Abstract
Purpose of the review
Critically ill patients are prescribed sedatives and analgesics to decrease pain and anxiety, improve patient – ventilator dyssynchrony and ensure patient safety. These medications may themselves lead to delirium and ICU-acquired weakness, which are associated with worse clinical outcomes. This review will focus on the epidemiology of these two disease processes and discuss strategies aimed at reducing these devastating complications of critical illness.
Recent Findings
Delirium and ICU-acquired weakness are associated with longer hospital stay, increased cost and decreased quality of life after discharge from the ICU. Delirium has also shown to be associated with increased mortality. Strategies aimed at reducing sedative exposure through protocols and coordination of daily sedation and ventilator cessation trials, avoiding benzodiazepines in favor of alternative sedative regimens and early mobilization of patients have all shown to significantly improve patient outcomes.
Summary
Delirium and ICU-acquired weakness are complications of critical illness associated with worse clinical outcomes and functional decline in survivors. An evidence-based approach based on the following tenets; minimization of sedative medication, particularly benzodiazepines, delirium monitoring and management and early mobilization may mitigate these complications.
Keywords: Delirium, ICU-acquired weakness, Early Ambulation, Sedatives and Analgesics
Introduction
Pain, anxiety and delirium are common occurrences in an Intensive Care Unit (ICU) secondary to existing diseases, surgical procedures, trauma, invasive monitors, endotracheal intubation, and nursing interventions (1–3). Inadequately treated pain may lead to increased stress response, with resultant tachycardia, increased oxygen consumption, hypercoagulability, immunosuppression, hypermetabolism, and increased endogenous catecholamine activity (4–7). Insufficient pain relief can also contribute to deficient sleep, disorientation, anxiety, and long-term effects such as post-traumatic stress disorder (8).
Analgesia and sedation is therefore used in the ICU to provide comfort and ensure patient safety, especially in those who are mechanically ventilated. Although analgesia and sometimes sedation is necessary in an ICU, when over used without goals and targets, they predispose patients to untoward complications of increased time on mechanical ventilation, longer times in the ICU, more radiological testing for altered mental status, ICU-Acquired Weakness and greater likelihood of delirium (9, 10).
This state of the art review will focus on: delirium, representing an acute or newly acquired cognitive dysfunction, and ICU-Acquired Weakness, an ICU associated physical disability, as two areas for intervention, to improve functional outcomes in our critically ill patients. A detailed description of the epidemiology of delirium in trauma patients with emphasis on its relationship with analgesic and sedative medications will be provided, followed by brief overview of ICU-Acquired Weakness. Finally we will provide an evidence based approach to assist the readers in incorporating best analgesia, sedation and delirium practices, including early ambulation, to improve outcomes in their critically ill patients.
DELIRIUM
Delirium is a manifestation of acute brain dysfunction, characterized by an acute disturbance of consciousness accompanied by inattention, disorganized thinking, and perceptual disturbances that fluctuates over a short period of time (11).
Prevalence and outcomes
Delirium is commonly under-diagnosed in the ICU and has a reported prevalence of 20% to 80%, depending on the severity of illness and the need for mechanical ventilation (12–15). While majority of the studies evaluating delirium have been in medical ICU patients, four recent studies have shown delirium rates to be between 50–80% in surgical, trauma ICU and burn ICU patients (16–19). Delirium can be categorized into subtypes according to psychomotor behavior manifested by patients. Hypoactive delirium patients are characterized by decreased physical and mental activity (lethargy) and inattention, is frequently overlooked by both physicians and nursing staff and may have higher mortality and morbidity (20, 21). On the other extreme are hyperactive delirium patients who are agitated and combative. Patients exhibiting both characteristics have mixed delirium. Evaluation of the subtypes of delirium has revealed that hypoactive delirium is often the more prevalent form of delirium in surgical and trauma patients; Pandharipande and colleagues (22) found that 64% of surgical and 60% of trauma ICU patients had hypoactive delirium. Angels et. al. (18) in their smaller study of trauma patients found the incidence of hypoactive delirium to be 46%, hyperactive delirium to be 15%, and mixed at 39%, while Agarwal et. al. (19) reported rates of 71% hypoactive delirium, 22% mixed delirium, and 6% pure hyperactive delirium in their study looking at burn trauma patients.
