INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked progressive muscular dystrophy, caused by loss of the dystrophin protein at the myofiber membrane.1,2 Pharmacologic treatment of DMD patients with glucocorticoids can improve patient strength and prolong ambulation, with concomitant improvements in quality-of-life scales.3–9 As such, glucocorticoid treatment for DMD is recommended in standard-of-care guidelines, and as an American Academy of Neurology practice parameter.10–12 The majority of trials and treatment recommendations have used an oral dose of prednisone at 0.75 mg/kg/d. However, alternative dosing regimens have been reported as changing the efficacy versus side-effect profiles, including weekend dosing,13,14 lower doses,15 and alternative-day doses (10 mg/kg/wk divided over 2 weekend days).16–18 In each study, a goal was to achieve a better balance of efficacy (increased strength and delay of disease progression) with fewer side effects (bone fragility, weight gain, mood changes).19–21 It is pertinent to note that muscle weakness and wasting is an acknowledged side effect of chronic glucocorticoid administration in many indications, such as critical care medicine, and is the most common drug-induced form of muscle weakness.22 Glucocorticoids have a direct molecular effect on myofibers, stimulating the catabolic AKT1/FOXO1 pathway, decreasing protein synthesis and increasing the rate of protein catabolism, resulting in weakness and atrophy.23 Thus it is likely that DMD patients treated with glucocorticoids show the clinical outcome of increased muscle strength mitigated to some extent by the side effect of muscle catabolism. Clearly any effort to reduce side effects such as weight gain and short stature may also lead to lessening of the side effect of muscle weakness, whereby the balance would then be tipped to greater efficacy.
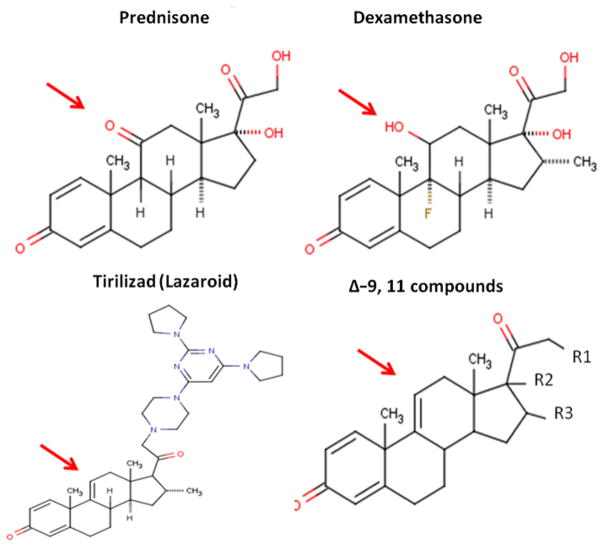
Fluorinated glucocorticoids, such as dexamethasone, are considerably more potent, with higher affinity to the glucocorticoid receptor (Fig. 1). However, these tend to also exacerbate side effects, and are generally avoided in indications of chronic use, such as muscular dystrophy. On the other hand, less potent nonfluorinated varieties of glucocorticoids have been tried, such as deflazacort. Deflazacort trials in DMD have suggested similar efficacy to that of prednisone (albeit at a higher dose), with an improvement in some side-effect profiles.3,24–26
Fig. 1.
Chemical structures of glucocorticoids and dissociative steroids. The arrow indicates the position of the key 9,11 alterations distinguishing classic glucocorticoids (prednisone, dexamethasone) from dissociative steroids (Δ-9,11 analogues).
FINDING THE OPTIMUM REGIMEN OF CORTICOSTEROIDS FOR DMD (FOR-DMD) CLINICAL TRIAL
To study the balance of efficacy and side effects, depending on steroid type (prednisone vs deflazcort) and dosing regimen (daily vs 10 days on, 10 days off), the FOR-DMD trial was designed and implemented. FOR-DMD is a multicenter, double-blind, parallel-group, 36- to 60-month study, comparing 3 corticosteroid regimens in wide use in DMD:
Daily prednisone (0.75 mg/kg/d)
Intermittent prednisone (0.75 mg/kg/d, 10 days on, 10 days off)
Daily deflazacort (0.9 mg/kg/d).
