Abstract
Despite significant advances in the virologic management of HIV infection over the last two decades, effective treatments for HIV-associated neurocognitive disorders (HAND) remain elusive. While pharmacological interventions have yielded some success in improving neurocognitive outcomes in HIV, there is a dearth of rigorous studies examining the efficacy of cognitive rehabilitation for remediating HIV-associated neurocognitive impairment. This qualitative review summarizes and critiques the emerging literature on cognitive and behavioral treatments for HAND, which provides many reasons for optimism, but also has major limitations that underscore the scope of the work that lies ahead. Considering the notable real-world consequences of HAND, the development, validation, and clinical deployment of cognitive neurorehabilitation interventions tailored to the needs of persons living with HIV infection is a priority for clinical neuroAIDS investigators. In describing potential future directions for this endeavor, particular attention was paid to the application of cognitive neuropsychological principles in developing theory-driven approaches to managing HAND, improving everyday functioning, and enhancing HIV health outcomes.
Keywords: HIV, AIDS dementia complex, cognition, cognitive rehabilitation
The first scientific evidence of objective neuropsychological deficits in persons living with HIV infection was published a quarter-century ago (Grant, et al., 1987). Subsequent to that seminal study, there has been an abundance of clinical research characterizing the impact of HIV infection on the brain, including its neuroepidemiology (e.g., incidence and prevalence), neural and cognitive mechanisms, and impact on real-world outcomes. However, there has been a paucity of literature dedicated to cognitive and behavioral approaches to treating HIV-associated neurocognitive disorders (HAND). As such, the next challenge for clinical neuroAIDS researchers is to translate the wealth of observational knowledge regarding HAND into effective, theory-driven, and evidence-based treatments that can improve health outcomes in persons living with HIV infection. In that regard, the aims of this qualitative review are to: 1) briefly review the current state of the HAND literature, including its diagnosis, profile, and effects on everyday functioning; 2) describe current pharmacological management strategies for managing HAND; 3) critically evaluate the limited literature on computerized (and emerging theory-driven) cognitive neurorehabilitation approaches to improving HAND; and 3) outline possible future directions for cognitive rehabilitation research in the context of HIV infection over the next decade.
CLINICAL FEATURES OF HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS (HAND)
Diagnosis and Neuroepidemiology of HAND
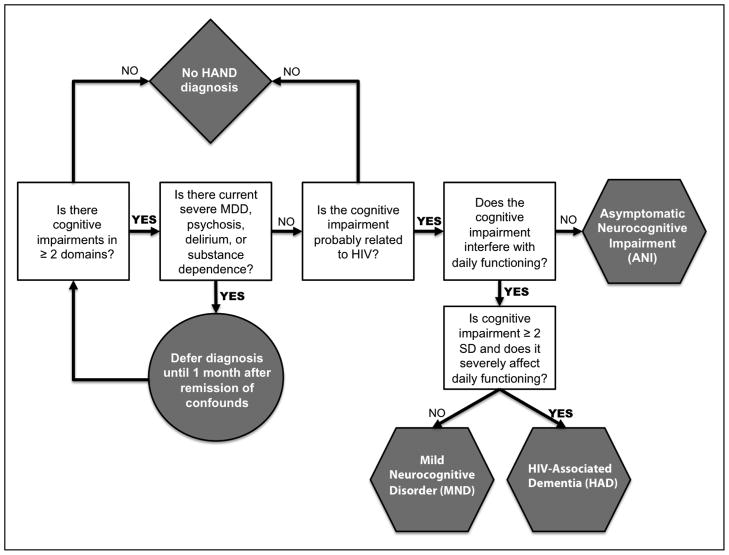
An important determinant for dedicating scientific resources for the development of an intervention is whether or not the condition of interest is of public health importance (e.g., prevalent and adversely impacts patients, their caregivers, and the healthcare system). HAND occurs in an estimated 30–50% of individuals with HIV (e.g., Heaton, et al., 2010), meaning that approximately 350,000–575,000 persons in the United States alone may suffer from HAND. Annual incidence rates of HAND range from approximately 10 to 25% (e.g., Robertson, et al., 2007). Established risk factors for HAND include older age (e.g., Valcour, et al., 2004), lower cognitive reserve (Morgan, Woods, Smith, et al., 2012), histories of immune suppression (Ellis, et al., 2011), and a host of comorbidities, including substance use disorders (Rippeth, et al., 2004) and co-infection with hepatitis C (e.g., Cherner, et al., 2005). The updated research nosology for HAND was determined by an NIH working group in Frascati (Antinori, et al., 2007), taking into consideration the many clinical and scientific advancements in treatment (i.e., the introduction of combined antiretroviral therapy [cART]) and neuropsychological assessment (e.g., new tests and improved normative standards) of HIV infection, as well as emerging appreciation of important comorbidities (e.g., hepatitis C co-infection). The Frascati criteria allow for three diagnostic categories: Asymptomatic Neuropsychological Impairment (ANI), Mild Neurocognitive Disorder (MND), and HIV-Associated Dementia (HAD) (see Figure 1). For each of these HAND diagnoses, an individual must demonstrate at least mild neuropsychological impairment (i.e., > 1 SD below the appropriate normative mean) in at least two cognitive domains that is attributable, at least in part, to HIV infection. Meeting this cognitive criterion alone qualifies for a diagnosis of ANI, which comprises approximately 50% of HAND diagnoses and approximately 15–30% of cases of HIV infection overall (Grant, et al., 2005). ANI has been the most controversial addition to the HAND nosology (Gisslen, Price, & Nilsson, 2011), as it does not require the presence of functional decline. Dissenting opinions have posited that ANI will cause unnecessary stress for individuals now deemed “neurocognitively impaired” despite no reported symptoms and few viable treatment options (Gisslen, et al., 2011). However, Blackstone and colleagues (2012) recently demonstrated that many of these “asymptomatic” individuals might actually evidence functional impairment when examined with more sensitive assessment tools (e.g., performance-based everyday functioning tests). Therefore, consideration of ANI diagnoses may be useful in spotlighting individuals who may be unaware of their functional disability (e.g., due to limited insight) or at risk for incident functional decline (Heaton, et al., 2012) and may therefore benefit from cognitive neurorehabilitation.
Figure 1.
Decision-tree outlining the consensus Frascati diagnostic criteria for HIV-Associated Neurocognitive Disorders (HAND) as adapted from Antinori et al. (2007) and Woods et al. (2009). MDD = major depressive disorder. SD = standard deviation.
Declines in everyday functioning are central to the diagnostic criteria of both MND and HAD, which necessitate the presence of syndromic, acquired mild-to-moderate (i.e. > 1 SD below the normative mean) or moderate-to-severe (i.e., at least 2 SDs below demographically-adjusted normative means) neurocognitive deficits in 2 or more domains, respectively. The functional dependence criteria for MND are milder than those for HAD and may be determined by evidence of two or more of the following that are not exclusively attributable to a comorbid condition: 1) self- or proxy-report of declines in ≥2 instrumental activities of daily living (IADLs; e.g., financial management); 2) unemployment or a significant reduction in job responsibilities secondary to reduced cognitive abilities; 3) decline in vocational functioning (e.g., increased errors, decreased productivity or efficiency); 4) self- or proxy-report of increased problems in ≥2 cognitive ability areas in day-to-day life (NB. this criterion can only be used if based in the absence of current depression, which may increase false positive self-reports of complaints); or 5) scores > 1 SD below mean on a performance-based laboratory measure of everyday functioning (e.g., medication management). A diagnosis of HAD requires substantial functional decline, as marked by: 1) unemployment due to cognitive impairment; 2) self- or proxy-report of dependence in > 2 IADLs related to cognitive problems; 3) self- or proxy-report of declines in ≥4 cognitive ability areas in day-to-day life (NB. As with a diagnosis of MND, this criterion only applicable if based exclusively on the self-report in the absence of current depression); 4) performance that is > 2 SD below the mean on a performance-based laboratory measure of everyday functioning (or > 1 SD below the mean on two functional tests). MND and HAD are present in approximately 5–20% and 1–2% of HIV-infected adults, respectively (Woods et al., 2009), and represent a significant subpopulation for whom aggressive treatment for neurocognitive impairment is most needed to improve everyday functioning.
Neuropsychological Profile of HAND
In order to identify sensitive and specific targets for prevention and/or remediation of HAND it is essential to first understand its neural mechanisms, affected brain systems, and cognitive architecture (see Figure 2). HIV is lentivirus that is highly neurotropic, and is able to infiltrate the central nervous system (CNS) through “Trojan Horse” mechanism via infected monocytes and cluster of differentiation 4 (CD4+) lymphocytes (Hult, Chana, Masliah, & Everall, 2008). Although the virus does not directly infect neurons, it often causes damage to neural tissue through both direct (e.g., viral proteins) and indirect (e.g., inflammatory) processes (Kaul, Garden, & Lipton, 2001). HIV-associated neuropathology is diverse in the era of cART is characterized by synaptodendric injury and can include HIV encephalitis (HIV-E), vasculopathy, and gliosis (Everall et al., 2009). HIV can affect many different neural pathways, but primarily impacts both the structure (e.g., white matter hyperintensities) and function (e.g., abnormal brain perfusion) of fronto-striatal-thalamo-cortical (FSTC) circuitry, in addition to medial temporal lobe regions (Thompson et al., 2005).
Figure 2.
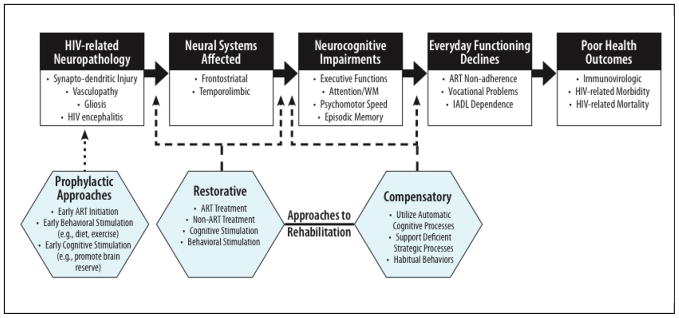
A preliminary conceptual model depicting possible targets for prevention and rehabilitation of the neuropathogenesis, affected brain systems, neurocognitive deficits, and real-world impact of HIV-Associated Neurocognitive Disorders (HAND). ART = antiretroviral therapy. IADL = instrumental activities of daily living. WM = working memory.