Historically, delirium was considered an inconsequential occurrence during critical illness. Unfortunately, recent investigations have shown that delirium is independently associated with worse outcomes as seen with other organ dysfunctions. The presence of delirium is a strong predictor of longer hospital stay, greater times on mechanical ventilation, higher costs and more alarmingly, increased risk of death (17, 23–26**). Additionally, each additional day with delirium increases the risk of dying by 10% (12, 27*). Longer periods of delirium are also associated with greater degrees of cognitive decline, when patients are evaluated after one year (25*).
Diagnosis of Delirium
Numerous national and international surveys have highlighted the importance of recognizing delirium in the ICU (28–35). Most of these surveys show a disconnect between the perceived importance of delirium and the accuracy of diagnosis, and implementation of management and treatment techniques. Therefore, ICU providers must move beyond just knowledge and awareness about delirium, towards implementation of validated screening tools to diagnose delirium. It is especially important to develop instruments that can be used readily by nurses at the bedside who may not have experience or skill in psychiatric assessment.
Delirium Assessment Instruments
Devlin et al. (36, 37) have shown that using validated assessment tools can improve the ability of both physicians (36) as well as nurses (37) to detect delirium at the bedside. We will discuss 2 instruments that have been extensively studied and implemented in ICUs for use in non-verbal patients.
The Confusion Assessment Method for ICU (CAM – ICU):(38)
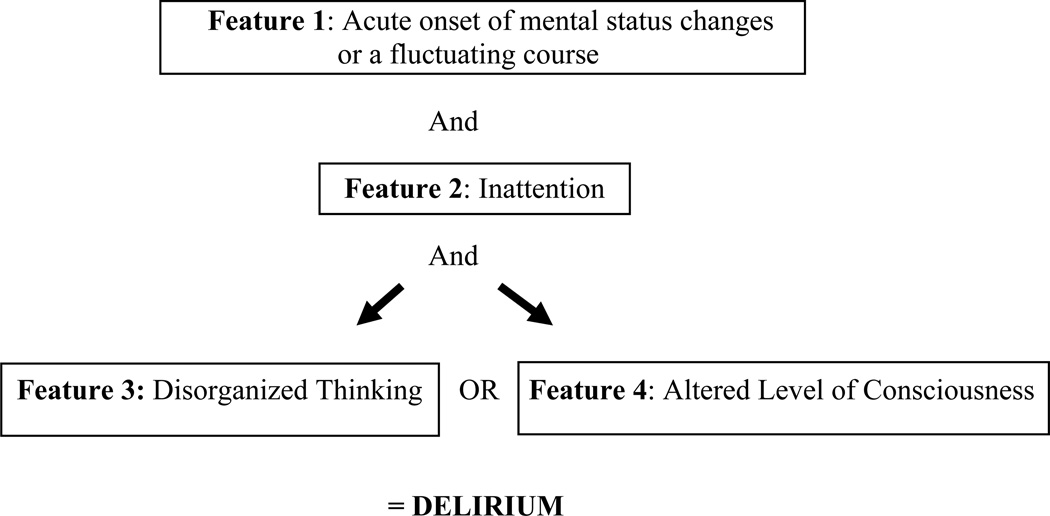
The Confusion Assessment Method for ICU (CAM-ICU) (Figure 1) is a reliable and validated (38–40) instrument for diagnosing delirium. It has a high specificity and sensitivity, is easy to use, and takes approximately 60–90 seconds to administer. Patients are first evaluated for a level of consciousness and if they respond to verbal commands (e.g. a Richmond Agitation-Sedation score of −3 or higher) (41) then they are assessed for delirium. Large scale implementation studies (42, 43) have been performed, including in trauma patients, showing good reliability and compliance of delirium monitoring by bedside nurses.