The hypothesis being tested is that daily corticosteroids (prednisone or deflazacort) will be of greater benefit than intermittent corticosteroids (prednisone) in terms of function and subject/parent satisfaction. A secondary outcome is to study whether daily deflazacort will be associated with a better side-effect profile than daily prednisone.
The primary outcome variable will be a 3-dimensional (multivariate) outcome consisting of the following 3 components (each averaged over all postbaseline follow-up visits through month 36): (1) time to stand from lying (log-transformed), (2) forced vital capacity, and (3) subject/parent global satisfaction with treatment, as measured by the Treatment Satisfaction Questionnaire for medication.
Secondary outcome variables will include regimen tolerance, adverse event profile, and secondary functional outcomes including the 6-minute walk test, quality of life, and cardiac function. The analyses will be adjusted for covariates, namely country/ region, baseline time to stand from lying, baseline forced vital capacity (FVC), and initial weight band. A sample size of 100 subjects per group (300 in total) will provide adequate power to detect differences that are thought to be of minimal clinical significance between any 2 of the 3 treatment groups, assuming a 10% rate of subject withdrawal.
The trial will randomize 300 boys aged 4 to 7 years to 0.75 mg/kg/d prednisone; 0.75 mg/kg/d prednisone for 10 days alternating with 10 days off; or 0.9 mg/kg/d deflazacort. All boys will complete a minimum 3 years (36 months) treatment period. All boys entering the trial will remain on the study drug until the last boy completes the 36 months of study; this may be up to 60 months.
Eligible boys will be those with confirmed DMD (defined as male with clinical signs compatible with DMD and confirmed DMD mutation in the dystrophin gene [out-of-frame deletion or point mutation or duplication] or absent/<3% dystrophin on muscle biopsy); age at least 4 years and under 8 years; ability to rise independently from the floor; willingness and ability of parent or legal guardian to give informed consent; willingness and ability to comply with scheduled visits, drug administration plan, and study procedures; and ability to maintain reproducible FVC measurements.
The study is funded by the National Institutes of Health (Kate Bushby and Robert Griggs, study Chairs), and will begin enrollment in 2012.
DEVELOPMENT OF DISSOCIATIVE STEROIDS FOR DMD
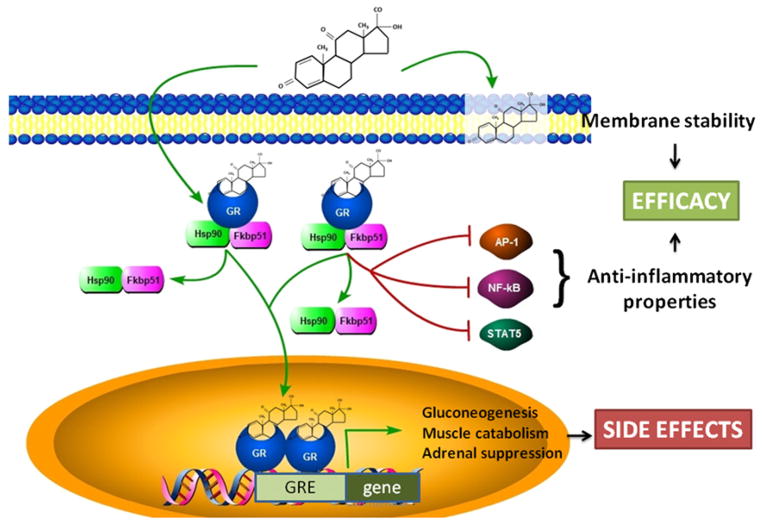
An alternative approach to optimizing dosing regimens of traditional glucocorticoid drugs is to change the chemistry of the drug, with the goal of broadening the therapeutic window (increasing efficacy while decreasing side effects). Glucocorticoid drugs are recognized to have 2 subactivities: serving as a ligand for steroid hormone receptors, and nonreceptor-mediated effects on plasma membranes. The ligand/ receptor complex has 2 further subactivities: transactivation and transrepression properties. Transactivation (also termed cis-regulation) is the best characterized molecular response, whereby ligand/glucocorticoid receptor complexes translocate from the cytoplasm to the nucleus and then interact directly with DNA and gene promoters (Fig. 2). With transactivation, the ligand/receptor dimers typically bind to a DNA sequence motif (glucocorticoid response elements [GRE]), and activate transcription of the nearby gene (hence the designation “transactivation”). Of importance, there is increasing evidence that the transactivation subactivity is associated more with side effects rather than with drug efficacy.27
Fig. 2.