Consistent with this preferential impact of HIV on frontostriato-thalamo-cortical neural circuitry (see Ellis, Calero, & Stockin, 2009), the neurocognitive profile of HAND is often marked by impairments in domains of executive functions, episodic learning and memory, psychomotor speed, and working memory (see Table 1 for tests sensitive to HAND), while deficits in simple attention, sensory perception, receptive language, and visuoperceptual functions are less common. Executive dysfunction is among the most widely studied and well-documented neurocognitive deficits in HIV, particularly in the cART era (e.g., Heaton et al., 2011). Many individuals with HIV evidence deficits on a wide variety of higher-order processes, including abstraction and novel problem solving (e.g., Heaton, et al., 1995), cognitive flexibility (e.g., Reger, Welsh, Razani, Martin, & Boone, 2002), pre-potent response inhibition (e.g., Martin, et al., 2004), and planning (e.g., Cattie, Woods, Iudicello, Posada, & Grant, 2012). Emergent data also indicate that individuals infected with HIV may be prone to risky decision-making (Hardy, Hinkin, Levine, Castellon, & Lam, 2006), particularly those with HAND (Iudicello, Woods, Cattie, Doyle, & Grant, 2012), perhaps as a function of cognitive impulsivity (Martin, et al., 2004).
Table 1.
A selection of neuropsychological tests that have demonstrated construct validity in HIV disease, with note taken to those with evidence of sensitivity to neurocognitive change.
| DOMAIN | TEST |
|---|---|
| Neurocognitive Ability | |
| Executive Functions | Halstead Category Test Stroop Color and Word Test Trailmaking Test, Part B Wisconsin Card Sorting Test |
| Learning & Episodic Memory | Brief Visuspatial Memory Test-Revised California Verbal Learning Test (2nd ed.) Cogstate Maze Learning Testa Hopkins Verbal Learning Test-Revised Rey Auditory Verbal Learning Test Rey Complex Figure Test Wechsler Memory Scale (3rd ed.) |
| Language | Action (verb) Fluency Animal Fluency Boston Naming Test Controlled Oral Word Association Test |
| Information Processing Speed |
Cogstate Simple Card Detectiona
Digit Vigilance Test Symbol Digit Modalities Test Trailmaking Test, Part A Useful Field of View Testb WAIS-III Processing Speed Index |
| Attention and Working Memory | Paced Serial Addition Test WAIS-III/WMS-III Working Memory Index |
| Motor Skills | Finger Tapping Grooved Pegboard Timed Gait |
| Everyday Functioning | |
| Questionnaires | Modified Lawton & Brody ADL Medical Outcomes Survey-HIV SF-36 Patients’ Assessment of Own Functioning Inventory |
| Laboratory | Medication Management Task - Revised Timed IADL Testb Valpar standardized work samples ACTG Adherence Questionnaire |
Note. Becker et al., 2012 did not report individual test performances to determine sensitivity to neurocognitive change.
Evidenced change post-intervention in Boivin et al., 2010.
Evidenced change post-intervention in Vance et al., 2012.
The executive aspects of other cognitive ability areas are also frequently affected among persons living with HIV. For instance, the pattern of episodic memory impairment in HAND is most consistent with the prototypical mixed encoding and retrieval profile that is often observed in populations with compromised frontostriatal systems (e.g. Parkinson’s disease), with impairment most evident on more executively demanding free recall tasks but normalized performance on more structured recognition trials (Woods, et al., 2005). Given the frequently observed pattern of executive dysfunction, learning and memory deficits in HAND are also marked by limited use of higher-order strategic organizational encoding strategies (e.g., Delis, et al., 1995), including semantic clustering during list learning (e.g., Gongvatana, Woods, Taylor, Vigil, & Grant, 2007). Beyond retrospective memory impairment, HIV-infected individuals experience mild-to-moderate impairment on performance-based tests of prospective memory (i.e., “remembering to remember”), marked by a pattern of dysfunctional strategic encoding, monitoring, and retrieval of future intentions (e.g., Carey, Woods, Rippeth, Heaton, & Grant, 2006). Within the broad area of language functioning, HIV is associated with comparably mild-to-moderate impairment on measures of letter and category fluency (Iudicello, et al., 2007), thereby suggesting a common mechanism of deficient strategic search and retrieval from lexico-semantic memory stores rather than a degradation of those networks (Woods, et al., 2004). The process of switching between lexico-semantic categories during verbal fluency appears to be particularly affected in HAND, especially during alternating fluency trials (Iudicello, et al., 2008). Although simple attention (e.g., forward digit span) is generally spared in non-demented persons with HIV infection, deficits on measures of complex attention and working memory are considerably more prevalent (e.g., Heaton, et al., 1995; Reger, et al., 2002).
Taken together, the neurocognitive profile of HIV infection points toward a primarily dysexecutive syndrome, which may impact various domains of functioning through deficient higher-order strategic abilities (e.g., semantic clustering in verbal learning; Woods, et al., 2004) as well as weakened mechanisms for cognitive control (e.g., greater intraindividual variability; Morgan, Woods, Delano-Wood, Bondi, & Grant, 2011), while sparing relatively less cognitively demanding (e.g., so-called “automatic”) processes. Such information about neurocognitive strengths and weaknesses in HIV may be of value in determining both targets (e.g., domains and processes) and approaches (e.g., compensatory aids) for neurorehabilitation of HAND. More specifically, compensatory techniques that aim to capitalize on relatively intact automatic processes (e.g., basic attention, procedural learning) may effectively circumvent relative deficits in strategic neurocognitive processes in order to improve overall functioning (see Figure 2).
Although a review of the limitations of the HAND literature is beyond the scope of this paper, a few notable gaps in our current knowledge warrant consideration in the context of the burgeoning efforts toward designing effective rehabilitation strategies in HIV. First, we still have only a relatively rudimentary understanding about the natural course of HAND (i.e., from the acute and early period of HIV infection to death). Needed are prospective, longitudinal studies that can provide reliable estimates of incidence and functional recovery, as well as the predictors, mediators and moderators (e.g., cognitive, demographic, and medical) factors of neurocognitive stability, improvement, and decline (e.g., Mateen et al., 2012). Another fundamental problem is the lack of reliable, sensitive screening tools for clinics to identify HIV-infected individuals for whom treatment for HAND may be indicated (see Valcour et al., 2011).
Everyday Functioning Impact of HAND
Given that HIV-associated neurocognitive deficits are a significant, independent risk factor for a variety of adverse everyday functioning outcomes, developing effective interventions to improve HAND may translate into real-world benefits for patients and care providers alike. For instance, neurocognitively impaired HIV+ individuals show deficits across a range of laboratory-based functional tasks compared to their uninfected peers (e.g., cooking, financial and medication management; Heaton, et al., 2004). Additionally, HIV-associated neurocognitive impairment is associated with a greater likelihood of being unemployed (Woods, Weber, Weisz, Twamley, & Grant, 2011), decreases in job-related abilities (Heaton, et al., 2004), and difficulties of returning to work after disability (van Gorp, et al., 2007). Executive dysfunction (e.g., Hinkin, et al., 2002) and impairments in episodic memory (e.g., Woods, et al., 2009) are reliably associated with lower antiretroviral (ARV) adherence independent of other neuropsychiatric factors and HIV disease severity. HAND (specifically executive dysfunction and visual inattention) is also associated with both simulator and on-road automobile driving difficulties, including a greater number of driving accidents (Marcotte, et al., 1999; Marcotte, et al., 2006; Marcotte, et al., 2004). Lastly, greater severity of neurocognitive impairment in HIV infection is independently associated with lower health-related quality of life (Doyle, Weber, Atkinson, Grant, & Woods, 2012; Tozzi, et al., 2004) and higher risk for mortality (Ellis, et al., 1997; Wilkie, et al., 1998). Given the persistence of HAND in the cART era and its unique role in a wide range of real-world outcomes, the development, validation, and clinical deployment of targeted, effective cognitive neurorehabilitation treatments are needed and of clear importance to health outcomes in HIV disease.
Clinical Course of HAND
The natural history of neuroAIDS suggests that HAND may be responsive to cognitive rehabilitation and that such efforts may yield positive public health gains. Unlike neurodegenerative disorders (e.g., Alzheimer’s disease), HAND it is not invariably progressive. In fact, observational longitudinal studies show that it is common for individuals with HAND to remain cognitively stable over time (Cysique, Franklin, et al., 2011), and that approximately 21% may also evidence notable improvements (Robertson, et al., 2007). Recognizing the malleability of HAND, the Frascati diagnostic criteria include an “in remission” qualifier for individuals with prior diagnoses of HAND who no longer meet the neurocognitive and/or functional criteria. There are a variety of clinical factors that may relate to the amelioration of neurocognitive deficits, including comorbidity burden (e.g., hepatitis C co-infection), but opportunities for proactive modification of the course of HAND also exist. Such opportunities represent a unique environment for cognitive rehabilitation in the setting of neurological disease, whereby it may be possible to intervene to successfully improve cognition in a relatively young population with a manageable chronic illness. This is especially relevant in the cART era in which persons are now living with HIV infection into their 60s and 70s (CDC, 2010). With longer life expectancy and improved health outcomes, it is no longer assumed that HIV-infected individuals will need to collect disability payments as income, but instead, many may seek to continue working until retirement age (van Gorp, et al., 2007). It is this prosperous turn in the course of the HIV epidemic that has rendered the need to develop empirically supported methods to improve HAND even more dire.