Figure 1. Confusion Assessment Method in the ICU.
Copyright © 2002, E. Wesley Ely, MD, MPH and Vanderbilt University, all rights reserved
Intensive Care Delirium Screening Checklist:(44)
This is a validated (44, 45), eight item based screening checklist for delirium (Table 1). The patient is evaluated for inattention, disorientation, hallucination, delusion or psychosis, psychomotor agitation or retardation, inappropriate speech or mood, sleep – wake cycle disturbance, and fluctuation of the above symptoms. Each item is scored as absent or present (0 or 1) and summed. A score ≥ 4 indicates delirium, while 0 indicates no delirium. Patients with scores between 1 and 3 are considered to have subsyndromal delirium (46). Patients with subsyndromal delirium have some but not all features of delirium and have outcomes that are in between those of patients with no delirium and those with delirium (46).
Table 1.
The Intensive Care Delirium Screening Checklist (ICDSC)
| Patient evaluation | |
|---|---|
| Altered level of consciousness (A–E) | * |
| Inattention | Difficulty in following a conversation or instructions. Easily distracted by external stimuli. Difficulty in shifting focuses. Any of these scores 1 point. |
| Disorientation | Any obvious mistake in time, place or person scores 1 point. |
| Hallucinations-delusion-psychosis | The unequivocal clinical manifestation of hallucination or of behavior probably due to hallucination or delusion. Gross impairment in reality testing. Any of these scores 1 point. |
| Psychomotor agitation or retardation | Hyperactivity requiring the use of additional sedative drugs or restraints in order to control potential danger to oneself or others. Hypoactivity or clinically noticeable psychomotor slowing. |
| Inappropriate speech or mood | Inappropriate, disorganized or incoherent speech. Inappropriate display of emotion related to events or situation. Any of these scores 1 point. |
| Sleep/wake cycle disturbance | Sleeping less than 4 h or waking frequently at night (do not consider wakefulness initiated by medical staff or loud environment). Sleeping during most of the day. Any of these scores 1 point. |
| Symptom fluctuation | Fluctuation of the manifestation of any item or symptom over 24 h scores 1 point. |
| Total score (0–8) | |
Level of consciousness
A: No response, score: None.
B: Response to intense and repeated stimulation (loud voice and pain), score: None.
C: Response to mild or moderate stimulation, score 1.
D: Normal wakefulness, score: 0.
E: Exaggerated response to normal stimulation, score: 1.
(Adapted from Bergeron et al.)(13)
Pathophysiology of Delirium
The pathogenesis of delirium is poorly understood. Numerous hypotheses exist which include neurotransmitter imbalance [e.g., dopamine, γ-aminobutyric acid (GABA), and acetylcholine]; inflammatory perturbations (e.g., tumor necrosis factor-α, interleukin-1, and other cytokines /chemokines); impaired oxidative metabolism; cholinergic deficiency and changes in various amino acid precursors (47, 48).