Molecular action of glucocorticoids and dissociative steroids. Classic pharmacologic glucocorticoids have anti-inflammatory, membrane fluidity, and glucocorticoid response element (GRE)-mediated transcriptional activities. Dissociative steroids retain membrane and anti-inflammatory subactivities associated with efficacy, but do not retain the GRE-mediated transcriptional activities associated with side-effect profiles. GR, glucocorticoid receptor.
Clinical efficacy, on the other hand, is increasingly associated with the second, trans-repression subactivity. Transrepression involves ligand/receptor interactions with other cellular signaling proteins, such as nuclear factor (NF)-κB, activator protein 1, and STAT5 complexes, with downstream changes in cell signaling, and more indirect effects on gene transcription (non–GRE-mediated).27,28 Transrepression has been associated with anti-inflammatory activity and clinical efficacy.
All steroid hormones, including glucocorticoids, are lipophilic, and readily traverse lipid bilayers (cell membranes). Some steroid drugs have been optimized for membrane activities, such as the lazaroids (see Fig. 1). Lazaroids, including the Δ-9,11 modification thought to block binding of the drug to the receptor, were optimized for effects on cell membranes (prevention of lipid peroxidation), and tested clinically for neuroprotection.29–31 In DMD, there are well-documented changes in myofiber membrane function and integrity, and steroids are likely to modify this defect (for better or worse). Consistent with this, recent studies of lazaroids in myogenic cells in culture32 and ischemia/reperfusion injury in vivo have shown benefit of lazaroid drugs.33
In an effort to improve upon glucocorticoid therapy for DMD, the authors studied drugs with the Δ-9,11 chemistry (see Fig. 1). The goal was to determine whether this chemistry represented a dissociative steroid (eg, separation of the transactivation [side effects] and transrepression [efficacy]) (see Fig. 2). A Δ-9,11 drug, anecortave, did in fact bind the glucocorticoid receptor, albeit at lower affinity than pharmacologic glucocorticoids.34 Of importance, the ligand/glucocorticoid receptor complex was found to translocate to the nucleus, but showed no activity in binding to GRE elements and activating GRE-mediated gene transcription. Thus, the Δ-9,11 drug appeared to have lost the transactivation subactivity associated with many deleterious side effects (see Fig. 2).
To determine whether the Δ-9,11 drug retained transrepression (the subactivity associated with glucocorticoid efficacy), the authors studied anti-inflammatory effects using NF-κB reporter assays.35 NF-κB inhibitory activity was found to be retained by the Δ-9,11 drug, at a potency similar to that of prednisone.34 We were also interested in the effects of the drugs on the phospholipids that make up the membrane. Phospholipid bilayers have a hydrophilic head on the inner and outer walls of the membrane and hydrophobic tails. Lipids such as cholesterol are known to compress head groups, strengthen the bilayer, and decrease permeability when incorporated into a lipid bilayer. Indeed, our delta 9,11 steroids exert a similar and more profound effect on phospholipid bilayers than either prednisolone or cholesterol. The D-ring functionality (17-hydroxy-20-keto-21-hydroxy) orients within the phospholipid head groups while the hydrophobic ABC and most of the D ring orients in the lipid core. Since the C-17 C-20 bond can rotate, our compounds are operationally cone-like wedges in the phospholipid. They compress the head groups and decrease permeability while disordering the hydrophobic core which, among other things, protects against lipid peroxidation by decreasing the repeat number in lipid peroxidation chain reactions. This phenomenon for the delta 9,11 steroids has been described.36 These result encouraged the authors to conduct a preclinical study of the dystrophin-deficient mdx mouse model of DMD. Evidence of efficacy in vivo was found, whereby daily oral delivery of Δ-9,11 analogue reduced muscle inflammation and improved multiple functional assays. Of note, no side effects of reductions in body weight or spleen size seen with prednisone treatment were observed, suggesting that the Δ-9,11 drug had indeed lost side effects. These data suggest that the Δ-9,11 chemistry holds promise as a dissociative steroid, with retention of efficacy via transrepression, and loss of side effects via reductions in transactivation subactivities. Current studies are focused on testing a series of Δ-9,11 compounds to optimize the potency, bioavailability, and toxicity profiles (lead compound selection), as well as testing of the optimized lead compound in animal models of multiple chronic inflammatory conditions, including other types of muscular dystrophy.
