Abstract
Neuropeptide FF (NPFF) was first isolated from the bovine brain in 1985 and is linked with a variety of biological activities. NPFF, which belongs to the RF-amide family of peptides, interacts with two distinct G-protein-coupled receptors, NPFF1 and NPFF2. These receptors are distributed throughout the body. The NPFF system was initially described as an anti-opioid system and, while the NPFF system does affect the opioid system, it also has been implicated in pain modulation, changes in arterial blood pressure, feeding behavior and regulation of core body temperature and of monoamine systems. Most of this pharmacology has been realized from the peptide NPFF itself or through peptide analogs. The quest for nonpeptide tools for this receptor system has been limited by low selectivity and poor pharmacokinetic properties. Herein, we summarize the current knowledge from the scientific and patent literature that demonstrates a clear need for future medicinal chemistry efforts.
With the discovery and isolation of neuropeptide FF (NPFF) from bovine brain in 1985 [1], NPFF (FLFQPQRFa) has been linked with a variety of biological activities. It is part of a family of peptides that have dipeptide Arg-Phe-NH2 at their C-terminal, known as RF-amides [2]. Other members of the mammalian RF-amide family include prolactinreleasing peptides, kisspeptin/metastin and QRFP/P518/26RFa [3]. Two subtypes of the G protein-coupled receptor for NPFF exist [4]. Endogenous peptides NPSF and NPVF show preference for NPFF1 in vivo [5], while endogenous peptide NPFF prefers NPFF2 [6]. Table 1 describes the sequences of NPSF, NPVF and NPFF for human, bovine, mouse and rat species. NPFF1 and NPFF2 are distributed throughout the body with NPFF2 present in both the spine and brain, while NPFF1 appears only in the brain [7,8].
Table 1.
Sequence of endogenous neuropeptide FF and RF-related peptides.
| Peptide | Species | Sequence |
|---|---|---|
| Precursor: pro-NPFFA | ||
|
| ||
| NPFF | Human | SQAFLFQPQRF |
| Bovine | SPAFLFQPQRF | |
| Mouse | SPAFLFQPQRF | |
| Rat | NPAFLFQPQRF | |
|
| ||
| NPAF (NPSF for the last 8 residues† | Human | AGEGLNSQFWSLAAPQRF |
| Bovine | AGEGLSSPFWSLAAPQRF | |
| Mouse | QFWSLAAPQRF | |
| Rat | EFWSLAAPQRF | |
|
| ||
| Precursor: pro-NPFFB | ||
|
| ||
| RFRP-1 | Human | MPHSFANLPLRF |
| Bovine | MPPSAANLPLRF | |
| Mouse | VPHSAANLPLRF | |
| Rat | VPHSAANLPLRF | |
|
| ||
| RFRP-3 (NPVF) | Human | VPNLPQRF |
| Bovine | AMAHLPLRLGKNRED | |
| SLSRWVPNLPQRF | ||
| Mouse | VNMEAGTRSHFPSLPQRF | |
| Rat | ANMEAGTMSHFPSLPQRF | |
Note that ‘NPSF’ has been used in the literature to describe both an eight amino acid short version of NPAF and a 37 amino acid extended version of rat RFRP-1.
NPFF: Neuropeptide FF; RFRP: RF-related peptide.
Reproduced with permission from [6].
Although initially described as solely an anti-opioid system [9,10], NPFF has a myriad of pharmacological effects. The complex nature of NPFF pharmacology depends on which subtype is targeted, the route of administration and opioid activity [11]. For example, the anti-opioid properties of NPFF are seen when the route of administration of NPFF is intracerebroventricular; yet, if the route of administration is changed to intrathecal, NPFF has shown to extend morphine-induced analgesia [12]. In addition to affecting the opioid system, NPFF has been connected to pain modulation, changes in arterial blood pressure [8], regulation of monoamine systems [8], reduction in food intake [13,14] and regulation of core temperature [15,16]. The majority of the pharmacological functions have been discovered through several groups’ efforts to create peptide ligands for NPFF1 and NPFF2 receptors.
