 |
| 12 | rs7535818 | RGS1 | 0.163 | 0.06 | 0.020 |
| 13 | rs1323291 | RGS1 | 0.084 | 0.06 | 0.029 |
| 14 | rs2984921 | RGS1 | 0.270 | 0.72 | 0.45 |
| 15 | rs2760508 | RGS1 | 0.208 | 0.62 | 0.35 |
| 16 | rs4631672 | RGS13 | 0.482 | 0.83 | 0.72 |
| 17 | rs6674661 | RGS13 | 0.244 | 0.97 | 0.70 |
| 18 | rs10801138 | RGS13 | 0.455 | 0.77 | 0.94 |
| 19 | rs9287126 | RGS13 | 0.464 | 0.29 | 0.24 |
| 20 | rs10489519 | Intergenic | 0.395 | 0.32 | 0.15 |
| 21 | rs2746073 | RGS2 | 0.257 | 0.97 | 0.86 |
| 22 | rs10801156 | RGS2 | 0.260 | 0.85 | 0.56 |
| 23 | rs1856840 | RGS2 | 0.360 | 0.86 | 0.67 |
| 24 | rs6661522 | UCHL5 | 0.490 | 0.99 | 0.82 |
| 25 | rs10921309 | TROVE2 | 0.438 | 0.95 | 0.99 |
| 26 | rs10737620 | TROVE2 | 0.278 | 0.25 | 0.13 |
| 27 | rs6703727 | GLRX2; CDC73 | 0.258 | 0.33 | 0.40 |
| 28 | rs10733078 | GLRX2; CDC73 | 0.305 | 0.73 | 0.52 |
| 29 | rs10921318 | CDC73 | 0.309 | 0.49 | 0.35 |
| 30 | rs10754051 | CDC73 | 0.427 | 0.86 | 0.66 |
| 31 | rs7546869 | CDC73 | 0.301 | 0.25 | 0.10 |
P-values are calculated using χ2-tests.
Boldface indicates P<0.1 (stage 1 threshold).
Locus, genes within 50 kb of marker; MAF, minor allele frequency.
As we used tagging SNPs that are unlikely to be the functional variants themselves, we constructed haplotype blocks using the default confidence interval procedure in Haploview 3.2 to better understand the LD structure around these markers. Markers 10, 11, and 12 occurred on a single haplotype block in our sample, although the LD between these and marker 9 was high, consistent with CEU HapMap data, suggesting one large block structure across the SNPs in this region. We therefore tested the association of the four-marker haplotypes created from combinations of markers 9–12. In Table 2, we present the results, by stage, for these four-marker haplotypes as calculated using UNPHASED (results for three-marker and five-marker sliding window haplotypes produced similar patterns). As indicated, the most consistent result across stages is for the common ‘protective’ C-T-G-G haplotype, showing higher frequencies in controls than cases (P = 0.0036 in the combined sample). The less common T-T-A-A haplotype showed a nominal association with an increased risk (P = 0.029). As we are testing five markers in both stages (or five haplotypes derived from four of these markers), we applied a corrected P-value of 0.01 to indicate significance, which is fulfilled by the C-T-G-G haplotype. Permutation testing estimates the empirical 5% of the best P-value equals 0.00787, indicating that this haplotype (with P = 0.0036) occurs less than 5% of the time by chance.
Table 2.
Haplotype analysis results for RGS1 block single-nucleotide polymorphisms in stage 1, stage 2, and the combined samplesa
| Stage | Markers | Global P-value |
Haplotype | Frequency cases | Frequency controls | Haplotype P-value |
|---|---|---|---|---|---|---|
| 1 (N = 376) | 9, 10, 11, 12 | 0.062 | T-G-A-A | 0.72 | 0.66 | 0.076 |
| T-T-A-A | 0.024 | 0.023 | 0.96 | |||
| C-G-A-A | 0.042 | 0.032 | 0.48 | |||
| C-T-A-A | 0.068 | 0.079 | 0.64 | |||
| C-T-G-G | 0.12 | 0.18 | 0.027 | |||
| 2 (N = 752) | 9, 10, 11, 12 | 0.00029 | T-G-A-A | 0.64 | 0.68 | 0.087 |
| T-T-A-A | 0.046 | 0.021 | 0.0092 | |||
| C-G-A-A | 0.046 | 0.029 | 0.14 | |||
| C-T-A-A | 0.087 | 0.073 | 0.37 | |||
| C-T-G-G | 0.14 | 0.18 | 0.043 | |||
| Combined (N = 1128) | 9, 10, 11, 12 | 0.0024 | T-G-A-A | 0.67 | 0.68 | 0.71 |
| T-T-A-A | 0.038 | 0.022 | 0.029 | |||
| C-G-A-A | 0.045 | 0.029 | 0.091 | |||
| C-T-A-A | 0.079 | 0.075 | 0.69 | |||
| C-T-G-G | 0.13 | 0.18 | 0.0036 |
Boldface indicates P < 0.05 for specific haplotypes.