PHARMACOTHERAPY OF HAND
Antiretroviral Therapies
The landscape of neuroAIDS treatment changed dramatically with the introduction of combination antiretroviral therapy (cART) in the mid 1990s. HIV-associated morbidities (e.g., opportunistic infections) and mortalities declined radically (Centers for Disease Control, 2010), largely due to cART’s effectiveness in suppressing HIV RNA in plasma and restoring immune functioning (Department of Health and Human Services, 2012), which has greatly improved health-related quality of life. The immunovirologic benefits of cART extend to the prevalence and severity of neurological complications associated with HIV infection; for example, the estimated prevalence of CNS opportunistic infection and frank HAD has decreased significantly since the pre-cART era (Heaton, et al., 2010; Sacktor, Lyles, et al., 2001). Although the 30–50% prevalence estimates of HAND have remained largely stable over time, the majority of cases are now less severe than previously observed, suggesting a persistent level of mild-to-moderate, yet clinically relevant, CNS involvement (Heaton, et al., 2011). Interestingly, the prevalence of HAND appears to have increased somewhat in individuals with less severe immunovirologic HIV disease (Heaton, et al., 2011). Beyond the lessened severity of HAND, the profile of neurocognitive impairment appears to have evolved since the introduction of cART Heaton and colleagues (Heaton, et al., 2011) recently reported a shift from primary deficits in the domains psychomotor speed and coordination in the pre-cART era to impairments of episodic memory and executive functions in the present treatment era.
Our increased understanding of the role of immunovirologic factors (e.g., nadir CD4 counts) in the development of HAND has helped to inform changes in cART management guidelines. Previously, it was commonplace for physicians to initiate cART only after a patient dropped below very low immunologic thresholds (e.g., a current CD4 count below 200), given the numerous toxicities and complications that can accompany cART (e.g., metabolic syndrome) (Department of Health and Human Services, 2002). Yet with the recognition that histories of significant immunocompromise (e.g., low nadir CD4) increase risk of poorer neurocognitive outcomes, regardless of current disease severity, questions emerged about the potential neuroprotective value of earlier HIV treatment (Ellis, et al., 2011). Further supporting the potential value of early treatment, neurocognitive impairment can emerge during the acute and early period of HIV infection (i.e., within the first year of seroconversion), particularly among persons with higher levels of viremia (Weber, et al., In press). As a result of these converging findings, current practice standards typically suggest that physicians prescribe cART after the diagnosis of HIV is made in order to prevent the neuroinflammatory cascades associated with poor immune functioning (Department of Health and Human Services, 2012), and perhaps as a prophylaxis to HAND. In fact, the initiation of cART has been associated with improvements in white matter integrity (e.g., Wright et al., 2012) and neurocognition (e.g., Joska, Fincham, Stein, Paul, & Seedat, 2010), particularly in ART-naïve individuals (e.g., Letendre, et al., 2004) and those who experience restoration of CD4 counts (Al-Khindi, Zakzanis, & van Gorp, 2011). Although a recent meta-analysis suggests that the benefits of cART on HAND effects may be quite modest (mean Cohen’s ds range: 0.17–0.24), particularly among older adults (Al-Khindi et al., 2011).
Antiretroviral Central Nervous System (CNS) Penetration
Nevertheless, the literature on cART’s effectiveness in restoring neurocognitive functioning in HIV in the modern era is highly variable, likely due to a variety of factors including issues related to study design (e.g., inclusion of persons without HAND) and measurement of cognition (e.g., limited test batteries). One very compelling candidate hypothesis for these discrepant findings is the notion of drug penetrance in the central nervous system (CNS) (Letendre, et al., 2008). The CNS may contain a reservoir of virus that can remain largely untouched by many antiretrovirals (ARVs) (Iglesias-Ussel & Romerio, 2011), which is particularly important due to the risk of poor health outcomes linked to detectable HIV RNA in the cerebrospinal fluid (CSF; e.g., HAND; Ellis, et al., 2002). ARVs vary in their ability to cross the blood-brain barrier and act upon the CNS viral reservoir. Letendre and colleagues (2008; 2004) developed and validated the use of a CNS penetration effectiveness (CPE) score, which rank orders cART regimens in terms of their impact on the CNS based on characteristics of drug pharmacological characteristics (e.g., molecular weight), delivery (e.g., drug concentration in CSF) and neurological outcomes (e.g., levels of HIV in CSF). For example, an ARV with a smaller molecular weight that produced high concentrations in CSF and showed evidence of reducing the prevalence of neurocognitive impairment received a higher CPE score, relative to a drug with opposing characteristics. Individual ARV CPE ranks were then summed for a given patient’s entire regimen to produce an overall score (see Letendre, et al., 2008 for details). When examined across a cross-sectional cohort of 467 HIV-infected adults on cART, lower CPE ranks were associated with greater CSF viral load, such that low CPE scores were associated with a 3-fold increase in the odds of having HIV detectable in CSF (Letendre, et al., 2008). These results are consistent with this group’s previous examinations into the effects of CNS penetration of cART on cognition, whereby individuals who initiated cART regimens with better CNS penetration yielded greater levels of viral suppression in the CSF, which in turn was associated with improvement in global neurocognitive functioning (Letendre, et al., 2004); see also Smurzynski, et al., 2011). A recent quantitative review of the CPE literature showed broadly medium effect sizes between penetrance and neurocognitive outcomes in studies with rigorous methodologies, with no evidence to suggest that higher CNS penetration produces negative effects on cognition (Cysique, Waters, & Brew, 2011). Extending this work, Shikuma and colleagues (2012) recently demonstrated that ARV regimens with greater efficacy on monocytes/macrophages, which can transmigrate into the CNS and play a role in neuroAIDS, were associated with better neurocognitive performance in a large cross-sectional cohort. However, as this research is still in its infancy, it remains hampered by some methodological limitations, including cross-sectional designs, low statistical power, and suboptimal randomization protocols (Cysique, Waters, et al., 2011). Results from well-designed randomized clinical trials of CNS and monocyte efficacy (some of which are already underway) will be necessary to support an evidence-based practice recommendation in clinic.
Non-Antiretroviral Pharmacotherapy of HAND
Paralleling the numerous ARV-focused studies, clinical research on a variety of non-ARV medications for HAND has also produced largely mixed results. A handful of psychiatric medications used as adjunctive therapies have shown early promise in managing HAND, suggesting that these drugs prescribed for disorders often comorbid in HIV infection may disrupt neurotoxic pathways in the CNS (Ances, Letendre, Alexander, & Ellis, 2008). For instance, a single-arm study demonstrated that administration of lithium, which in vitro studies suggest may be neuroprotective in HIV (Everall, et al., 2002), was associated with restoration of global neurocognitive functioning among 21 HIV-infected participants (Letendre, et al., 2006). This same group later reported that HIV-infected adults prescribed serotonin reuptake inhibitors (SRIs; i.e., citalopram, sertraline, or trazodone) were less likely to have detectable CSF viral load and performed significantly better on neurocognitive testing than those not prescribed SRIs (Letendre, Ances, Gibson, & Ellis, 2007).
Similar efforts have been made to test the efficacy of neuroenhancing drugs on HAND, but with limited success. Of note, at least five studies, even dating into the pre-cART era, have attempted to harness the promising psychostimulant properties of methylphenidate on HAND. However, many of these studies have produced negligible effects (e.g., no differential improvement relative to placebo; van Dyck, et al., 1997) or effects only on psychological symptoms (e.g., depression; Fernandez, et al., 1995). In one of the few positive study outcomes, Hinkin and colleagues (2001) found improvements on computerized tasks involving choice reaction time and dual task (i.e., simple and choice) reaction time in a single-blind, placebo-controlled, crossover study in HIV-infected individuals with depression and cognitive slowing, suggesting that there may be specific aspects of cognition that are responsive to methylphenidate (e.g., attention, psychomotor speed). However, most non-ARV medications that showed initial developmental promise in pilot trials have not maintained the observed effects in larger, more rigorous clinical trials. For instance, Sacktor and colleagues (2001) found improvements in verbal memory and psychomotor abilities in 14 HIV-infected individuals treated with transdermal selegiline in a randomized, placebo-controlled, double-blind study. When expanded to a larger sample with MRS-determined neural damage, no differences between treatment arms were observed after three and six months of treatment with selegiline (Schifitto, et al., 2009). A similar story has emerged with memantine, in which previous studies that demonstrated attenuation of hippocampal damage in a rodent model of HIV encephalitis (Anderson, Gendelman, & Xiong, 2004) did not translate to cognitive improvements in a larger clinical trial in HIV-infected adults (Schifitto, et al., 2007).
One inherent problem in the exclusive use of pharmacotherapy to improve HAND is that neurocognitive deficits often are independently associated with medication nonadherence in HIV (Hinkin, et al., 2002). In other words, one of the primary symptoms of HAND (i.e., non-adherence) may directly interfere with the fidelity and efficacy of its prescribed treatment (i.e., an adjunct medication). It is estimated that 40–50% of individuals with HIV are nonadherent to their ARVs (Nieuwkerk, et al., 2001), which are essential to prevent negative health consequences such as viral rebound, development of drug-resistant viral strains, more rapid progression to AIDS, and death (Arnsten, et al., 2001; Bangsberg, et al., 2000; Bangsberg, et al., 2001). Beyond psychosocial factors such as social support and psychiatric illness, critical neurocognitive functions like executive functions and episodic memory (e.g., prospective memory) are major risk factors for nonadherence (Woods, et al., 2009). Therefore, efforts focused exclusively on pharmacological interventions for treatment of HAND may be at risk for failure due to suboptimal adherence. Additionally, as opposed to the markers used to judge ARV adherence via virologic control (e.g., plasma viral load), it may be difficult for physicians to gauge a patient’s adherence to such regimens, and patients may lack concrete indicators of treatment benefit (e.g., CD4 count) that is more common in the culture of cART. Such considerations open the door for development of primary (and supportive) cognitive and behavioral strategies (e.g., cognitive rehabilitation) designed to improve neurocognition in this population.