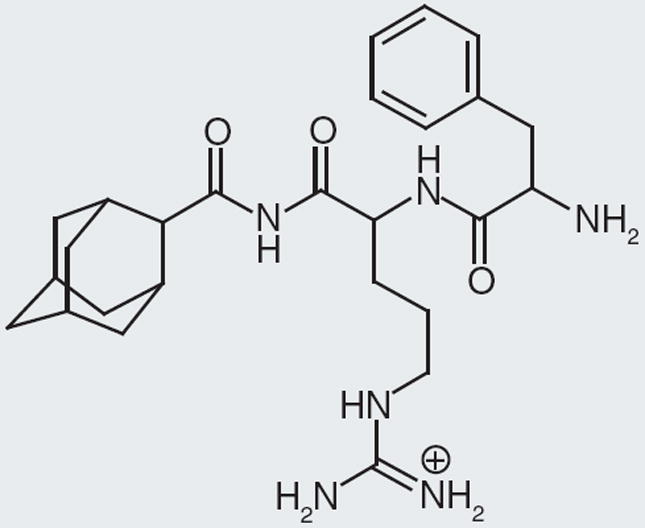
Initial work in the 1990s observed the importance of the N-terminal amino acids of endogenous NPFF by creating modified peptide analogs [17]. They discovered that the N-terminal was responsible for the high-affinity binding. Another group created affinity radioligands for NPFF receptors by modifying peptides (DYLMEFQPQRF- and YLFQPQRF-amide) [18]. Peptide work continued to produce both agonists and antagonists. BIBP3226 was initially described as a neuropeptide-Y (NPY) Y1 receptor antagonist [19,20]. It also proved to have pharmacological activity at NPFF receptors both in vitro and in vivo. For example, in vitro NPY-derived ligands BIBP3226 (a Ki value of approximately 100 nM for the NPFF2 receptor) and GR231118 (Ki = 50–70 nM for NPFF2 receptor) acted as an antagonist and agonist, respectively [21]. In vivo BIBP3226 was able to offset hypothermic effects of cerebrally injected NPFF and NPVF in mice and prevent antimorphine actions of NPFF in mouse tail-flick assay [22]. By contrast, it appears that the endogenous peptide NPVF is selective for NPFF1 versus NPFF2 receptors [5]. Likewise, peptide VPNLPQRF-NH2 shows strong selectivity for the NPFF1 receptor, while peptide EFWSLAAPQRF-NH2 has strong selectivity for the NPFF2 receptor (Table 2) [23]. The dipeptide known as RF9 is a nonselective antagonist for the NPFF receptors (Figure 1) [24]. RF9 can prevent NPFF-induced hypothermia [25] and does not lower body temperature itself. RF9 does appear to exert inhibitory effects on NPFF agonist-induced changes in body temperature and nociceptive tests, yet does not affect these changes when administrated alone [26]. Another interesting study showed that dansylated GSRF-NH2 and dansylated PQRF-NH2 acted as agonists, while dansylated GSR-NH2 and dansylated PQR-NH2 acted as antagonists on NPFF receptors [15,27]. Most recently, the peptide dNPA (d-Asn-Pro-(NHMe)Ala-Phe-Leu-Phe-Gln-Pro-Gln-Arg-Phe-NH2) was found to be a selective NPFF2 receptor agonist [28].
Table 2.
Peptides with selectivity for NPFF1 or NPFF2.
| Peptide sequence | Ki for NPFF1 (nM) | Ki for NPFF2 (nM) |
|---|---|---|
| VPNLPQRF-NH2 | 0.6 | 17.4 |
| EFWSLAAPQRF-NH2 | 20.8 | 0.21 |
NPFF: Neuropeptide FF.
Data from [23].
Figure 1. Nonselective dipeptide neuropeptide FF antagonist, RF9.
Nonpeptide NPFF ligands
Found mainly in the patent literature, nonpeptide small-molecule NPFF ligands offer a different viewpoint for probing the NPFF receptors and pharmacology of the system as a whole. One of the main benefits of these small-molecule ligands is their ability to better define the active site of NPFF1 or NPFF2 receptors; likewise, selectivity and affinity can be improved through relatively minor modifications in a parent structure. The majority of the small molecules described in the patent literature feature guanidine functionality. For instance, Acadia Pharmaceuticals has published structures featuring hydrazine/guanidine functionality; Actelion Pharmaceuticals and Synaptic Pharmaceuticals each published similar core structures with a guanidine moiety attached directly to a heterocycle (Figures 2 & 3). In contrast, two different Japanese patents show molecules that do not have a guanidine moiety but still retain their affinity for the NPFF system.
Figure 2. Actelion Pharmaceuticals compounds with single digit nanomolar IC50 at neuropeptide FF1 receptor.
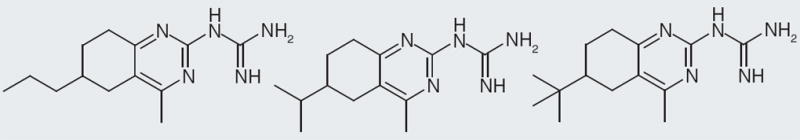
Figure 3. Synaptic Pharma Group neuropeptide FF ligand core structures.