Haplotype: ‘1’ represents the major allele and ‘2’ represents the minor allele, respectively, for each SNP marker in a haplotype.
Results shown only for common haplotypes (frequency >0.01).
Discussion
In this study, we examined whether human genes syntenic to the murine chromosome 1 emotionality region were associated with genetic susceptibility to human internalizing phenotypes, including anxiety disorders, major depression, and neuroticism. This susceptibility was indexed by a latent genetic factor common to these phenotypes derived from multivariate twin modeling. We entered the resulting sample of 589 high genetic risk and 539 low genetic risk individuals into a two-stage association study in which markers from the candidate loci were screened in stage 1, the positive results of which were tested for replication in stage 2. Individual markers and relevant haplotypes were analyzed.
Out of the 31 markers tested in this region, five in and around the RGS1 gene fulfilled the threshold screening criterion in stage 1 of P-value less than 0.1, but these associations were not replicated in stage 2. The C-T-G-G haplotype of four of these SNPs in high LD showed an association across the two stages with a lower internalizing risk. However, as individual SNPs did not show a consistent association, either (a) these haplotype results occurred by chance or (b) this haplotype is in LD with another functional variant that we did not test. RGS1 is a small gene (4.3 kb) that codes for one of the many members of the class of proteins known as regulators of G-protein signaling. These proteins attenuate the signaling activity of G-proteins by binding to activated, GTP-bound Ga subunits and increase the rate of conversion of GTP into GDP. As summarized on the UCSC Genome Browser website (www.genome.ucsc.edu), RGS1 has little known brain expression, limited to the hypothalamus and the corpus callosum. Extant research supports its role in B-cell inflammatory responses (Moratz et al., 2000), with possible involvement in conditions such as celiac disease (Hunt et al., 2008), melanoma progression (Rangel et al., 2008), and multiple sclerosis (International Multiple Sclerosis Genetics Consortium, 2010). To our knowledge, this is the first report of an association with a psychiatric phenotype.
In a previous study, Fullerton et al. (2008) identified human SNPs corresponding to functional and conserved regions in the murine emotionality locus and tested them for association in a large, extreme-selected sample for neuroticism. They reported a significant association for SNP rs6428058, about 600 kb upstream of RGS18 (outside of our selected region). They did not genotype any markers overlapping the RGS1 block implicated in the current study. We note that neither that study nor the current one found evidence supporting a role for the RGS2 gene in internalizing disorder susceptibility.
The results of this study should be interpreted in the context of several potential limitations. First, this sample, although chosen to maximize power to directly test an association with a common genetic risk for internalizing phenotypes and selected from the informative tails of a much larger sample, may nonetheless lack sufficient power to detect an association with common genetic polymorphisms that have modest effect sizes. In particular, although we attempted to balance type I and type II errors and maintain an available sample for replication using a two-stage study design with less stringent stage 1 screening P-values, the stage 1 sample size is nonetheless quite modest. Second, because of the large number of SNPs in the region and cost limitations of the study, we chose to genotype 31 tagging SNPs with less stringent criteria in the regions of the known genes only, and thus, could not capture all of the major allelic variation in the region.
In summary, this study finds a suggestive association between variants in the RGS1 gene and internalizing disorders. As with any novel genetic association finding, these results should be considered as tentative until adequate replication is shown.
Acknowledgements
The authors thank Drs Jonathan Flint and Saffron Willis-Owen for useful discussions on their chromosome 1 association data (Fullerton et al., 2008).