COGNITIVE NEUROREHABILITATION OF HAND
The absence of an optimal first-line clinical pharmacotherapy for HAND has led researchers to begin exploring cognitive and behavioral approaches. However, only three studies have been published to date on cognitive neurorehabilitation of HAND. In brief, the vast majority of cognitive rehabilitation approaches across etiologies fall into two categories: restorative and compensatory (see Figure 2). Restorative approaches rely upon the principle of neuroplasticity and propose that “drill-and-practice” of cognitive skills will encourage more effective neural organization and ultimately improved cognitive abilities (Wykes & Spaulding, 2011), based on principles of implicit and procedural learning (Squire, 1986). In contrast, compensatory cognitive approaches do not directly aim to correct underlying cognitive deficits, but instead seek to improve cognitive functioning by supporting damaged cognitive processes with both internal (e.g., chunking) or external (e.g., cueing reminders) strategies (Twamley, Jeste, and Bellack et al., 2003). All three of the HIV studies published thus far adopted a broad restorative approach (i.e., not targeted at a specific cognitive mechanisms) using proprietary computerized rehabilitation tools (e.g., Captain’s Log). In this section, we review and critique these three studies, along with a recent series of theory-driven laboratory investigations designed to improve cognition in HIV that might serve to inform future work on rehabilitation of HAND.
Captain’s Log in Ugandan Children (Boivin et al., 2010)
The first study published on cognitive rehabilitation of HAND was performed in a sample of HIV-infected children in Uganda (Boivin, et al., 2010). The authors aimed to establish feasibility and proof-of-concept of Captain’s Log (Sandford, 2007), a computerized cognitive rehabilitation training program, in 60 Ugandan children (M age = 10 years) with perinatal infection who had notable histories of immunosuppression (i.e., M nadir CD4 = 35) and high current HIV RNA levels in plasma. Although HAND diagnoses in this sample were not reported, the HIV-infected cohort demonstrated significantly lower neurocognitive performance at baseline relative to a seronegative comparison group. In a non-blinded fashion, HIV-infected participants were randomized into either a no-contact control (n=28) or intervention (n=30) condition. The intervention sample underwent 10 45-minute training sessions with Captain’s Log modules targeting attention, memory, visual motor, and logic over a period of 5 weeks. Captain’s Log trainings vary across the separate cognitive modules (e.g., Alternating Attention, Detailed Reading) but generally include computerized game-like interfaces in which positive feedback (e.g., money, points, or grade percentages) is provided continuously for on-task behaviors such as increased reaction time or accurate responding whereas random, off-task responding is negatively reinforced (e.g., play is halted and an error message presents). Adherence to the intervention was high, such that as a group children in the intervention attended 95% of the Captain’s Log sessions. Pre- and post-intervention neurocognitive assessments were performed using the Cogstate neuropsychological battery (Darby, Maruff, Collie, & McStephen, 2002). In an ANCOVA analysis with baseline Cogstate performance included as a covariate, the intervention group performed better significantly better than controls at post-intervention on maze learning (Cohen’s d=0.77) and card detection speed (Cohen’s d=0.69), but not maze chasing, card identification speed, one card learning, or working memory (median Cohen’s d=0.25; all ps >0.05). These results remained significant even after adjusting for relevant cofactors (e.g., age, gender, and SES). In addition, immune activation (i.e., CD4 and CD8) was associated with improvements in maze learning in the intervention group (r=0.38). These gains on measures of simple attention and information processing speed suggest the potential for neuroplasticity in children with HIV that may be harnessed through restorative cognitive neurorehabilitation, although the precise mechanism is unclear (e.g., typical neural development vs. neural recovery). It is also unclear whether these results could generalize across the lifespan in adults with HIV (i.e., for whom neuroplasticity may be lessened) in different viral clades (e.g., viral strains typically found in North America), or whether a longer period of intervention may be more efficacious across speeded measures and generalize to other domains or abilities.
SmartBrain in HIV Seropositive and Seronegative Adults (Becker et al., 2012)
Becker and colleagues (2012) published the second study on cognitive rehabilitation of HAND. The authors examined the efficacy of a multi-domain computerized cognitive stimulation program (i.e., SmartBrain) in a partially randomized, non-blinded sample of 30 HIV-infected adults and 30 seronegative comparison participants. Note that, although group assignment was initially random, it became non-random after 30 participants were enrolled in order to meet recruitment goals and overcome limitations in staff availability. The baseline neurocognitive global scores for the HIV-infected sample were broadly within normal limits (i.e., Heaton Global Impairment Rating of approximately 3). In regards to HIV disease characteristics, 60% of the sample were diagnosed with AIDS, 83% were currently prescribed cART, and the average current CD4 count was 523; however, mean viral loads were in the detectable range (i.e., 2.05 log10). Individuals assigned to the intervention group were asked to use SmartBrain from their home computer for 24 weeks, with initial session length at 10 minutes that increased weekly to a maximum of 30 minutes. SmartBrain (SmartBrain Technologies, 2013) contains 14 game-like modules in the domains of memory, attention, gnosis, and executive functions. For example, “Placing the Colors” is a visuospatial memory task in which individuals must recall the location of colored circles within a presented grid after a brief delay; the task includes continuous verbal feedback after each circle placement in order to facilitate learning. All intervention participants began training in each of the modules at the lowest difficulty level, which was then adjusted to meet an individual’s appropriate baseline performance, with subsequent performance-based adjustments over time. Pre- and post-intervention neurocognitive assessments consisted of an extensive battery of standard clinical tests of memory, language, visuoconstruction, psychomotor speed, fine-motor skills, and executive functions. Primary results from this study revealed no significant effects of SmartBrain on global cognitive outcomes, irrespective of HIV serostatus. However, adherence to the intervention was suboptimal such that only 54% of participants were able to use the program more than once. Post-hoc analyses revealed that the subgroup of subjects who engaged in the most training sessions demonstrated the most improvement on the neurocognitive battery, suggesting that this intervention may be effective for persons who are able to use it as directed.
InSight in HIV-infected Adults (Vance et al., 2012)
Most recently, Vance and colleagues (2012) used a computerized cognitive training program specifically designed to improve speed of information processing in a sample of 46 middle- and older-aged HIV-infected adults. In regards to HIV disease characteristics, the sample had an average current CD4 count of 452 (with 52% of the sample having nadir CD4 counts of less than 200). It was reported that 70% of subjects had detectable viral loads despite 96% of the cohort being prescribed cART. Global cognitive functioning of these participants was not reported. In a randomized, non-blinded fashion, participants assigned to the intervention group (n=22) underwent 10 1-hour training sessions with Posit Science’s “InSight” computer program (Posit Science, 2013). This program consists of five games designed to improve participants’ speed of information processing, with visually presented material that becomes increasingly difficult (i.e., faster). For example, in “Bird Safari”, individuals are shown a picture of a target bird, which has specific characteristics (e.g., color of wing tips). The individual is then shown a flock of birds in the sky and subsequently needs to identify the location of the target bird in the sky once the flock is removed. Additionally, performance is continuously monitored and used to adjust the difficulty of the tasks, such that better performance allowed an individual to work on faster items and vice versa. Pre- and post-intervention neurocognitive assessments occurred 5 weeks apart and included the Useful Field of View (UFOV) test, the Finger Tapping Test, the Wisconsin Card Sorting Test, and the Timed Activities of Daily Living Test. Adherence to the intervention was high such that participants in this group completed an average of 9.3 out of 10 training visits (range: 2–10). The intervention group yielded baseline-to-post-test improvements (relative to controls) on the Timed Activities of Daily Living Test (Cohen’s d=0.42) and the Useful Field of View Test (d=0.34). Of note, performance on the Wisconsin Card Sorting Test was not different across the groups (d=0.18), but the controls demonstrated greater improvement on the Finger Tapping Test at follow-up (d= −.30). This lack of training benefit on a test of executive functions was expected by the authors and demonstrates specificity of this intervention for informational processing speed abilities. However, given the short time span of this intervention, it will be important to determine whether the gains made from this training may maintain beyond the training period.
Summary, Methodological Limitations, and Conceptual Considerations
Taken together, these three studies provide promising early evidence for the potential efficacy of restorative cognitive rehabilitation approaches in HIV. All three studies demonstrated at least some positive effects of computerized training programs on neurocognition in HIV-infected persons across the lifespan. The magnitude of these effects was in the medium range and was particularly evident for visual learning (Boivin, et al., 2010) and information processing speed (i.e., Boivin, et al., 2010; Vance, et al., 2012), including speeded tests of everyday functioning ability (i.e., TIADL; Vance, et al., 2012) (see Table 1). Benefit from these interventions did not generalize to other ecologically-relevant neurocognitive domains, including executive functions (e.g., Vance, et al., 2012) and working memory (e.g., Boivin, et al., 2010). However, given the nature and design of these computerized programs (e.g., restorative approaches that focus on information processing speed training), this outcome would not necessarily be expected.
Importantly, these studies establish proof of concept for the use of cognitive rehabilitation in HAND. Beyond the primary cognitive outcomes, it is encouraging to observe relatively high adherence rates to two of the three time-intensive interventions (i.e., above 90%; Boivin, et al., 2010; Vance, et al., 2012), thereby supporting the feasibility and acceptability of these computerized approaches for persons living with HIV. The two studies with the highest adherence rates used interventions that were designed to be completed in the laboratory. On the contrary, Becker and colleagues (2012) used a non laboratory-based intervention that ultimately fared relatively less well in terms of adherence; that is, only 54% of individuals were able to log in, register, and repeatedly access the training (the number of training activities completed ranged from 0–941 [median=112]). This intervention was designed for home computer use via the Internet, which was intended to improve access, but a number of participants had problems using SmartBrain due to slow Internet connection speed and server downtime. Moreover, it has been estimated that as many as two-thirds of HIV-infected persons may need assistance in using the Internet (Mayben & Giordano, 2007). Beyond barriers to treatment due to the intervention itself, these feasibility complications spotlight the importance of considering cohort characteristics (e.g., low SES) in the design of cognitive interventions that will best interface with this population. Nevertheless, the authors observed a dose-response relationship with intervention use, suggesting that if these adherence barriers are overcome, an intervention like SmartBrain may be effective in improving HAND for at least some HIV-infected persons.