Taisho Pharmaceutical
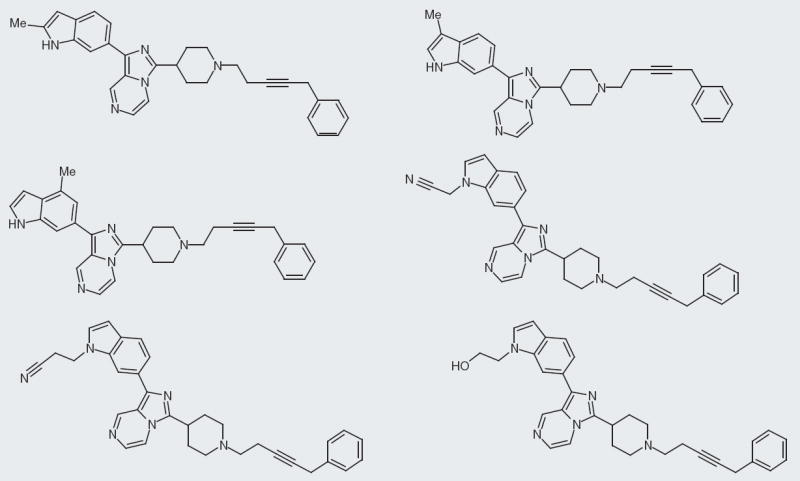
Taisho Pharmaceutical, a Japanese company, has a patent that describes compounds with IC50 values between 30 and 300 nM [101]. The IC50 values are for NPFF2 receptors in vitro. The core structure of their compounds is a fused imidazole-pyrazine with indole and piperidine substitutions (Figure 4). The SAR suggests that varying the carbon linker between the piperidine nitrogen and a substitution is tolerated within the reported IC50 values (Figure 5). Likewise, methylation at various positions off the indole ring maintains activity within the 30–300 nM range (Figure 6). Attachment of electron-withdrawing or electron-donating groups off the indole nitrogen also preserves activity. Changing the substitution of the piperidine nitrogen to a secondary or tertiary amine, or having an ether linkage still upholds IC50 in their reported range. Altering the indole ring substitution to other ring systems also keeps the IC50 values between 30 and 300 nM. While Taisho Pharmaceutical describes over 90 compounds in their patent, it does not provide specific IC50 values for specific compounds; therefore, it is difficult to fully appreciate and construct a complete SAR.
Figure 4. Taisho Pharmaceutical neuropeptide FF2 receptor compound general structures.
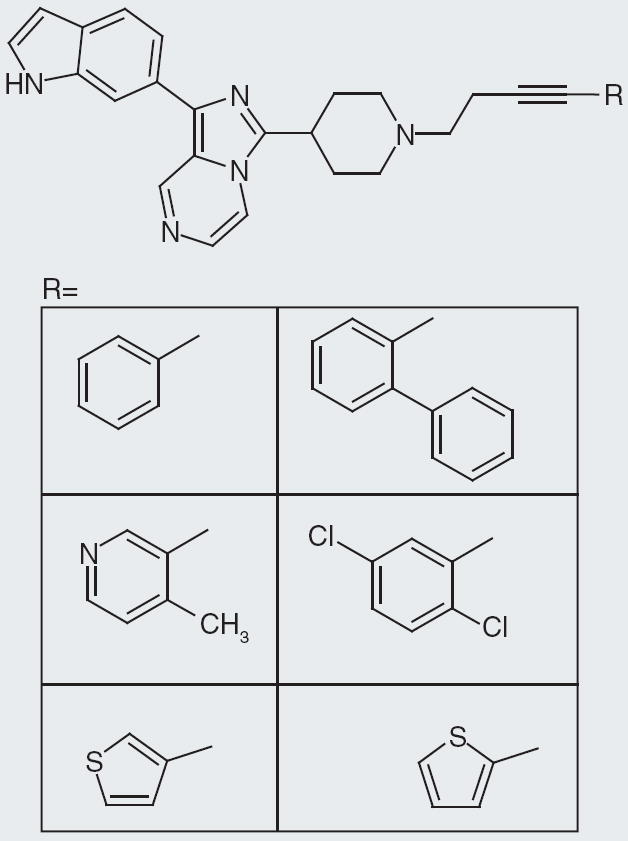
Figure 5. Changes in carbon linker between piperidine nitrogen and alkyne moiety.
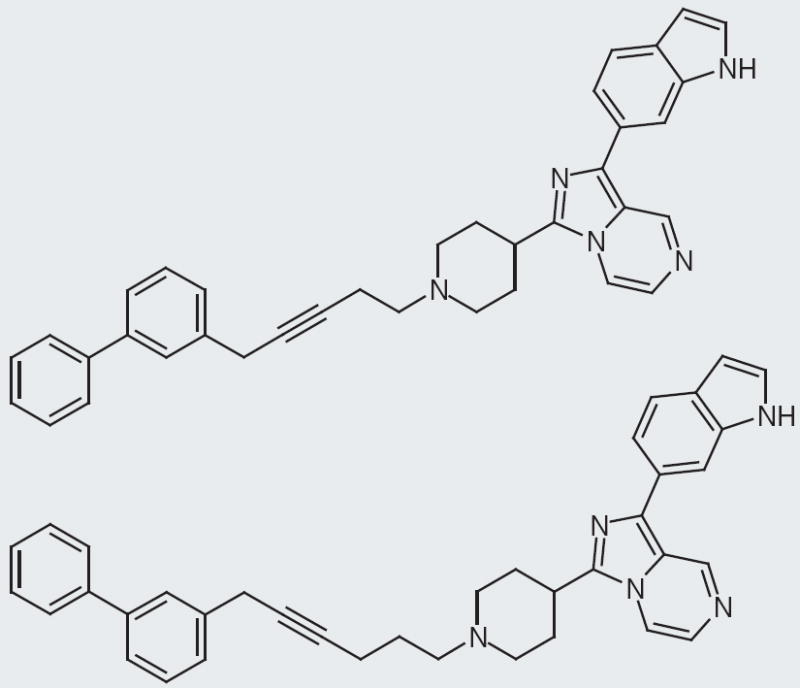
Figure 6. Various substitutions on the indole ring.