Risk Factors for Delirium
Risk factors for the development of delirium are multi-factorial and can be divided into host factors (age, baseline co-morbidities, baseline cognitive impairment and genetic predisposition), factors of acute illness (sepsis, hypoxemia and metabolic disturbances) and iatrogenic and environmental factors (metabolic disturbances, anticholinergic medications, sedatives and analgesic medications and sleep disturbances) (49–52). Primary central nervous system disease, shock, liver disease, acute respiratory distress syndrome, postoperative status, kidney disease, heart failure, and anemia have also been associated with delirium.(15)
Sedative and analgesics as risk factors for delirium
In studies specific to the trauma ICU, sedatives and analgesics were found to be risk factors for development of delirium by both Pandharipande (16) and Lat (17). Specifically, Pandharipande et. al. (16) reported that exposure to midazolam was an independent risk factor for the development of delirium in both surgical and trauma patients; the association between opioid medications and delirium was inconsistent with fentanyl being a risk factor for delirium in surgical ICU patients but not in trauma patients and morphine being associated with a lower risk of delirium in the trauma ICU patients. This difference can be explained by the fact that fentanyl as an infusion may be used additionally as a sedative rather than just an analgesic, leading to higher incidences of delirium. Morphine on the other hand was used in bolus doses for analgesia, and thus protective. Similar results were seen in a study performed in the burn trauma patients (19), where exposure to benzodiazepines was an independent risk factor for the development of delirium (odds ratio:6.8; [confidence interval: 3.1–15], P < 0.001). Similar to the trauma patients, exposure to both intravenous opiates (0.5, [0.4 – 0.6], P < 0.001) and methadone (0.7, [0.5 – 0.9], P = 0.2) were associated with lower odds of developing delirium, leading us to believe that appropriate pain control in populations at risk for severe pain (trauma, burn etc.) reduces delirium risk. In contrast, in the study by Lat et al (17) the patients who developed delirium were exposed to higher cumulative doses of both lorazepam and fentanyl; however this study did not assess the temporal relationship of drug administration and delirium as done in the study by Pandharipande and colleagues. Angles et. al. (18) showed that lower Glasgow coma score at admission, increased blood transfusion and higher multi organ failure scores (thus many markers of severity of illness) were associated with increased delirium. Their study could not assess whether sedatives and analgesics were associated with higher delirium rates.
ICU-ACQUIRED WEAKNESS
ICU-acquired weakness has been known to occur in 25% to 60% of patients who regain normal consciousness after an ICU stay (10). It is an acute onset neuromuscular functional impairment in critically ill patients, who have no prior history of neuromuscular disorders. Immobility and bed rest play an important role in the development of muscular weakness (53). In 2003 Herridge et. al. (54) studied 109 survivors of Acute Respiratory Distress Syndrome (ARDS) and followed them at 3, 6 and 12 months after discharge from the ICU. They found that most patients at one year have functional disability with muscle weakness and wasting being the most prominent feature. These findings have led to studies looking at the feasibility of starting physical therapy and mobility exercises on patients while on mechanical ventilation (55). Apart from immobility, other risk factors of ICU-acquired weakness include multiple organ failure, prolonged sedation with muscle inactivity, hyperglycemia, and the use of certain drugs like corticosteroids and neuromuscular blockers. Mechanical ventilation for greater than one week has been shown to be an independent risk factor for the disorder. Patients with ICU-acquired weakness have longer ICU and hospital stays, higher mortality and survivors experience disability for weeks to months (10).
MANAGEMENT
Over the past decade we have improved and mastered many lifesaving maneuvers in our ICU’s – aggressive resuscitation, source control of infection, antibiotics etc. Now it is important that we focus on improving patient outcome and recovery. To do this we need to focus on strategies to “liberate” our patients from mechanical ventilation then “animate” them by getting them out of bed early.
The ABCDE bundle for Optimal Management of Analgesia, Sedation and Delirium
To improve patient outcome and recovery we present an evidence based organizational approach referred to as the ABCDE bundle (Awakening and Breathing Trials, Choice of appropriate sedation, Delirium monitoring and Early mobility and Exercise).
Awaken the patient daily
Benzodiazepines are known to increase the risk of delirium in a dose-dependent manner (16, 52). Multiple studies have shown that protocolized target based sedation and daily spontaneous awakening trials reduce the number of days on mechanical ventilation. This strategy also exposes the patient to smaller cumulative doses of sedatives (9, 56).
Spontaneous Breathing Trials
This involves daily interruption of mechanical ventilation. Spontaneous breathing trials were shown to be superior to other varied approaches to ventilator weaning (57). Thus incorporation of spontaneous breathing trials into practice reduced the total time on mechanical ventilation.
