Abstract
Objectives: This study aims to examine the impact of external price referencing (EPR) on on-patent medicine prices, adjusting for other factors that may affect price levels such as sales volume, exchange rates, gross domestic product (GDP) per capita, total pharmaceutical expenditure (TPE), and size of the pharmaceutical industry.
Methods: Price data of 14 on-patent products, in 14 European countries in 2007 and 2008 were obtained from the Pharmaceutical Price Information Service of the Austrian Health Institute. Based on the unit ex-factory prices in EURO, scaled ranks per country and per product were calculated. For the regression analysis the scaled ranks per country and product were weighted; each country had the same sum of weights but within a country the weights were proportional to its sales volume in the year (data obtained from IMS Health). Taking the scaled ranks, several statistical analyses were performed by using the program “R”, including a multiple regression analysis (including variables such as GDP per capita and national industry size).
Results: This study showed that on average EPR as a pricing policy leads to lower prices. However, the large variation in price levels among countries using EPR confirmed that the price level is not only driven by EPR. The unadjusted linear regression model confirms that applying EPR in a country is associated with a lower scaled weighted rank (p=0.002). This interaction persisted after inclusion of total pharmaceutical expenditure per capita and GDP per capita in the final model.
Conclusions: The study showed that for patented products, prices are in general lower in case the country applied EPR. Nevertheless substantial price differences among countries that apply EPR could be identified. Possible explanations could be found through a correlation between pharmaceutical industry and the scaled price ranks. In conclusion, we found that implementing external reference pricing could lead to lower prices.
Keywords: medicines, prices, pricing policy, external price referencing, Europe
Introduction
The pharmaceutical market is characterized by low price elasticity and strong market power not only in Europe, but in all markets in which health insurance is widespread and patents are enforced. In countries, where medicines are subsidised, patients do not generally see the true price of a medicine, thus appearing to have low price elasticity and patent holders often have the market power [1, 2]. Even without price controls, this is not an unregulated market; European governments generally provide health coverage to their citizens and grant patents, so there is already extensive intervention in the market [3, 4]. Guaranteeing sustainable health coverage, in specific funding of public pharmaceutical expenditure, requires certain supply and demand side policy measures. Due to historic developments, cultural differences and different ways of health care funding, European countries have implemented various policies to contain pharmaceutical expenditure [5].
One of the supply side measures is regulating medicine prices. The most commonly used pricing policy in Europe (applied by 24 out of 28 European countries) is external price referencing (EPR), which is defined as “the practice of using the price(s) of a medicine in one or several countries in order to derive a benchmark or reference price for the purposes of setting or negotiating the price of the product in a given country” [3, 4, 6]. One may argue that due to the fact that EPR is frequently used this makes it a powerful tool to influence not only national medicine prices but also prices worldwide due to the interlinking of prices [7, 8]. Hence, it is necessary to understand whether European public authorities in charge of pricing of medicines are actually reaching the desired aim of EPR, thus stabilising and eventually lowering medicine prices. Of particular interest is to understand whether differences among lower or higher income countries and different EPR methodologies can be observed.
Only a very small number of studies addressed general aspects of EPR as a policy. Heuer [9] looked at the relationship of external price referencing and delays in the launch of medicines. In addition, Mariñoso [10] developed a scenario in which the potential drivers for a country to either engage in external price referencing or to directly negotiate prices were analysed. Only a few studies have explicitly analysed the impact of external price referencing on medicine prices. Stargardt [11] developed an analytic model to simulate the effect of a price reduction in Germany. They found that if there was a one EURO price reduction in Germany this would lead to a reduction of EURO 0.15 to EURO 0.36 in 15 European countries that use EPR and have Germany in their basket. Another perspective was developed by Richter [12], who argued in his study that pharmaceutical companies tend to keep prices higher in Germany for the reason that the prices in those countries would later become references for other countries.
Economic evidence on the impact of external reference pricing is scarce, but literature has generally shown that the introduction of EPR reduced prices. Windmeijer [13] measured the effects of the implementation of EPR in the Netherlands and found that EPR resulted in lower prices. Merkur [14] simulated the effect of EPR on medicine prices in Cyprus, after having found that Cyprus had relatively high prices compared to other European Union (EU) countries, they showed that EPR would lead to lower prices. Filko [15] stated in 2009 that due to the policy change to use EPR in Slovakia, which included the introduction of EPR based on the arithmetic mean of the six lowest countries within all other European Union countries as well as the implementation of the EURO, the proportion of pharmaceutical expenditure as percentage of total health care spending declined by approximately 25 percent. On the contrary Kaiser [16] came to the conclusion that in Denmark medicine prices decreased more than 26 percent after a policy change from EPR to internal price referencing. Further the patient co-payment went down by 3 percent and government expenditure by 5.6 percent and producer’s revenues by 5 percent.