Kyowa Hakko Kogyo
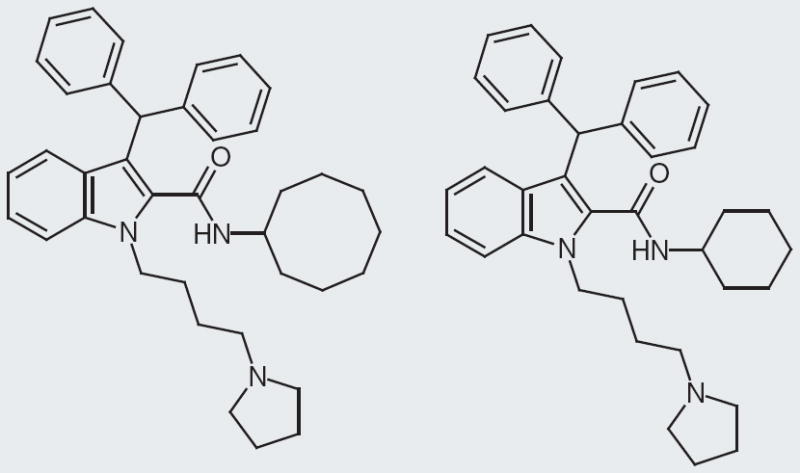
Kyowa Hakko Kogyo Company reports their NPFF small molecules at a fixed concentration of 3 μM and as a percentage binding of rat NPFF receptor [102]. The company’s molecules have an indole with a four-carbon spacer to a pyrrolidine as their common core structure (Figure 7). The best compound has a branched biphenyl in the 3-position and a cyclooctane ring in addition to the core structure (Figure 8). This compound has 100% binding at 3 μM. Interestingly, changing the structure from a cyclooctane ring to a cyclohexane drops the percentage binding almost 40%, to 61% (Figure 9). Yet, changing a cyclohexane to a cycloheptane ring improves the percentage binding to approximately 94%. Another interesting SAR point is the linker between the indole and the pyrrolidine, switching from a four-carbon linker to a three-carbon linker drops the activity from 100 to 41% binding at 3 μM. The change in linker does not always decrease percentage binding; in fact, going from a four- to three-carbon linker improves percentage binding in another case. While certain SAR features are clearer with Kyowa Hakko Kogyo compounds, unfortunately further clinical development seems unlikely due to the high lipophilicity of these compounds. Delivery, drug–drug interactions and plasma–protein binding all seem plausible barriers to developing these NPFF small molecules. Some of these challenges may have already been addressed as the patent reports that in vivo hot plate and tail-flick assays were performed; however, it was not clear which compound was tested.
Figure 7. Core structures of Kyowa Hakko Kogyo neuropeptide FF small-molecule ligand.
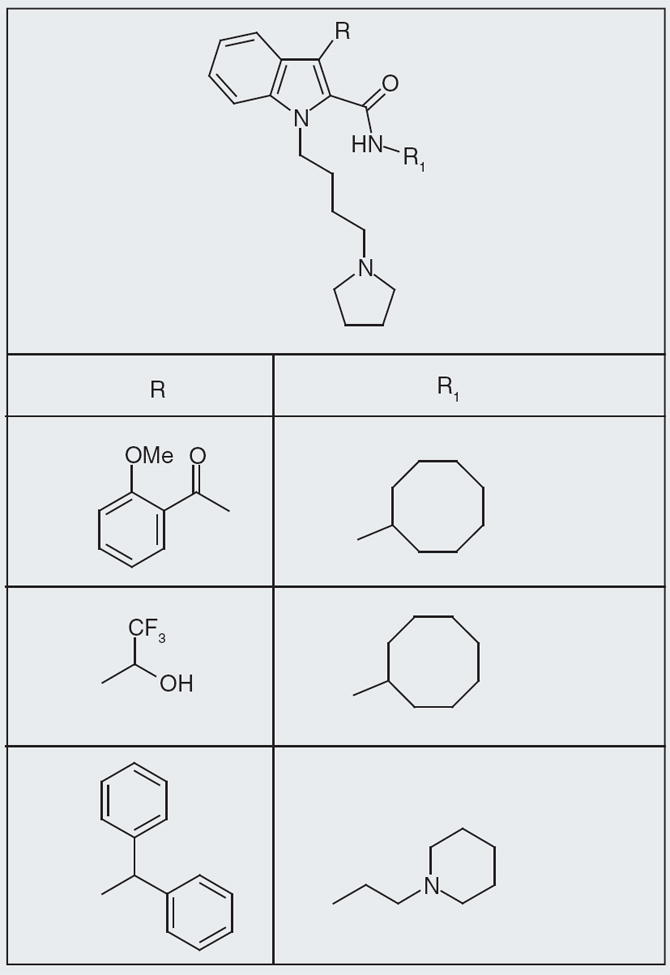
This is not an inclusive list of R and R1 substitutions included in the patent.