Co-ordination of daily awakening and daily breathing
The Awakening and Breathing controlled trial (58) combined the spontaneous awakening trial with the spontaneous breathing trial. This combination showed shorter duration of mechanical ventilation, a 4-day reduction in hospital length of stay, a remarkable 15% decrease in 1-year mortality and no long-term neuropsychological consequences of waking patients during critical illness (59).
Choosing the right sedative regimen in critically ill patients
Numerous studies have identified that benzodiazepines are associated with a worse clinical outcomes (50, 52, 60), necessitating attention to the choice of sedation one decides to utilize for ones patients. In a study comparing propofol to intermittent lorazepam, Carson et. al. (61) found that in patients requiring >48 hrs of mechanical ventilation, sedation with propofol resulted in shorter ventilator times than lorazepam, even when sedatives were interrupted daily. Breen et. al. (62) compared a sedation regimen using remifentanil to midazolam and found that the remifentanil-based sedation regimen decreased duration of mechanical ventilation and the time from the start of weaning to extubation. Two studies comparing sedation protocols using dexmedetomidine (α2 agonist) to benzodiazepine infusions showed similar results. The MENDS (63) showed more days alive without delirium or coma (7.0 vs 3.0; P = .01), with a lower risk of developing delirium on subsequent days if on dexmedetomidine compared to lorazepam (64). The SEDCOM (65) study also showed a decrease in delirium prevalence in the dexmedetomidine group (54% vs 76.6%, [95% CI, 14% to 33%]; P < .001), with shorter times on mechanical ventilation.
Delirium Management
The Society of Critical Care Medicine (SCCM) has published guidelines recommending routine monitoring for delirium in all ICU patients (4). Pharmacologic therapy for delirium should only be attempted after correcting any contributing factors or underlying physiologic abnormalities. Patients, who manifest delirium, should be treated with a traditional antipsychotic medication (haloperidol), per the SCCM guidelines (66). A recommended starting dose is 2 to 5 mg every 6 to 12 hours (IV or PO); the maximal effective doses are usually around 20 mg/day. Newer “atypical” antipsychotic agents (e.g., risperidone, ziprasidone, quetiapine, or olanzapine) also may prove helpful for the treatment of delirium (67). While the MIND study (68) showed no difference in the duration of delirium between haloperidol, ziprasidone or placebo, when used for prophylaxis and treatment, a smaller study done by Devlin et. al.(69) showed that quetiapine was more effective than placebo in resolution of delirium when supplementing ongoing haloperidol therapy. Data from the MENDS (63, 64) study and the SEDCOM trial (65) support the view that dexmedetomidine can decrease the duration and prevalence of delirium when compared to lorazepam or midazolam. Benzodiazepines remain the drugs of choice for the treatment of delirium tremens (and other withdrawal syndromes) and seizures.
Exercise and Early Mobility
Morris et. al.(70) showed that initiating physical therapy early during the patients ICU stay was associated with decreased length of stay both in an ICU as well as in the hospital. In this prospective cohort study the investigators assessed the effect of introducing a mobility protocol in the ICU. They found that patients in the study group patients received at least one more physical therapy session than the control group, (80% vs. 47%, p < .001), were out of bed earlier (5 vs. 11 days, p < .001), had therapy initiated more frequently (91% vs. 13%, p < .001), with no differences in the complication rates. Schweikert et. al. (71**) looked at the efficacy of combining daily interruption of sedation with physical and occupational therapy to see if it had an impact on the development of ICU Acquired Weakness and delirium in mechanically ventilated patients. They found that patients who underwent early mobilization had a significant improvement in functional status at hospital discharge. These patients also had a significant decrease in the duration of delirium (50%) in the ICU as well as during the hospital stay. At 28 days patients also had more ventilator free days (23.5 days in the physical therapy group vs 21.1 days in the control group), which was statistically significant. Needham et. al.(72) conducted a quality improvement project with the use of a multidisciplinary team that focused on reducing sedation use; they further increased the MICU staffing to include physical and occupational therapists. The authors reported that benzodiazepine use decreased with lower median daily sedative and analgesic doses. Patients also had improved sedation and delirium status (MICU days alert [30% vs 67%, P = 0.001] and not delirious [21% vs 53%, P = 0.003]). Rehabilitation treatments per patient increased (1 vs 7, P = 0.001) with a higher level of functional mobility (treatments involving sitting or greater mobility, 56% vs 78%, P = 0.03). A decrease in ICU and hospital length of stay was also noted.