This work was supported by NIH grants MH-40828, MH-65322, MH-20030, DA-11287, MH/AA/DA-49492 (K.S.K.), and NIH grant K08 MH-66277, a NARSAD Young Investigator Award (J.M.H.). Carol Prescott, PhD, provided critical help in the collection of the VATSPSUD sample. The authors acknowledge the contribution of the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR), for the ascertainment of participants for this study. The MATR is currently supported by UL1RR031990 from the National Center for Research Resources.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Revised. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Sanderson K, Beard J. Burden of disease. Methods of calculating disability from mental disorder. Br J Psychiatry. 1998;173:123–131. doi: 10.1192/bjp.173.2.123. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder and Stoughton; 1975. [Google Scholar]
- Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, Collins AC. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- Fullerton J. New approaches to the genetic analysis of neuroticism and anxiety. Behav Genet. 2006;36:147–161. doi: 10.1007/s10519-005-9000-4. [DOI] [PubMed] [Google Scholar]
- Fullerton JM, Willis-Owen SA, Yalcin B, Shifman S, Copley RR, Miller SR, et al. Human-mouse quantitative trait locus concordance and the dissection of a human neuroticism locus. Biol Psychiatry. 2008;63:874–883. doi: 10.1016/j.biopsych.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJ, Kendler KS, Chen X. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006a;11:752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006b;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40:395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium. IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis susceptibility loci. Genes Immun. 2010;11:397–405. doi: 10.1038/gene.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genet Epidemiol. 1984;1:89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: understanding the causes of psychiatric and substance use disorders. New York: Guilford Press; 2006. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of comorbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med. 2006;37:453–462. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2009;26:309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leygraf A, Hohoff C, Freitag C, Willis-Owen SA, Krakowitzky P, Fritze J, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? J Neural Transm. 2006;113:1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5’ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, et al. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol. 2000;164:1829–1838. doi: 10.4049/jimmunol.164.4.1829. [DOI] [PubMed] [Google Scholar]
- Mouri K, Hishimoto A, Fukutake M, Nishiguchi N, Shirakawa O, Maeda K. Association study of RGS2 gene polymorphisms with panic disorder in Japanese. Kobe J Med Sci. 2010;55:E116–E121. [PubMed] [Google Scholar]
- Otowa T, Shimada T, Kawamura Y, Sugaya N, Yoshida E, Inoue K, et al. Association of RGS2 variants with panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:430–434. doi: 10.1002/ajmg.b.31178. [DOI] [PubMed] [Google Scholar]
- Rangel J, Nosrati M, Leong SP, Haqq C, Miller JR, III, Sagebiel RW, Kashani-Sabet M. Novel role for RGS1 in melanoma progression. Am J Surg Pathol. 2008;32:1207–1212. doi: 10.1097/PAS.0b013e31816fd53c. [DOI] [PubMed] [Google Scholar]
- Robles JR, van den Oord EJ. Iga972: a cross-platform application for optimizing LD studies using a genetic algorithm. Bioinformatics. 2004;20:3244–3245. doi: 10.1093/bioinformatics/bth348. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, True WR, Xian H, Lyons MJ, Eisen SA, Goldberg J, et al. Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. J Affect Disord. 2000;57:25–35. doi: 10.1016/s0165-0327(99)00031-2. [DOI] [PubMed] [Google Scholar]
- Schork NJ, Nath SK, Fallin D, Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, et al. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Arch Gen Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) New York: Biometrics Research Department, New York State Psychiatric Institute; 1985. [Google Scholar]
- The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Van den Oord EJCG. A comparison between different designs and tests to detect QTLs in association studies. Behav Genet. 1999;29:245–256. [Google Scholar]
- Van den Oord EJ, Sullivan PF. False discoveries and models for gene discovery. Trends Genet. 2003;19:537–542. doi: 10.1016/j.tig.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Van Gestel S, Houwing-Duistermaat JJ, Adolfsson R, van Duijn CM, Van Broeckhoven C. Power of selective genotyping in genetic association analyses of quantitative traits. Behav Genet. 2000;30:141–146. doi: 10.1023/a:1001907321955. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents. 10 Years later. Arch Gen Psychiatry. 1997;54:932–940. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]