Additional methodological, statistical, and conceptual limitations to these first few studies on cognitive neurorehabilitation of HAND also deserve discussion as we look toward the future. First, none of these studies utilized HAND diagnoses as an inclusion criterion. This is important because only about half of HIV-infected adults in the US evidence diagnosable cognitive impairment; in other words, these interventions may have been performed on HIV+ individuals who are not necessarily in need of cognitive rehabilitation, therefore diminishing the likelihood of detecting a clinically meaningful effect that is specific to HAND. Future research will benefit from examining the impact of such intervention protocols in the context of HAND in order to guide efforts to identify which rehabilitation techniques are most helpful to those in greatest need of treatment. In fact, this differential effect has been seen in prior pharmacotherapy studies of HIV, where globally null effects of Peptide T were moderated by neurocognitive impairment, such that only individuals with HAND evidenced neurocognitive gains with treatment (Heseltine, et al., 1998). That is not to say variability and improvement in the normal range is not a relevant consideration; indeed, neurocognitive vulnerabilities may exist within the broadly “cognitively normal” HIV-infected population and can adversely affect daily functioning (Morgan, Woods, & Grant, 2012). For instance, excess performance variability within or between neurocognitive domains may be a harbinger for incident HAND (e.g., Bielak, Hultsch, Strauss, Macdonald, & Hunter, 2010). Instead, the primary point is simply that careful consideration of HAND-related inclusion criteria in study design and intervention selection, and interpretation is essential.
Another notable methodological limitation of this literature is that none of the studies reviewed employed an active control condition, but instead used no-contact control groups. Inclusion of an active control group would allow for comparison of the intervention of interest to an unrelated condition that would not be expected to produce cognitive gains and equates groups on exposure to general therapeutic factors (e.g., generalized cognitive activity, therapeutic contact). As such, a control condition is critical to help isolate the “active ingredient” of the intervention of interest. For instance, the Boivin et al. (2010) study used computer-based neurocognitive outcomes to assess the effectiveness of a computerized cognitive intervention, which exposed the treatment group to numerous additional hours of computer familiarity. In such resource-limited environments, it is possible that children in the intervention group improved over time simply due to their increased exposure to the computerized testing format. Although determining an appropriate active control condition depends heavily on distinguishing it from the rehabilitation target, examples of candidate active control conditions that have been utilized in other populations include TV watching (e.g., Rass, et al., 2012), generalized compensatory cognitive training (e.g., Pijnenborg, Van der Gaag, Bockting, Van der Meer, & Aleman, 2011), and low cognitive demand computer activities (e.g., Dickinson, et al., 2010). Another approach to remedy such methodological complications would be use of a cross-over design, whereby individuals are randomly assigned to receive an intervention or control condition for a period of time and then switch to the alternate condition (e.g., Bergquist, et al., 2009). However, this design may be less applicable for interventions for which the benefits of the protocol are thought to be long lasting, or in which the individual could employ lessons learned while engaging in the control condition (e.g., continued use of newly acquired compensatory strategies beyond the intervention). Lastly, an important future methodological direction will be inclusion of long-term follow-up evaluations to determine whether cognitive interventions produce lasting benefits, as evidenced by sustained neurocognitive gains at follow-up sessions beyond the limits of the intervention period.
Another consideration for future studies is that the use of generalized cognitive training protocols and analysis of global cognitive indices may not identify the distal and proximal mechanisms of neurocognitive improvement (i.e., effective aspects of the treatment as well as domains of cognition in HIV that are amenable to remediation). Furthermore, each of these studies employs restorative (i.e., training-based) cognitive rehabilitation computer program based on a bottom-up, largely non-specific rationale. Information processing speed was the primary focus of the studies reviewed above (e.g., Vance, et al., 2012). However, processing speed deficits are not only highly non-specific, but they are also less prevalent in the current era of HAND (Heaton, et al., 2011). An alternative approach may be to use a top-down, theory-driven application of cognitive psychology principles to guide intervention implementation, as detailed below. Future research might aim to design treatments to improve more prevalent cognitive deficits with greater ecological relevance (e.g., executive functions, episodic memory). The signal detected by interventions designed to improve these neurocognitive domains may yield a robust and impactful picture of treatment efficacy in HAND.
Finally, the relatively small sample sizes and analytic approaches used in these three studies may have, in some instances, increased the risk of Type II error and diminished the potential clinical impact of the findings. Future studies with larger samples may benefit from more elegant statistical modeling that may be more sensitive to subtle treatment effects (e.g., repeated-measures ANOVA). Additionally, cognitive rehabilitation outcomes studies should also consider the application of standard clinical trials reporting (e.g., intent-to-treat analyses) and analyses that provide greater clinical information. For example, the calculation of effect sizes per cognitive domain may lend valuable insight into relative gains, as well as inform power analyses for prospective studies (Bezeau & Graves, 2001). Similarly, indices of reliable change should be considered in order to determine the prevalence (and clinical characteristics) of individuals whose neurocognitive functioning improves (or declines) beyond the range of normal variability in repeated testing (Duff, 2012).
FUTURE DIRECTIONS
Beyond the restorative approaches to cognitive rehabilitation utilized by the three studies detailed above, there are a number of other established methodologies to improve neurocognition that have shown effectiveness in clinical populations (e.g., schizophrenia). Compensatory cognitive training, for example, operates under the assumption that while cognitive deficits themselves may be less amenable to remediation, the functional effects of such impairment may be overridden through the use of compensatory aids (Twamley, Vella, Burton, Heaton, & Jeste, 2012). These strategies often include environmental adaptations and tools (e.g., use of calendars and alarms) as well as internally-driven strategies to support impaired neurocognitive processes (e.g., chunking). Studies on the efficacy of compensatory cognitive training frequently demonstrate small-to-medium effects on laboratory-based neurocognitive tests (Wykes, Huddy, Cellard, McGurk, & Czobor, 2011), with somewhat greater benefits on measures of everyday and psychosocial functioning (e.g., Velligan, et al., 2000). Furthermore, a multimodal approach involving both cognitive interventions and pharmacotherapy (i.e., combined compensatory and restorative approach) may prove to be particularly efficacious; for instance, prescription of a high-CPE cART regimen may improve core cognitive functions to levels that allow for enhanced benefits from restorative or compensatory cognitive rehabilitation interventions.
It also remains to be determined whether prophylactic efforts may be made to stave off HAND in at-risk, but neurocognitively normal individuals (see Figure 2). First, identification of protective factors may help inform protocols for maintenance of cognitive health. One important factor to consider is cognitive reserve, or the combination of innate cognitive ability and life experience to stave off incident neurocognitive impairments associated with aging or neurological disease (Stern, 2012). Cognitive reserve has been identified as a protector for neurocognitive impairment (Basso & Bornstein, 2000), particularly as it relates to syndromic HAND (Morgan, Woods, Smith, et al., 2012). Using this logic, it is possible that cognitive enrichment and mental activity may delay the development of cognitive impairment (e.g., Treiber, et al., 2011) via increased neuroplasticity (Park & Bischof, 2011). Outside the scope of cognition-based interventions, a range of lifestyle modifications (e.g., diet high in omega-3 fatty acids; Luchtman & Song, 2013) may also help to prevent neurocognitive impairment. One example of such an intervention is the implementation of physical activity regimens, which is theorized to prevent cognitive decline in part through the increased production of neuroprotective factors (e.g., BDNF; Foster, Rosenblatt, & Kuljis, 2011). Physical activities as basic as increased walking have yielded positive results on neurocognitive performance in healthy older adults (Jak, 2012). If ultimately proven to be effect effective, cognitive and lifestyle interventions such as these may ultimately play a role in HIV standard of care treatment as a prophylaxis for HAND.
An Approach to Translating Observational Neuropsychological Studies into Interventions
Although the paucity of literature on the rehabilitation of HAND to date is somewhat discouraging, it also affords researchers tremendous creative opportunities to draw upon the advances in the rehabilitation of other conditions to develop novel, effective, theory-driven approaches targeted at the unique and specific features of HAND in the cART era (e.g., deficits in episodic memory and executive functions). There are two primary types of research that may help guide future cognitive rehabilitation efforts for HAND from the applied cognitive neuropsychology literature: 1) observational studies in which spontaneous compensatory strategy use has been examined, and 2) studies examining rigorous, theory-driven experimental manipulations of neurocognitive paradigms (e.g., memory and executive functions) designed to improve performance.