Conclusion
ICU delirium and ICU-acquired weakness are associated with higher costs, hospital length of stay and worse outcomes. These devastating complications of critical illness may be preventable or their duration and course mitigated via close attention to practices in the ICU. We propose implementation of a bundle of processes— awakening and breathing coordination, choice of appropriate sedative, delirium monitoring, and exercise/early mobility—or the ABCDE bundle, to improve functional and cognitive abilities of survivors of critical illness.
Key Bullet Points.
Delirium and ICU-acquired weakness are complications of critical illness in ICU survivors.
Delirium occurs in majority of mechanically ventilated patients and is associated with longer hospital stay, increased cost, and decreased quality of life after discharge from the ICU as well as increased mortality.
ICU-Acquired Weakness is also associated with longer ICU and hospital stays, higher mortality and ICU survivors experience disability for weeks to months.
To improve patient outcome and recovery we present an evidence based organizational approach referred to as the ABCDE bundle (Awakening and Breathing Trials, Choice of appropriate sedation, Delirium monitoring and Early mobility and Exercise).
Acknowledgements
Funding
Dr. Girard is supported by the National Institutes of Health (AG034257) and the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Drs Pandharipande and Girard have received honorarium from Hospira Inc. Dr. Pandharipande has also received honorarium from GlaxoSmithKline and Orion Pharma.
References
- 1.Jaber S, Chanques G, Altairac C, Sebbane M, Vergne C, Perrigault P, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. 2005;128(4):2749. doi: 10.1378/chest.128.4.2749. [DOI] [PubMed] [Google Scholar]
- 2.Cohen I, Gallagher T, Pohlman A, Dasta J, Abraham E, Papadokos P. Management of the agitated intensive care unit patient. Critical Care Medicine. 2002;30(1):S97. doi: 10.1097/00003246-200201002-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande P, Jackson J, Ely EW. Delirium: acute cognitive dysfunction in the critically ill. CurrOpinCrit Care. 2005;11(4):360–368. doi: 10.1097/01.ccx.0000170503.76528.4b. [DOI] [PubMed] [Google Scholar]
- 4.Jacobi J, Fraser G, Coursin D, Riker R, Fontaine D, Wittbrodt E. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult.[see comment][erratum appears in Crit Care Med 2002 Mar;30(3):726] Critical Care Medicine. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Gehlbach BK, Kress JP. Sedation in the intensive care unit. CurrOpinCrit Care. 2002;8:290–298. doi: 10.1097/00075198-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Weinert CR, Sprenkle M. Post-ICU consequences of patient wakefulness and sedative exposure during mechanical ventilation. Intensive Care Med. 2008;34(1):82–90. doi: 10.1007/s00134-007-0829-2. [DOI] [PubMed] [Google Scholar]
- 7.Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, et al. Patients' recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kapfhammer HP, Rothenhausler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 10.de Jonghe B, Lacherade J-C, Sharshar T, Outin H. Intensive care unit-acquired weakness: Risk factors and prevention. Critical Care Medicine. 2009;37(10):S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric A. Diagnostic and statistical manual of mental disorders. Fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 13.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 14.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 15.Girard T, Pandharipande P, Ely E. Delirium in the intensive care unit. Critical Care. 2008;12(Suppl 3):S3. doi: 10.1186/cc6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandharipande P, Cotton B, Shintani A, Thompson J, Pun B, Morris J, et al. Prevalence and Risk Factors for Development of Delirium in Surgical and Trauma Intensive Care Unit Patients. Journal of Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Critical Care Medicine. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 18.Angles EM, Robinson TN, Biffl WL, Johnson J, Moss M, Tran ZV, et al. Risk factors for delirium after major trauma. The American journal of surgery. 2008;196(6):864–870. doi: 10.1016/j.amjsurg.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal V, O'Neill P, Cotton B, Pun B, Haney S, Thompson J, et al. Prevalence and Risk Factors for Development of Delirium in Burn Intensive Care Unit Patients. Journal of Burn Care & Research. 2010;31(5):706–715. doi: 10.1097/BCR.0b013e3181eebee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meagher DJ, Hanlon DO, Mahony EO, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000;12:51–56. doi: 10.1176/jnp.12.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted postacute facility patients. JGerontolA BiolSciMedSci. 2007;62(2):174–179. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman PB, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 25. Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010 Jul;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. This propspective cohort study of Medical ICU patients showed that duration of delirium is idependently associated with log-term cognitive impairment which is a common health problem among ICU survivors.