Figure 8. Best Kyowa Hakko Kogya compound; 100% binding at 3 μM at rat neuropeptide FF receptor.
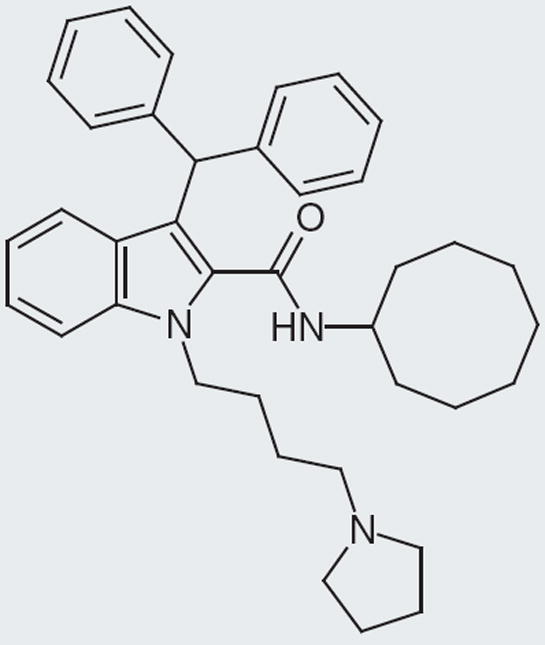
Figure 9. Varying ring size from an eight- to a six-member ring dramatically decreases percentage binding to rat neuropeptide FF receptor.
Acadia Pharmaceuticals
Acadia Pharmaceuticals published selective NPFF2 receptor agonists with a hydrazine-guanidine core structure [29]. They report their data as pEC50 and percentage efficacy for NPFF1 and NPFF2 receptors, although they do not specify from which species the NPFF receptors are derived. According to the patent [103], efficacy is the percentage maximal response compared with the maximum response elicited by NPFF; therefore, pEC50 is the negative log(EC50) and the EC50 is the molar concentration that produces 50% maximal response. A variety of substitutions of the hydrazine-guanidine core provided active and selective compounds. Of the compounds tested in vitro at both NPFF1 and NPFF2 receptors, two were taken on further to in vivo models of pain (Figure 10). Compound 3093 was tested in a thermal hyperalgesia assay and chronic constriction injury assay [30,31] where it showed a dose-dependent reduction in hyperalgesia. Interestingly, compound 1045 with a dichlorobenzylidene residue was not effective in multiple in vivo models; yet, compound 3093 with the dibromobenzylidene residue was effective in some of the same in vivo models of pain and inflammation. In addition, compound 3099 with a chlorotrifluromethylbenzyldiene moiety was also effective in in vivo models.
Figure 10. Compounds 1045, 3093 and 3099 are selective neuropeptide FF2 receptor agonists from Acadia Pharmaceuticals.
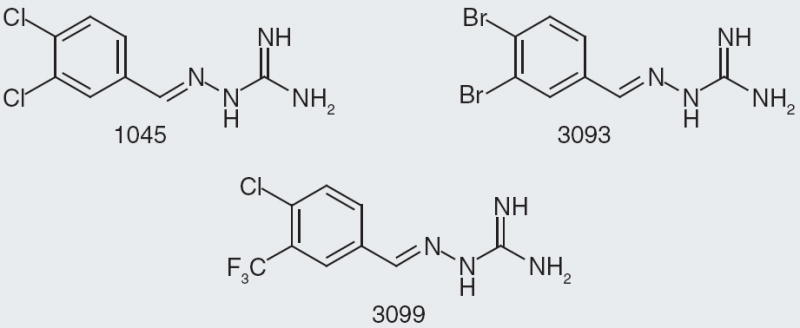
Compounds 3093 and 3099 were successful at reducing tactile allodynia in spinal nerve ligation model of pain in rats.