SUMMARY
DMD is among the most common of the muscular dystrophies, leading to shortened life span and considerable disability. Glucocorticoids are considered the standard of care, yet dose regimens have not been optimized, and the balance of efficacy and side effects for specific types of glucocorticoids requires further study. The FOR-DMD trial promises to shed light on dose optimization, as well as the therapeutic window of prednisone versus deflazacort. An alternative approach to optimizing currently available steroid regimens is to develop new drugs that are able to broaden the therapeutic window (increased efficacy with decreased side effects). Initial studies of Δ-9,11 modifications of the steroid backbone suggests that this chemistry produces a dissociative steroid, whereby anti-inflammatory activity is retained (transrepression) and membrane stabilization properties enhanced, while side effects are mitigated (loss of transactivation subactivity). Current studies are focusing on lead compound optimization using transactivation and membrane stability assays.
KEY POINTS.
Current standard of care of Duchenne muscular dystrophy (DMD) includes pharmacologic treatment with oral glucocorticoids.
Gains in strength and slowed progression of disease afforded by glucocorticoids are offset, in part, by the wide range of side effects of drug treatment.
Dose optimization studies are limited, and new larger clinical studies are needed to best balance efficacy and side effects (therapeutic window), as are studies of glucocorticoid alternatives to prednisone.
The FOR-DMD trial funded by the National Institutes of Health is under way to compare different dose regimens and types of glucocorticoids (prednisone, deflazacort).
A novel dissociative steroid, a Δ-9,11 drug, is under clinical development for DMD. This drug promises to broaden the therapeutic window and reduce side-effect profiles.
Acknowledgments
The project described is supported by grant number U01NS061799 from the National Institute of Neurological Disorders and Stroke and U54HD053177 from the National Institute for Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institute of Child Health and Human Development, or the National Institutes of Health. The authors acknowledge funding to TREAT-NMD by the EU FP6. Newcastle upon Tyne is a partner in the MRC Centre for Neuromuscular Diseases. ReveraGen receives funding from the National Institutes of Health TRND program of the National Center for Advancing Clinical Sciences, the Muscular Dystrophy Association Venture Philanthropy Fund, the US Department of Defense CDRMP, and Foundation to Eradicate Duchenne (FED).
References
- 1.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman EP, Bertelson CJ, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–17. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Angelini C, Pegoraro E, Turella E, et al. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve. 1994;17(4):386–91. doi: 10.1002/mus.880170405. [DOI] [PubMed] [Google Scholar]
- 4.Biggar WD, Harris VA, Eliasoph L, et al. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuro-muscul Disord. 2006;16:249–55. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Griggs RC, Moxley RT, III, Mendell JR, et al. Prednisone in Duchenne dystrophy: a randomized, controlled trial defining the time course and dose response: Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 6.Griggs RC, Moxley RT, III, Mendell JR, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–7. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- 7.Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Markham LW, Kinnett K, Wong BL, et al. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–70. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–7. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 10.Moxley RT, III, Ashwal S, Pandya S, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 11.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 12.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–89. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 13.Connolly AM, Schierbecker J, Renna R, et al. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12:917–25. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 14.Escolar DM, Hache LP, Clemens PR, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77(5):444–52. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation of Duchenne muscular dystrophy: interesting results in a trial of prednisone. Arch Neurol. 1987;44:812–7. doi: 10.1001/archneur.1987.00520200016010. [DOI] [PubMed] [Google Scholar]