- 26. Shehabi Y, Riker R, Bokesch P, Wisemandle W. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care unit patients. Critical Care Medicine. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. In this prospective cohort analysis of ventilated and lightly sedated ICU patients, delirium was shown to be the strongest independent predictor of mortality and duration on mechanical ventilation after adjusting for relevant co-variates.
- 27. Pisani M, Kong S, Kasl S, Murphy T, Araujo K, Van Ness P. Days of delirium are associated with 1-year mortality in an older intensive care unit population. American Journal of Respiratory and Critical Care Medicine. 2009:200904. doi: 10.1164/rccm.200904-0537OC. This cohort study demonstrated a dose response curve between days of delirium and the risk of dying at one year.
- 28.Ely EW, Stephens RK, Jackson JC, Thomason JW, Truman B, Gordon S, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32(1):106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 29.Cheung C, Alibhai S, Robinson M, Tomlinson G, Chittock D, Drover J, et al. Recognition and labeling of delirium symptoms by intensivists: Does it matter? Intensive Care Medicine. 2008;34(3):437–446. doi: 10.1007/s00134-007-0947-x. [DOI] [PubMed] [Google Scholar]
- 30.Devlin J, Fong J, Howard E, Skrobik Y, McCoy N, Yasuda C, et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. American Journal of Critical Care. 2008;17(6):555. [PubMed] [Google Scholar]
- 31.Van Eijk M, Kesecioglu J, Slooter A. Intensive care delirium monitoring and standardised treatment: a complete survey of Dutch intensive care units. Intensive and Critical Care Nursing. 2008;24(4):218–221. doi: 10.1016/j.iccn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Cadogan F, Riekerk B, Vreeswijk R, Rommes J, Toornvliet A, Honing M, et al. Current awareness of delirium in the intensive care unit: a postal survey in the Netherlands. The Netherlands journal of medicine. 67(7):296–300. [PubMed] [Google Scholar]
- 33.Salluh J, Dal-Pizzol F, Mello P, Friedman G, Silva E, Teles J, et al. Delirium recognition and sedation practices in critically ill patients: A survey on the attitudes of 1015 Brazilian critical care physicians. Journal of Critical Care. 2009;24(4):556–562. doi: 10.1016/j.jcrc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Mac Sweeney R, Barber V, Page V, Ely EW, Perkins GD, Young JD, et al. A national survey of the management of delirium in UK intensive care units. QJM. 2010 Apr;103(4):243–251. doi: 10.1093/qjmed/hcp194. [DOI] [PubMed] [Google Scholar]
- 35.Patel R, Gambrell M, Speroff T, Scott T, Pun B, Okahashi J, et al. Delirium and sedation in the intensive care unit: Survey of behaviors and attitudes of 1384 healthcare professionals*. Critical Care Medicine. 2009;37(3):825. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin J, Fong J, Schumaker G, O'Connor H, Ruthazer R, Garpestad E. Use of a validated delirium assessment tool improves the ability of physicians to identify delirium in medical intensive care unit patients. Critical Care Medicine. 2007;35(12):2721. doi: 10.1097/01.ccm.0000292011.93074.82. [DOI] [PubMed] [Google Scholar]
- 37.Devlin J, Marquis F, Riker R, Robbins T, Garpestad E, Fong J, et al. Combined didactic and scenario-based education improves the ability of intensive care unit staff to recognize delirium at the bedside. Critical Care. 2008;12(1):R19. doi: 10.1186/cc6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Ely E, Inouye S, Bernard G, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 40.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 41.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 42.Pun B, Gordon S, Peterson J, Shintani A, Jackson J, Foss J, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers*. Critical Care Medicine. 2005;33(6):1199. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 43.Soja S, Pandharipande P, Fleming S, Cotton B, Miller L, Weaver S, et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Medicine. 2008;34(7):1263–1268. doi: 10.1007/s00134-008-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergeron N, Dubois M, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 45.Radtke F, Franck M, Oppermann S, Lütz A, Seeling M, Heymann A, et al. The Intensive Care Delirium Screening Checklist (ICDSC)--translation and validation of intensive care delirium checklist in accordance with guidelines. Anaesthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie: AINS. 2009;44(2):80. doi: 10.1055/s-0029-1202647. [DOI] [PubMed] [Google Scholar]