Actelion Pharmaceuticals
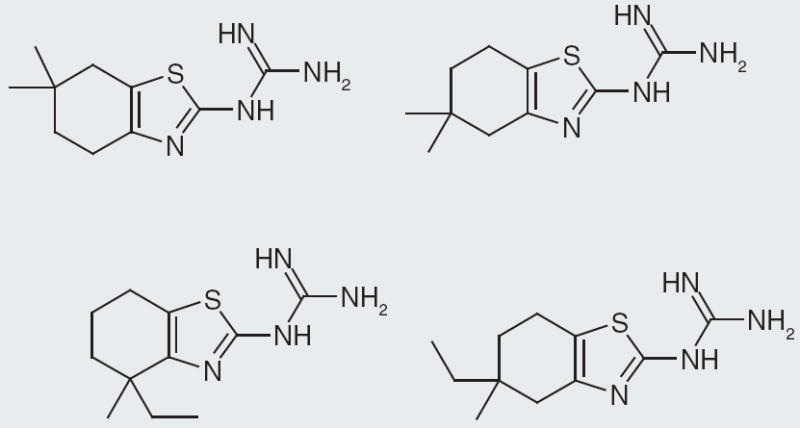
Actelion Pharmaceuticals present their compounds that have nanomolar binding to NPFF1 receptor expressed in Chinese hamster ovary cells. The core structure is a tetrahydroquinazoline with a guanidine at the 2-position and a methyl at the 4-position [104]. Among these compounds, the ones with single-digit nanomolar binding contain an aliphatic substitution of at least two carbons at the 6-position (Figure 2). In a second patent [105], Actelion Pharmaceuticals describe compounds having a tetrahydrobenzothiazole with a guanidine at the 2-position. Nanomolar binding for NPFF1 receptor is reported for some of their best compounds. Substitutions at the 5-, 4- and 6-position are explored and are tolerated maintaining single-digit binding affinity. A few interesting SAR features are identified through exploration of substitutions around the tetrahydrobenzyl portion of the compound. For instance, a tert-butyl group at the 6-position affords 10 nM affinity, yet moving it to the 4-position creates an even stronger binding affinity at 2 nM (Figure 11). Similarly, changing a dimethyl substitution from the 6- to the 5-position changes the binding from 4 to 2 nM, while changing a methyl, ethyl disubstitution from the 4- to the 5-position increases the binding from 5 to 0.2 nM (Figure 12). While these data prove interesting, it is difficult to know how selective these compounds are, since no NPFF2 receptor data are presented in the patents.
Figure 11. Changing a tert-butyl substitution from the 6- to the 4-position increases neuropeptide FF1 receptor-binding affinity.
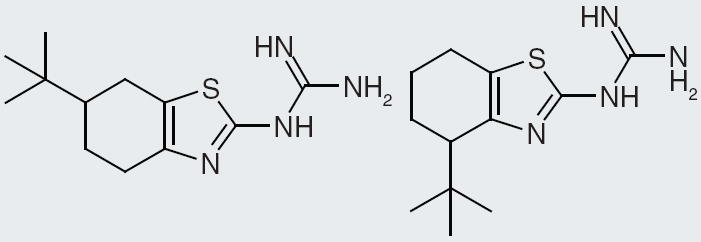
Figure 12. Changes from the 6- or 4-position to the 5-position on the tetrahydrobenzothiazole ring improves binding affinity at neuropeptide FF1 receptor.
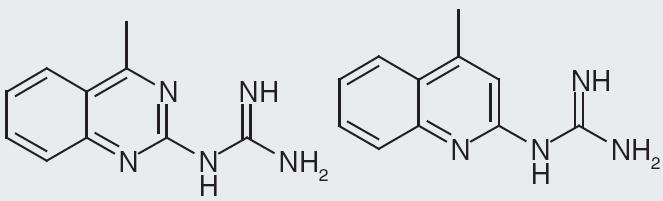
Synaptic Pharma Group
Synaptic Pharma group describes compounds that are agonists, antagonists and mixed agonists/antagonists at NPFF1 and NPFF2 receptors. They describe compounds in both rat and human NPFF1 and NPFF2 receptor binding. The core structure of their compounds are a quinazoline with a guanidine at the 2-position and a methyl substitution at the 4-position; the second core is a quinoline with guanidine at the 2-position and methyl at the 4-position (Figure 3) [106,107]. Aliphatic and aromatic substitutions at the 6- and 7-position are explored. Larger aliphatic or bicyclic substitutions seem to create compounds that bind to NPFF1 and NPFF2 receptors without much selectivity; while smaller substitutions at the 6- or 7-position create compounds with at least tenfold selectivity between the two subtypes. The company describe their compounds as agonist or antagonist according to intrinsic activity and Ki values; an agonist has >15% intrinsic activity, an antagonist has ≤15% intrinsic activity and a Ki value ≤1.2 μM at the rat NPFF receptors [108]. According to Synaptic Pharma Group’s claims, compounds that act as an agonist at NPFF1 and NPFF2 receptors would be suitable for treatment of incontinence and pain; likewise, compounds that act as NPFF1 and NPFF2 receptor antagonists could have proopioid effects. Additionally, compounds that are NPFF1 receptor agonists might be used to treat obesity, according to the company’s patents [108].
Conclusion
Despite a variety of small molecules published in patent literature, a straightforward SAR is still absent. Lipophilic substitutions, a nitrogen-containing heterocycle and guanidine substitution are common in most but not all of the structures. Selectivity still poses problems; it can be accomplished to some extent, but not always greater than a factor of ten between the two subtypes. Some of the compounds that have in vitro data, have such large aliphatic substitutions that solubility and delivery could pose problems for further development of such compounds. Although some of the patents describe various animal data, none describe whether their compounds were tested at off-target receptors or systems. This lack of data causes pause for the validity that their compounds are solely working through the NPFF system. Another concern is delivery methods, especially because even endogenous NPFF is known to cause varying effects depending on its delivery method in testing. Moreover, the lipophilicity of some of the compounds described could hinder further development; likewise, another hurdle in further development of some of these compounds is the guanidine moiety. Plasma–protein binding, interactions with other biological systems and drug–drug interactions are all factors that will need to be addressed as further development of small-molecule NPFF ligands continues.