Observational studies of compensatory strategy use in HAND provide an opportunity to evaluate techniques that are naturally beneficial for some individuals with HIV infection (e.g., may have good acceptability). For example, the smaller impairment effect sizes that are typically observed for structured, lower cognitively loaded tasks (e.g., recognition trials) versus organizationally demanding tasks (e.g., semantic clustering during list learning) among individuals with HIV infection (Delis, et al., 1995) may suggest that deploying such higher-level strategies may bolster cognitive performance (see Figure 2). Relatedly, Woods and colleagues (2010) demonstrated that older HIV-infected individuals who spontaneously used a chunking strategy on a test of visual working memory were less likely to commit errors when asked to choose novel designs. This finding is consistent with the broader clinical literature in that the effectiveness of spontaneous strategy deployment to remediate working memory dysfunction has been observed in healthy older adults (e.g., Wegesin, Jacobs, Zubin, Ventura, & Stern, 2000) as well as across numerous clinical populations (e.g., mild traumatic brain injury; Cicerone, 2002). It is theorized that the use of metacognitive (self-recognized and implemented) strategies minimizes the complexity of the working memory task (e.g., concurrent processing) and allows for deeper levels of encoding, which in turn liberates cognitive resources that may be used to facilitate higher levels of overall performance. Although important in laboratory performance, it remains unclear whether an individual’s spontaneous use of a particular compensatory strategy in the laboratory will generalize to regular strategy use in the performance of tasks of everyday functioning. Along these lines, Weber and colleagues (2012) demonstrated that older HIV-infected adults who reported regularly utilizing compensatory strategies to aid them in daily activities were significantly more likely to remember to perform a naturalistic prospective memory task (i.e., remembering to call the examiner) relative to non-strategy users. The knowledge gained from such observational, cross-sectional studies is tempered by the obvious design limitations (e.g., lack of randomization) that hinder clear interpretation for findings. For instance, Blackstone and colleagues (2012) found that HIV-infected adults who reported greater use of adherence compensatory strategies also had worse medication adherence, reported increased psychiatric distress, and were dependent in their daily functioning. If these data were taken from a prospective experimental design, they could suggest that compensatory strategies produce worse functioning outcomes. However, given the cross-sectional nature of these data, it is not possible to determine whether individuals with poor everyday functioning are benefitting at all from such strategies (relative to their baseline) and/or are using them effectively. Regardless of these limitations, findings from these cross-sectional studies can undoubtedly inform more rigorous prospective experimental efforts in the cognitive rehabilitation of HAND.
Complicating the implementation and use of compensatory strategy in adults infected with HIV is the role of accurate metacognition and insight, as an individual must be capable of recognizing his/her own cognitive strengths and weaknesses as well as task characteristics that signal that strategy use is necessitated. Metacognition involves the capacity for introspection including accurate perception and assessment of one’s everyday performance and its consequences. Up to 50% of people living with HIV show poor insight into their neurocognitive abilities, in which cognitive performance (e.g., executive and memory) does not correspond with functional complaints (i.e., both over- and under-estimating abilities). Notably, integrity of such metacognitive abilities has been associated with better rehabilitation motivation and outcomes (Prigatano & Wong, 1999) as well as safe and independent functioning outside of rehabilitation settings (e.g., vocational success) in other populations. Metacognitive trainings employed in other populations aim to teach individuals to self-regulate and monitor thoughts and actions in order to gain control over one’s own learning and behaviors (Sohlberg & Turkstra, 2011), and have proven successful for improving IADL functioning in individuals with executive dysfunction (e.g., TBI). Future research on such metacognition interventions in HIV may therefore be indicated, not only to improve awareness and decrease functional errors, but also to possibly enhance the effectiveness of other cognitive rehabilitation efforts.
Experimental cognitive theory paradigms, on the other hand, provide an opportunity to explore specific mechanisms that may be leveraged to enhance HIV-associated neurocognitive impairments and minimize their functional consequences. Although such approaches have been widely used in other clinical populations (e.g., multiple sclerosis), we are aware of only two such studies in HIV infection. One such technique is the application of the generation effect, more frequently studied in healthy young adults, to improve verbal recall in this population. A recent study demonstrated the effectiveness of a self-generation intervention in HIV (Weber, Woods, Kellogg et al., 2012), whereby participants were presented with word pairs in either a didactic format (i.e., explicitly presented) or a self-generated condition, which is theorized to facilitate memory recall by deepening encoding of the information to be remembered (Slamecka & Graf, 1978). In the self-generation condition, individuals were presented with a complete first word of the pair, but only the first letter of the second word is presented (Basso, Lowery, Ghormley, Combs, & Johnson, 2006). To generate the second word, the participant is provided a semantic cue that delineates a relationship between the paired word associates. For example, a didactic presentation of a word pair might be “drive–car”, whereas the parallel self-generation condition would be “drive–c___”. In the latter case, an individual would be asked to generate a word beginning with ‘c’ that is semantically related to the word “drive”. Self-generated encoding has also been used in numerical formats (e.g., addition; 2+2 = __), which has demonstrated robust effects on improving memory (average effect size = 0.92; Bertsch et al., 2007). In this study, self-generation improved recall and recognition memory performance in HIV-infected adults by over one standard deviation. Moreover, on self-generated trials, the HIV sample performed comparably to the level of seronegative adults without any aid, suggesting that this manipulation may help to normalize their episodic memory deficits. Clinically, self-generation could be applied by encouraging patients to generate the aspects of their treatment that they will need to remember (e.g., appointment times, treatment goals).
Another area in which this experimental approach has show modest success in HIV infection is category cueing to promote enhanced verbal fluency, which stemmed from studies indicating that lexicosemantic switching is the primary cognitive mechanism driving HIV-associated noun fluency deficits (Iudicello, et al., 2008; Woods, et al., 2004). Iudicello and colleagues (2012) devised a manipulation to a standard fluency paradigm in which individuals were prompted with a lexicosemantic subcategory every 15 seconds (e.g., supermarket items; see Drane, et al., 2006 for details), which was theorized to minimize demands on switching (i.e., strategic search and retrieval mechanisms). In a within-subject design, HIV-infected adults generated more total words when explicitly cued to switch than in the control non-cued condition (Hedge’s g=0.22). Of clinical relevance, the HIV group’s performance in the cued trial did not differ significantly from that of demographically comparable seronegatives in the standard condition, suggesting a possible normalization of the fluency deficit. This study helps illustrate the use of observational mechanistic findings at the domain level to inform the development of experimental approaches to overcome those deficits via simple compensatory manipulations in the laboratory. Importantly, supporting such processes may not only improve cognitive scores in HIV-infected persons, but may also be effective in achieving normalization of performance.
Of course, this work is rooted in a tradition of translating cognitive neuropsychological theory into effective cognitive remediation paradigms, which are also employed in other populations, such as multiple sclerosis (MS), and may serve to inspire future work in HIV. For example, Goverover and colleagues (2009) demonstrated that the spacing effect, the distribution of information over time, was effective in improving delayed retrospective memory performance in adults with MS. The benefit of manipulating the form of spaced learning was highlighted by Sumowski and colleagues (2010). This study observed enhanced performance on tests of delayed verbal recall when participants were tested with the learned material over time (i.e., retrieval practice, or the testing effect), as compared to simply restudying the information. Additionally, the effectiveness of self-generation has been extended to the learning and memory of information involved in activities of daily living (e.g., managing finances; Goverover, Chiaravalloti, & DeLuca, 2008). Learning and memory performance in this population may also be enhanced using the Story Memory Technique, which utilizes visual imagery to enhance encoding and recall of verbal information (Chiaravalloti, DeLuca, Moore, & Ricker, 2005).
Similar lessons may be learned through examination of cognitive rehabilitation literature in traumatic brain injury, particularly as it relates to the improvement of executive functions. For example, a number of studies have validated the efficacy of Goal Management Training (GMT; Levine, et al., 2000; Robertson, 1996) to remediate executive deficits in traumatic brain injury. GMT is a structured, manualized rehabilitation protocol that trains individuals to examine all aspects of goal-directed behavior, including environmental assessment, goal selection, encoding and retention of those goals, and monitoring of the performed action for consistency with the stated goal. Across several studies, GMT has been associated with improved neurocognitive performance on standardized tests of executive functions (e.g., Tower Test; Levine, et al., 2011) as well as improved performance (e.g., reduced errors) on everyday tasks in the laboratory (e.g., classifying a list of objects; Levine, et al., 2000). Extending such executively-driven interventions to other cognitive domains, Fish and colleagues (Fish, et al., 2007) demonstrated that a content-free cueing paradigm (i.e., simply providing a previously learned acronym “STOP!” to initiate strategy use) improved everyday prospective memory performance through increased monitoring, theoretically due to the support of impaired strategic functioning. Overall, given the overlap between neurocognitive profiles in these populations, the efficacy of rehabilitation techniques such as these should be examined in the context of HAND.
Rehabilitating Everyday Functioning Outcomes
Although the translation of observational studies to experimental improvements in the laboratory represents an important first step in the rehabilitation of HAND, ensuring the ecological value of these findings as concerns real-world health outcomes is a major challenge. To date, there is one study that has used cognitive techniques to improve functional outcomes in HIV. Neundorfer and colleagues (Neundorfer, et al., 2004) demonstrated the effectiveness of a spaced retrieval intervention (coupled with external aids) in the self-reported success of pre-specified goals for everyday activities (e.g., remembering to take medications). In this intervention, individuals are asked to recall the content of the intention to be remembered when cued, with correct performance yielding increasingly longer duration between testing cues, until the patient is able to correctly recall the intention without error at 3 consecutive subsequent testing sessions. As compared to the spacing effect (e.g., Sumowski et al., 2010), which simply seeks to distribute learning over time in a static fashion, spaced retrieval acts as a behavior-shaping paradigm that rewards closer approximations to the correct response, in which practice of material is concordant with an individual’s success. Two months after their intervention, 60% participants were able to recall their first previously stated intention, and 89% were able to recall their second intention, suggesting that spaced retrieval and other cognitive techniques may be effective to improve real-life recall abilities in individuals with HIV. The importance of this translational bridge cannot be overstated, as cross-sectional research has clearly shown that many primary tasks of independent living and health maintenance are heavily reliant upon intact neurocognition in HIV infection. For instance, van Gorp and colleagues (2007) found that memory function was the strongest neuropsychiatric predictor in HIV-infected adults who were able to successfully return to work. The usefulness of this translation from the laboratory to life will be especially important within the domain of medication adherence. Currently, there exist numerous intervention studies that seek to address nonadherence using a range of techniques, from cognitive-behavioral therapy (e.g., Weber, et al., 2004) to personalized text messaging (e.g., Hardy, et al., 2011). While a proportion of these intervention studies have demonstrated efficacy in improving adherence and important disease indicators (e.g., plasma viral load), the impact of these interventions are often short-lived and limited to the duration of the intervention period (e.g., Hardy, et al., 2011). Clinical and theory-driven neuropsychological approaches to such research are largely absent, despite considerable evidence that memory and executive deficits play a major role the effectiveness of remembering to take one’s medication (Zogg, Woods, Sauceda, Wiebe, & Simoni, 2012). In one of the few adherence studies to use such an approach, Andrade and colleagues (2005) found that HIV-infected individuals with memory impairment benefited more significantly from recorded verbal prompts than in those with intact memory functioning. As in this study, examining neurocognition as a covariate in future such interventions will be helpful to determine whether these strategies are effective in those for whom it is most indicated, as well as to explore the cognitive mechanisms of intervention success. Not only will such analyses assist with the generalization of adherence interventions to HIV-infected individuals of varying cognitive capacity, but will also help to fine-tune and boost the efficacy of these interventions by identifying the most beneficial “active ingredient” in multi-component strategies such as texting.