- 46.Ouimet S, Riker R, Bergeon N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Medicine. 2007;33(6):1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 47.Inouye SK. Delirium in older persons. NEnglJMed. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 48.Girard TD, Ely EW, Rehm CG, Fuhrman TM. 12th Critical Care Refresher - Society of Critical Care Medicine. Mount Prospect, IL: Society of Critical Care Medicine; 2008. Delirium in the ICU; pp. 101–107. [Google Scholar]
- 49.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium: a review of 80 primary data collection studies. Arch Intern Med. 1995;155:461–465. doi: 10.1001/archinte.155.5.461. [DOI] [PubMed] [Google Scholar]
- 50.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 51.Marcantonio E, Juarez G, Goldman L, Mangione C, Ludwig L, Lind L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518. [PubMed] [Google Scholar]
- 52.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997 Jan 1;82(1):182–188. doi: 10.1152/jappl.1997.82.1.182. 1997. [DOI] [PubMed] [Google Scholar]
- 54.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 55.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 56.Kress J, Pohlman A, O'Connor M, Hall J. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 57.Ely E, Baker A, Dunagan D, Burke H, Smith A, Kelly P, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 58.Girard T, Kress J, Fuchs B, Thomason J, Schweickert W, Pun B, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 59.Jackson J, Girard T, Gordon S, Thompson J, Shintani A, Thomason J, et al. Long-term cognitive and psychological outcomes in the Awakening and Breathing Controlled trial. American Journal of Respiratory and Critical Care Medicine. 2010:200903. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105(5):380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 61.Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34(5):1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 62.Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497] Crit Care. 2005;9(3):R200–R210. doi: 10.1186/cc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 64.Pandharipande P, Sanders R, Girard T, McGrane S, Thompson J, Shintani A, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riker R, Shehabi Y, Bokesch P, Ceraso D, Wisemandle W, Koura F. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 66.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 67.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444–449. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 68.Girard TD, Carson SS, Pandharipande PP, Schmidt GA, Wright PE, Pun BT, et al. The modifying the incidence of delirium (MIND) trial: a randomized controlled trial of the feasibility, efficacy, and safety of antipsychotics for the prevention and treatment of ICU delirium. Am J Respir Crit Care Med. 2008;177:A817. [Google Scholar]
- 69.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo-controlled pilot study *. Critical Care Medicine. 2010;38(2):419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 70.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure *. Critical Care Medicine. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 71. Schweickert W, Pohlman M, Pohlman A, Nigos C, Pawlik A, Esbrook C, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. This is the only interventional study that tested a non pharmacologic intervention - early mobility - in ICU patients and showed a reduction in delirium and improvements in functional outcome.
- 72.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project. Archives of Physical Medicine and Rehabilitation. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]