Just as the pharmacological effects of the NPFF system continue to expand through peptide work, continuation of small-molecule development will further develop the SAR of the NPFF1 and NPFF2 receptor binding sites. Furthermore, small molecules can help to explain selectivity between the two subtypes of receptors. Additionally, with improvements in selectivity and affinity, imaging probes and therapeutic agents become possible. Although known since the mid-1980s, the NPFF system still remains a relatively underexplored system. With a constantly evolving pharmacological profile that suggests a strong relationship with the opioid system, a great deal of promise exists for modulators of the NPFF system.
Future perspective
Alternatives to the current standards in pain management are a necessity. Modulation of the NPFF system may provide a viable alternative to the opioid system. Current small molecules provide certain hints at the essential components of selective NPFF ligands. While most of the published small molecules contain a guanidine moiety, this portion may need to be changed through isosteric replacements. It may be that the bidentate interaction is necessary but an alternative to the guanidine might still provide this type of interaction without the pitfalls of the guanidine. Similarly, while lipophilic substitutions of varying size seem to be tolerated and can in fact aid in some cases, a balance must be struck between the lipophilic and hydrophobic portions of the molecules in order to increase the chances for viable therapeutic agents. In the end, a great deal of potential still exists in the field of small-molecule ligands for the NPFF system.
Executive summary.
-
▪
Neuropeptide receptors are G-protein-coupled receptors classified into two subtypes: neuropeptide FF (NPFF)1 and NPFF2.
-
▪
NPFF receptors may be involved in maintaining some aspects of homeostasis and in a number of disorders.
-
▪
Stable and selective nonpeptide probes for pharmacology are still lacking in the field.
-
▪
Future efforts will involve elucidation of the key pharmacophoric elements that allow for design of druggable nonpeptide compounds.
Acknowledgments
This work was supported in part by a grant from the National Institute on Drug Abuse (DA 029738).
Key Terms
- Neuropeptide FF
Neuropeptide of the RF-amide family linked to a variety of pharmacological functions including anti-opioid activity
- RF9
Dipeptide and a nonselective antagonist for neuropeptide FF receptors
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
-
▪
of interest
-
▪▪
of considerable interest
- 1▪▪.Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci USA. 1985;82(22):7757–7761. doi: 10.1073/pnas.82.22.7757. First description of the isolation of neuropeptide FF (NPFF) from bovine brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonin F. Neuropeptide FF receptors as therapeutic targets. Drugs Future. 2006;31(7):603. [Google Scholar]
- 3.Zajac J-M, Mollereau C. Introduction. Peptides. 2006;27(5):941–942. doi: 10.1016/j.peptides.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Bonini JA, Jones KA, Adham N, et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem. 2000;275(50):39324–39331. doi: 10.1074/jbc.M004385200. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Guan X-M, Martin WJ, et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276(40):36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- 6▪.Mouledous L, Mollereau C, Zajac J-M. Opioid-modulating properties of the neuropeptide FF system. Biofactors. 2010;36(6):423–429. doi: 10.1002/biof.116. Current view on the connection between NPFF and the opioid system. [DOI] [PubMed] [Google Scholar]
- 7.Goncharuk V, Jhamandas JH. Neuropeptide FF2 receptor distribution in the human brain: an immunohistochemical study. Peptides. 2008;29(9):1544–1553. doi: 10.1016/j.peptides.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Panula P, Aarnisalo AA, Wasowicz K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog Neurobiol. 1996;48(4–5):461–487. doi: 10.1016/0301-0082(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 9▪.Lameh J, Bertozzi F, Kelly N, et al. Neuropeptide FF receptors have opposing modulatory effects on nociception. J Pharmacol Exp Ther. 2010;334(1):244–254. doi: 10.1124/jpet.109.164384. Description of the current understanding of NPFF1 and NPFF2 receptor effects in vivo. [DOI] [PubMed] [Google Scholar]
- 10.Yang H-YT, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides. 2008;42(1):1–18. doi: 10.1016/j.npep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Roussin A, Serre F, Gouardã Res C, et al. Anti-analgesia of a selective NPFF2 agonist depends on opioid activity. Biochem Biophys Res Commun. 2005;336(1):197–203. doi: 10.1016/j.bbrc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 12.Roumy M, Zajac J-M. Neuropeptide FF, pain and analgesia. Eur J Pharmacol. 1998;345(1):1–11. doi: 10.1016/s0014-2999(97)01604-x. [DOI] [PubMed] [Google Scholar]