- 16.Fenichel GM, Mendell JR, Moxley RT, III, et al. A comparison of daily and alternate-day prednisone therapy in the treatment of Duchenne muscular dystrophy. Arch Neurol. 1991;48:575–9. doi: 10.1001/archneur.1991.00530180027012. [DOI] [PubMed] [Google Scholar]
- 17.Beenakker EA, Fock JM, Van Tol MJ, et al. Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Arch Neurol. 2005;62:128–32. doi: 10.1001/archneur.62.1.128. [DOI] [PubMed] [Google Scholar]
- 18.Sansome A, Royston P, Dubowitz V. Steroids in Duchenne muscular dystrophy: pilot study of a new low-dosage schedule. Neuromuscul Disord. 1993;3:567–9. doi: 10.1016/0960-8966(93)90117-3. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DJ, James KA, Miller LA, et al. MD STARnet. Use of corticosteroids in a population-based cohort of boys with Duchenne and Becker muscular dystrophy. J Child Neurol. 2010;25(11):1319–24. doi: 10.1177/0883073810362762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve. 2007;36(4):424–35. doi: 10.1002/mus.20812. [DOI] [PubMed] [Google Scholar]
- 21.Schara U, Mortier J, Mortier W. Long-term steroid therapy in Duchenne muscular dystrophy-positive results versus side effects. J Clin Neuromuscul Dis. 2001;2(4):179–83. doi: 10.1097/00131402-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78(1):41–4. doi: 10.1016/j.jbspin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nat Med. 2004 Jun;10(6):584–5. doi: 10.1038/nm0604-584. [DOI] [PubMed] [Google Scholar]
- 24.Mesa LE, Dubrovsky AL, Corderi J, et al. Steroids in Duchenne muscular dystrophy—deflazacort trial. Neuromuscul Disord. 1991;1(4):261–6. doi: 10.1016/0960-8966(91)90099-e. [DOI] [PubMed] [Google Scholar]
- 25.Bonifati MD, Ruzza G, Bonometto P, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344–7. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Biggar WD, Politano L, Harris VA, et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscul Disord. 2004;14(8–9):476–82. doi: 10.1016/j.nmd.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 28.Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 29.Taylor BM, Fleming WE, Benjamin CW, et al. The mechanism of cytoprotective action of lazaroids I: Inhibition of reactive oxygen species formation and lethal cell injury during periods of energy depletion. J Pharmacol Exp Ther. 1996;276:1224–31. [PubMed] [Google Scholar]
- 30.Bracken MB, Shephard MJ, Holford TR, et al. Administration of methylpredniso-lone for 24 or 48 h or tirilazadmesylate for 48 h in the treatment of acute spinal cord injury; results of the third national acute spinal cord injury randomized controlled trial. JAMA. 1997;277:1597–604. [PubMed] [Google Scholar]
- 31.Kavanagh RJ, Kam PC. Lazaroids: efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Br J Anaesth. 2001;86:110–9. doi: 10.1093/bja/86.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Passaquin AC, Lhote P, Rüegg UT. Calcium influx inhibition by steroids and analogs in C2C12 skeletal muscle cells. Br J Pharmacol. 1998;124:1751–9. doi: 10.1038/sj.bjp.0702036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campo GM, Squadrito F, Campo S, et al. Antioxidant activity of U-83836E, a second generation lazaroid, during myocardial ischemia/reperfusion injury. Free Radic Res. 1997;27:577–90. doi: 10.3109/10715769709097861. [DOI] [PubMed] [Google Scholar]
- 34.Baudy AR, Reeves E, Damsker JM, et al. Δ-9,11 modification of glucocorticoids dissociate NF-κB inhibitory efficacy from GRE-associated side effects. J Pharmacol Exp Ther. 2012;343:225–32. doi: 10.1124/jpet.112.194340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baudy AR, Saxena N, Gordish H, et al. A robust in vitro screening assay to identify NF-kappaB inhibitors for inflammatory muscle diseases. Int Immunopharmacol. 2009;9:1209–14. doi: 10.1016/j.intimp.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epps DE, McCall JM. Physical and Chemical Mechanisms of the Antioxidant Action of Tirilazad Mesylate. Handbook of Novel Antioxidants Antioxid Health Dis. 1997;4:95–137. [Google Scholar]