CONCLUSION AND CALL TO ACTION
The development, validation, and clinical deployment of efficacious treatments for HAND, including cognitive neurorehabilitation approaches, is a clear priority for clinical neuroAIDS investigators. HIV-associated neurocognitive impairments remain prevalent in the cART era and adversely affect a host of everyday activities and HIV health outcomes. Although only a few studies have begun to evaluate the effectiveness of cognitive approaches to treating HAND, their initial results are encouraging, provide important information regarding proof-of-principle, and can directly inform future work. We are aware of a few NIH-funded cognitive rehabilitation studies in neuroAIDS that are presently under way, ranging from training fellowships to Phase I clinical trials. For example, our group recently launched a three-phase, multi-site, translational study to target (in the laboratory), develop (in the clinic), and test (in the field) a theory-based prospective memory intervention for cART non-adherence in HIV-infected youth at risk for substance abuse. The long-term translational goal of such work is to integrate empirically-based cognitive treatments of HAND into clinics, which may be challenging given the difficulties inherent in screening for HAND (Valcour, Paul, Chiao, Wendelken, & Miller, 2011) and the limited resources for (and awareness of) cognitive rehabilitation services (Herlihy, Samarawickrama, Gibson, Taylor, & O’Flynn, 2012). Nevertheless, the scientific quest to discover effective cognitive, behavioral, and/or pharmacological therapies for HAND remains paramount and carries with it the potential to positively impact the lives of persons living with HIV infection, their care providers, and the healthcare system.
Acknowledgments
This research was supported by National Institutes of Health grants F31-DA034510, T32-DA031098, R01-MH073419, R01-DA034497, P50-DA026306, and P30MH62512. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
The authors report no conflicts of interest.
References
- Al-Khindi T, Zakzanis KK, van Gorp WG. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review. Journal of the International Neuropsychological Society. 2011;17:956–969. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- Ances BM, Letendre SL, Alexander T, Ellis RJ. Role of psychiatric medications as adjunct therapy in the treatment of HIV associated neurocognitive disorders. Int Rev Psychiatry. 2008;20(1):89–93. doi: 10.1080/09540260701877670. [DOI] [PubMed] [Google Scholar]
- Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004;24(32):7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AS, McGruder HF, Wu AW, Celano SA, Skolasky RL, Jr, Selnes OA, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41(6):875–882. doi: 10.1086/432877. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Clague H, Charlebois ED, Ciccarone D, Chesney M, et al. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26(5):435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. J Clin Exp Neuropsychol. 2000;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Becker JT, Dew MA, Aizenstein HJ, Lopez OL, Morrow L, Saxton J, et al. A pilot study of the effects of internet-based cognitive stimulation on neuropsychological function in HIV disease. Disability and Rehabilitation. 2012;34(21):1848–1852. doi: 10.3109/09638288.2012.667188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist T, Gehl C, Mandrekar J, Lepore S, Hanna S, Osten A, et al. The effect of internet-based cognitive rehabilitation in persons with memory impairments after severe traumatic brain injury. Brain Inj. 2009;23(10):790–799. doi: 10.1080/02699050903196688. [DOI] [PubMed] [Google Scholar]
- Bertsch S, Pesta BJ, Wiscott R, McDaniel MA. The generation effect: a meta-analytic review. Mem Cognit. 2007;35(2):201–210. doi: 10.3758/bf03193441. [DOI] [PubMed] [Google Scholar]
- Bezeau S, Graves R. Statistical power and effect sizes of clinical neuropsychology research. J Clin Exp Neuropsychol. 2001;23(3):399–406. doi: 10.1076/jcen.23.3.399.1181. [DOI] [PubMed] [Google Scholar]
- Bielak AA, Hultsch DF, Strauss E, Macdonald SW, Hunter MA. Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology. 2010;24(6):731–741. doi: 10.1037/a0019802. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18(1):79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Woods SP, Weber E, Grant I, Moore DJ. Memory-Based Strategies for Antiretroviral Medication Management: An Evaluation of Clinical Predictors, Adherence Behavior Awareness, and Effectiveness. AIDS Behav. 2012 doi: 10.1007/s10461-012-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24(5):667–673. doi: 10.1037/a0019312. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I. Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol. 2006;28(4):536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Iudicello JE, Posada C, Grant I. Elevated Neurobehavioral Symptoms Are Associated With Everyday Functioning Problems in Chronic Methamphetamine Users. J Neuropsychiatry Clin Neurosci. 2012;24(3):331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 2010. [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J, Moore NB, Ricker JH. Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler. 2005;11(1):58–68. doi: 10.1191/1352458505ms1118oa. [DOI] [PubMed] [Google Scholar]
- Cicerone KD. Remediation of “working attention” in mild traumatic brain injury. Brain Inj. 2002;16(3):185–195. doi: 10.1080/02699050110103959. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D, Jr, Abramson I, Ellis RJ, Letendre S, Collier A, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33(5):505–522. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol. 2011;11:148. doi: 10.1186/1471-2377-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59(7):1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- Delis DC, Peavy G, Heaton R, Butters N, Salmon DP, Taylor M, et al. Do patients with HIV-associated minor cognitive/motor disorder exhibit a Äúsubcortical, Äú memory profile? evidence using the California Verbal Learning Test. Assessment. 1995;2(2):151–165. [Google Scholar]
- Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012 from http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- Department of Health and Human Services. Panel on Clinical Practice for Treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. 2002 From: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL04232001006.pdf.
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010;167(2):170–180. doi: 10.1176/appi.ajp.2009.09020264. [DOI] [PubMed] [Google Scholar]
- Doyle K, Weber E, Atkinson JH, Grant I, Woods SP. Aging, Prospective Memory, and Health-Related Quality of Life in HIV Infection. AIDS Behav. 2012;16(8):2309–2318. doi: 10.1007/s10461-011-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Lee GP, Cech H, Huthwaite JS, Ojemann GA, Ojemann JG, et al. Structured cueing on a semantic fluency task differentiates patients with temporal versus frontal lobe seizure onset. Epilepsy Behav. 2006;9(2):339–344. doi: 10.1016/j.yebeh.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248–261. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol Rev. 2009;19(2):144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Moore DJ, Childers ME, Letendre S, McCutchan JA, Wolfson T, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59(6):923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21(3):493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Everall IP, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. Journal of NeuroVirology. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Levy JK, Samley HR, Pirozzolo FJ, Lachar D, Crowley J, et al. Effects of methylphenidate in HIV-related depression: a comparative trial with desipramine. Int J Psychiatry Med. 1995;25(1):53–67. doi: 10.2190/16FH-9ECT-Y280-VV45. [DOI] [PubMed] [Google Scholar]
- Fish J, Evans JJ, Nimmo M, Martin E, Kersel D, Bateman A, et al. Rehabilitation of executive dysfunction following brain injury: “content-free” cueing improves everyday prospective memory performance. Neuropsychologia. 2007;45(6):1318–1330. doi: 10.1016/j.neuropsychologia.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Foster PP, Rosenblatt KP, Kuljis RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis. 2011;11:356. doi: 10.1186/1471-2334-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Woods SP, Taylor MJ, Vigil O, Grant I. Semantic clustering inefficiency in HIV-associated dementia. J Neuropsychiatry Clin Neurosci. 2007;19(1):36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Goverover Y, Arango-Lasprilla JC, Hillary FG, Chiaravalloti N, Deluca J. Application of the spacing effect to improve learning and memory for functional tasks in traumatic brain injury: a pilot study. American Journal of Occupational Therapy. 2009;63(5):543–548. doi: 10.5014/ajot.63.5.543. [DOI] [PubMed] [Google Scholar]
- Goverover Y, Chiaravalloti N, DeLuca J. Self-generation to improve learning and memory of functional activities in persons with multiple sclerosis: meal preparation and managing finances. Arch Phys Med Rehabil. 2008;89(8):1514–1521. doi: 10.1016/j.apmr.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107(6):828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Grant I, Sacktor N, McArthur J, Gendelman H, Everall I, Lipton S, et al. HIV neurocognitive disorders. The neurology of AIDS. 2005:357–373. [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20(3):355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy H, Kumar V, Doros G, Farmer E, Drainoni ML, Rybin D, et al. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDS. 2011;25(3):153–161. doi: 10.1089/apc.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Woods SP, Marra CM, Clifford D, Gelman B, et al. Asymptomatic Mild HIV-associated Neurocognitive Disorder Increases Risk for Future Symptomatic Decline: A CHARTER Longitudinal Study. Paper presented at the Conference on Retroviruses and Opportunistic Infections.2012. [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Herlihy D, Samarawickrama A, Gibson S, Taylor C, O’Flynn D. HIV-associated neurocognitive disorder: rate of referral for neurorehabilitation and psychiatric co-morbidity. Int J STD AIDS. 2012;23(4):285–286. doi: 10.1258/ijsa.2009.009379. [DOI] [PubMed] [Google Scholar]