- 13.Sunter D, Hewson AK, Lynam S, Dickson S. Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci Lett. 2001;313(3):145–148. doi: 10.1016/s0304-3940(01)02267-4. [DOI] [PubMed] [Google Scholar]
- 14.Murase T, Arima H, Kondo K, Oiso Y. Neuropeptide FF reduces food intake in rats. Peptides. 1996;17(2):353–354. doi: 10.1016/0196-9781(95)02137-x. [DOI] [PubMed] [Google Scholar]
- 15.Fang Q, He F, Wang Y-Q, et al. Pharmacological effects of the dansylated neuropeptide FF analogues on body temperature and morphine analgesia. Neuropeptides. 2007;41(5):339–347. doi: 10.1016/j.npep.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Desprat C, Zajac JM. Hypothermic effects of neuropeptide FF analogues in mice. Pharmacol Biochem Behav. 1997;58(2):559–563. doi: 10.1016/s0091-3057(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 17.Gicquel S, Mazarguil H, Desprat C, et al. Structure-activity study of neuropeptide FF: contribution of N-terminal regions to affinity and activity. J Med Chem. 1994;37(21):3477–3481. doi: 10.1021/jm00047a005. [DOI] [PubMed] [Google Scholar]
- 18.Devillers JP, Mazarguil H, Allard M, Dickenson AH, Zajac JM, Simmonnet G. Characterization of a potent agonist for NPFF receptors: binding study on rat spinal cord membranes. Neuropharmacology. 1994;33(5):661–669. doi: 10.1016/0028-3908(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 19.Doods HN, Wienen W, Entzeroth M, et al. Pharmacological characterization of the selective nonpeptide neuropeptide Y Y1 receptor antagonist BIBP 3226. J Pharmacol Exp Ther. 1995;275(1):136–142. [PubMed] [Google Scholar]
- 20.Rudolf K, Eberlein W, Engel W, et al. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271(2–3):R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 21.Mollereau C, Gouardères C, Dumont Y, et al. Agonist and antagonist activities on human NPFF2 receptors of the NPY ligands GR231118 and BIBP3226. Br J Pharmacol. 2001;133(1):1–4. doi: 10.1038/sj.bjp.0704049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Q, Guo J, He F, Peng Y-L, Chang M, Wang R. In vivo inhibition of neuropeptide FF agonism by BIBP3226, an NPY Y1 receptor antagonist. Peptides. 2006;27(9):2207–2213. doi: 10.1016/j.peptides.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Quelven I, Roussin A, Zajac J-M. Comparison of pharmacological activities of neuropeptide FF1 and neuropeptide FF2 receptor agonists. Eur J Pharmacol. 2005;508(1–3):107–114. doi: 10.1016/j.ejphar.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Simonin FDR, Schmitt M, Laulin J-P, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci USA. 2006;103(2):466–471. doi: 10.1073/pnas.0502090103. Description of NPFF receptor antagonist RF9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y-Q, Guo J, Wang S-B, Fang Q, He F, Wang R. Neuropeptide FF receptors antagonist, RF9, attenuates opioid-evoked hypothermia in mice. Peptides. 2008;29(7):1183–1190. doi: 10.1016/j.peptides.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Fang Q, Wang Y-Q, He F, Guo J, Chen Q, Wang R. Inhibition of neuropeptide FF (NPFF)-induced hypothermia and anti-morphine analgesia by RF9, a new selective NPFF receptors antagonist. Regul Pept. 2008;147(1-3):45–51. doi: 10.1016/j.regpep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Fang Q, Guo J, Peng Y-L, et al. In vitro and in vivo studies of dansylated compounds, the putative agonists and antagonists on neuropeptide FF receptors. Peptides. 2006;27(6):1297–1304. doi: 10.1016/j.peptides.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Moulédous L, Frances B, Zajac J-M. Modulation of basal and morphine-induced neuronal activity by a NPFF2 selective agonist measured by c-Fos mapping of the mouse brain. Synapse. 2010;64(9):672–681. doi: 10.1002/syn.20774. [DOI] [PubMed] [Google Scholar]
- 29.Gaubert G, Bertozzi F, Kelly NM, et al. Discovery of selective nonpeptidergic neuropeptide FF2 receptor agonists. J Med Chem. 2009;52(21):6511–6514. doi: 10.1021/jm9011998. [DOI] [PubMed] [Google Scholar]
- 30.Bennett GJ, Chung JM, Honore M, Seltzer ZE. Current Protocols in Pharmacology. John Wiley & Sons Inc.; San Francisco, CA, USA: 2001. Models of neuropathic pain in the rat. [DOI] [PubMed] [Google Scholar]
- 31.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
Patents
- 101.Nakamura T, Saito S, Yoshinaga M, Ohta H, Kawamura M, Ishizaka T. WO2009038012. 2009
- 102.Osakada N, Shinoda K, Kunori S, et al. WO2004080965. 2004
- 103.Sully AL, Davis RE, Vanover KE, et al. WO2005031000. 2005
- 104.Fecher A, Frezt H, Hilpert K, Breu V, Giller T, Valdenaire O. WO2005023781. 2005
- 105.Caroff E, Steger M, Valdenaire O, et al. WO2004083218. 2004
- 106.Kawakami J, Wetzel J, Boteju LW, Konkel MJ, Wan H, Noble SA. US20030139431. 2003
- 107.Forray J, Craig D, Kawakami J, et al. WO2003026657. 2003
- 108.Kawakami J, Konkel MJ, Boteju LW, Wetzel J, Noble SA, Wan H. WO2003026667. 2003