- Heseltine PN, Goodkin K, Atkinson JH, Vitiello B, Rochon J, Heaton RK, et al. Randomized double-blind placebo-controlled trial of peptide T for HIV-associated cognitive impairment. Arch Neurol. 1998;55(1):41–51. doi: 10.1001/archneur.55.1.41. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. J Neuropsychiatry Clin Neurosci. 2001;13(2):248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- Iglesias-Ussel MD, Romerio F. HIV reservoirs: the new frontier. AIDS Rev. 2011;13(1):13–29. [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Cattie JE, Doyle K, Grant I. Risky decision-making in HIV-associated neurocognitive disorders (HAND) Clin Neuropsychol. 2012 doi: 10.1080/13854046.2012.740077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I. Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. J Clin Exp Neuropsychol. 2012;34(5):476–488. doi: 10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Parsons TD, Moran LM, Carey CL, Grant I. Verbal fluency in HIV infection: a meta-analytic review. J Int Neuropsychol Soc. 2007;13(1):183–189. doi: 10.1017/S1355617707070221. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL, et al. Cognitive mechanisms of switching in HIV-associated category fluency deficits. J Clin Exp Neuropsychol. 2008;30(7):797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273–291. doi: 10.1007/7854_2011_141. [DOI] [PubMed] [Google Scholar]
- Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 2010;14(2):371–378. doi: 10.1007/s10461-009-9538-x. [DOI] [PubMed] [Google Scholar]
- Letendre S, Ances B, Gibson S, Ellis RJ. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2007;15(2):32–39. [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56(3):416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, et al. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20(14):1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, et al. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6(3):299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchtman DW, Song C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology. 2013;64:550–565. doi: 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc. 1999;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol. 2006;28(1):13–28. doi: 10.1080/13803390490918048. [DOI] [PubMed] [Google Scholar]
- Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ, et al. A multimodal assessment of driving performance in HIV infection. Neurology. 2004;63(8):1417–1422. doi: 10.1212/01.wnl.0000141920.33580.5d. [DOI] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic S, et al. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. J Int Neuropsychol Soc. 2004;10(2):298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP the Multicenter AIDS Cohort Study (MACS) Investigators. Neurologic disorders incidence in HIV+ vs HIV- men: Multicenter AIDS Cohort Study, 1996–2011. Neurology. 2012;79:1873–1880. doi: 10.1212/WNL.0b013e318271f7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayben JK, Giordano TP. Internet use among low-income persons recently diagnosed with HIV infection. AIDS Care. 2007;19(9):1182–1187. doi: 10.1080/09540120701402806. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25(5):645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Grant I. Intra-individual neurocognitive variability confers risk of dependence in activities of daily living among HIV-seropositive individuals without HIV-associated neurocognitive disorders. Arch Clin Neuropsychol. 2012;27(3):293–303. doi: 10.1093/arclin/acs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I. Lower Cognitive Reserve Among Individuals with Syndromic HIV-Associated Neurocognitive Disorders (HAND) AIDS Behav. 2012;16(8):2279–2285. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neundorfer M, Camp C, Lee M, Skrajner M, Malone M, Carr J. Compensating for Cognitive Deficits in Persons Aged 50 and Over with HIV/AIDS. Journal of HIV/AIDS & Social Services. 2004;3(1):79–97. [Google Scholar]
- Nieuwkerk PT, Sprangers MA, Burger DM, Hoetelmans RM, Hugen PW, Danner SA, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Arch Intern Med. 2001;161(16):1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- Park D, Bischof G. Neuroplasticity, aging, and cognitive function. Handbook of Psychology of Aging. 2011:109–119. [Google Scholar]
- Pijnenborg GH, Van der Gaag M, Bockting CL, Van der Meer L, Aleman A. REFLEX, a social-cognitive group treatment to improve insight in schizophrenia: study protocol of a multi-center RCT. BMC Psychiatry. 2011;11:161. doi: 10.1186/1471-244X-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posit Science. Insight Brain Fitness Program. Retrieved January 21, 2013 from http://www.positscience.com/brain-training-products/insight.
- Prigatano GP, Wong JL. Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Archives of Physical Medicine and Rehabilitation. 1999;80(1):77–84. doi: 10.1016/s0003-9993(99)90311-8. [DOI] [PubMed] [Google Scholar]
- Rass O, Forsyth JK, Bolbecker AR, Hetrick WP, Breier A, Lysaker PH, et al. Computer-assisted cognitive remediation for schizophrenia: a randomized single-blind pilot study. Schizophr Res. 2012;139(1–3):92–98. doi: 10.1016/j.schres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robertson I. Goal management training: A clinical manual. Cambridge: PsyConsult; 1996. [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Tarwater PM, Skolasky RL, McArthur JC, Selnes OA, Becker J, et al. CSF antiretroviral drug penetrance and the treatment of HIV-associated psychomotor slowing. Neurology. 2001;57(3):542–544. doi: 10.1212/wnl.57.3.542. [DOI] [PubMed] [Google Scholar]
- Sandford JA. Captain’s Log Computerized Cognitive Training System. Richmond, VA: Brain Train; 2007. [Google Scholar]
- Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21(14):1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Yiannoutsos CT, Ernst T, Navia BA, Nath A, Sacktor N, et al. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology. 2009;73(23):1975–1981. doi: 10.1212/WNL.0b013e3181c51a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma CM, Nakamoto B, Shiramizu B, Liang CY, Degruttola V, Bennett K, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17(7):1233–1242. doi: 10.3851/IMP2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamecka NJ, Graf P. The Generation Effect: Delineation of a Phenomenon. Journal of Experimental Psychology: Human Learning and Memory. 1978;4(6):592–604. [Google Scholar]
- SmartBrain Technologies. SmartBrain Interactive System for Cognitive Stimulation and Brain Training Proven Efficacy. Retrieved January 21, 2013 from http://www.smartbrain.net/smartbrain/previo_en.html.
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg MM, Turkstra LS. Optimizing cognitive rehabilitation: Effective instructional methods. New York: Guilford Press; 2011. [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Deluca J. Retrieval practice improves memory in multiple sclerosis: clinical application of the testing effect. Neuropsychology. 2010;24(2):267–272. doi: 10.1037/a0017533. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004;15(4):254–259. doi: 10.1258/095646204773557794. [DOI] [PubMed] [Google Scholar]
- Treiber KA, Carlson MC, Corcoran C, Norton MC, Breitner JC, Piercy KW, et al. Cognitive stimulation and cognitive and functional decline in Alzheimer’s disease: the cache county dementia progression study. J Gerontol B Psychol Sci Soc Sci. 2011;66(4):416–425. doi: 10.1093/geronb/gbr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophrenia Bulletin. 2003;29:359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, Jeste DV. Compensatory cognitive training for psychosis: effects in a randomized controlled trial. J Clin Psychiatry. 2012;73(9):1212–1219. doi: 10.4088/JCP.12m07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis. 2011;53(8):836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck CH, McMahon TJ, Rosen MI, O’Malley SS, O’Connor PG, Lin CH, et al. Sustained-release methylphenidate for cognitive impairment in HIV-1-infected drug abusers: a pilot study. J Neuropsychiatry Clin Neurosci. 1997;9(1):29–36. doi: 10.1176/jnp.9.1.29. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Rabkin JG, Ferrando SJ, Mintz J, Ryan E, Borkowski T, et al. Neuropsychiatric predictors of return to work in HIV/AIDS. J Int Neuropsychol Soc. 2007;13(1):80–89. doi: 10.1017/S1355617707070117. [DOI] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, Ball KK. Speed of Processing Training With Middle-Age and Older Adults With HIV: A Pilot Study. J Assoc Nurses AIDS Care. 2012;23(6):500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Huntzinger C, Ritch J, Ledbetter N, Prihoda TJ, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157(8):1317–1323. doi: 10.1176/appi.ajp.157.8.1317. [DOI] [PubMed] [Google Scholar]
- Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, et al. Substance Use is a Risk Factor for Neurocognitive Deficits and Neuropsychiatric Distress in Acute and Early HIV Infection. Journal of Neurovirology. doi: 10.1007/s13365-012-0141-y. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Woods SP, Kellogg E, Grant I, Basso MR. Self-generation enhances verbal recall in individuals infected with HIV. J Int Neuropsychol Soc. 2012;18(1):128–133. doi: 10.1017/S135561771100124X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Christen L, Christen S, Tschopp S, Znoj H, Schneider C, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9(1):85–95. [PubMed] [Google Scholar]
- Wegesin DJ, Jacobs DM, Zubin NR, Ventura PR, Stern Y. Source memory and encoding strategy in normal aging. J Clin Exp Neuropsychol. 2000;22(4):455–464. doi: 10.1076/1380-3395(200008)22:4;1-0;FT455. [DOI] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Eisdorfer C, Feaster D, Morgan R, Fletcher MA, et al. Mild cognitive impairment and risk of mortality in HIV-1 infection. J Neuropsychiatry Clin Neurosci. 1998;10(2):125–132. doi: 10.1176/jnp.10.2.125. [DOI] [PubMed] [Google Scholar]
- Woods SP, Conover E, Rippeth JD, Carey CL, Gonzalez R, Marcotte TD, et al. Qualitative aspects of verbal fluency in HIV-associated dementia: a deficit in rule-guided lexical-semantic search processes? Neuropsychologia. 2004;42(6):801–809. doi: 10.1016/j.neuropsychologia.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH. Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Int Neuropsychol Soc. 2009;15(1):42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, et al. Construct validity of Hopkins Verbal Learning Test-Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20(8):1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Woods SP, Weber E, Cameron MV, Dawson MS, Delano-Wood L, Bondi MW, et al. Spontaneous strategy use protects against visual working memory deficits in older adults infected with HIV. Arch Clin Neuropsychol. 2010;25(8):724–733. doi: 10.1093/arclin/acq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weber E, Weisz BM, Twamley EW, Grant I. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56(1):77–84. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS. 2012;26:1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy: What works and could we do better? Schizophrenia Bulletin. 2011;37:S80–S90. doi: 10.1093/schbul/sbr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: a review of an emerging literature. J Behav Med. 2012;35(1):47–62. doi: 10.1007/s10865-011-9341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]