Building upon the findings of these previous studies, this research aims to examine the impact of EPR on medicine prices using a sample of 14 originator products in 14 European countries. The medicines prices were obtained from a period of 2007-2008. It is known that price levels may vary across countries as a result of differences in factors such as national pricing and reimbursement policies, sales volume, exchange rates, gross domestic product (GDP) per capita, total pharmaceutical expenditure (TPE) and size of the pharmaceutical industry [17]. While conducting the present study, these additional factors were also taken into account. This study provides policy makers and scientists in the field of pharmaceutical policy with detailed information as to whether the desired effect of EPR – to have lower medicine prices – was achieved for originator products in 2007 and 2008.
Methods
Selection of countries and products
A basket of fourteen European countries with different economic situations and from different parts of the (EU) was selected. Three EU Member States which currently do not apply external price referencing were also included. The countries included were Austria, Belgium, Denmark, Germany, Greece, Finland, France, Italy, the Netherlands, Norway, Portugal, Spain, Sweden, Slovak Republic.
A basket of fourteen products was chosen. The main criterion for choosing the products was the patent status of each medicine: it had to be on-patent in 2007 and 2008 in the countries under investigation; therefore the date of market authorisation was checked either at the website of the European Medicines Agency (centralised procedure) or at the Austrian Medicines Agency. In addition, the medicines were predominantly prescribed in the out-patient sector and were included in the reimbursement system. Finally, price data had to be available. As shown in table 1 products represent a broad spectrum of therapeutic areas such as obesity, diabetes, HIV/aids and others. The products are a combination of products that recently came on the market (and therefore have relatively low sales volumes) and others that have been on the market for nearly ten years (and therefore have relatively high sales volumes).
Data Sources
Policy data on EPR were obtained from the European Pharmaceutical Pricing and Reimbursement Information PPRI network [19] as well as from Leopold [7] which is a descriptive study on EPR in European countries.
Price data were provided by the Austrian Health Institute, which has been running a Pharmaceutical Price Information Service (PPI) for many years [20]. Based on Austrian law, the PPI service was set up in the late 1990’s as a supportive tool for the Austrian Price Commission and since 2004 to also check prices reported by manufacturers in the process of EPR in Austria. Prices are obtained from official price databases of Ministries of Health or Social Health Insurance Institutions. The validation of the PPI prices is high, as for the interpretation of the prices knowledge on the underlying pharmaceutical system is required which the Austrian Health Institute offers as well.
The price data referred to October / November 2007 and 2008 and represent ex-factory prices per unit. The price data were collected for the same product, the same strength, the same pharmaceutical form and, if available, the same pack size. In case the country did not use the EURO, the conversion rate was taken from the Austrian National Bank of the previous month in that year. Discounts or rebates were not considered.
As prices refer to the ex-factory price level, no value added tax (VAT) was included. For the price comparisons the prices were analysed in prices per units. If products were known to be used exclusively in hospitals in some countries, their prices were disregarded.
To perform the statistical analysis volume data of the 14 products in both years as well as data on economic variables such as national gross domestic product or total health expenditure were collected.
Volume data were provided by IMS Health Institute [21]. IMS Health collects pharmaceutical consumption data from wholesalers, hospitals and/or dispensing outlets such as pharmacies or drugstores. The volume data referred to annual sales data of 2007 and 2008 of the same products, same strengths and companies as the price data. IMS displays its volume data in standard units (SU). This is a measure used by IMS and is derived from the commonest dosage forms. It is measured differently depending on the formulation of the medicine. Usually one SU equals one capsule, one tablet, one prefilled syringe, one dose of inhaled medicine or 5 ml of an oral suspension etc. If IMS does not collect data from all suppliers in a country they project the sample of a particular distribution channel to the national level. These projections are validated annually.
Data on economic variables such as gross domestic product per capita, total pharmaceutical expenditure (TPE) per capita in EURO Purchasing Power Parities as well as data on inhabitants per 100,000 were extracted from the Organization of Economic Co-operation and Development (OECD) Health database and referred to 2006 [22]. The calculation of per capita Purchasing Power Parities (PPPa) followed the methodology suggested by OECD.
In addition to economic factors, it was tested whether there is a possible relationship between the importance of national pharmaceutical industry and medicine prices, as national governments seek to find the right balance between social/health policies and economic policies. The variable employment in pharmaceutical industry population was collected as a proxy for the importance of the pharmaceutical industry in a country. Data were taken from the European Federation of Pharmaceutical Industry Association (EFPIA) and referred to 2006 [23]. This was converted into a rate per 100,000 population.
Table 1: List of selected products included in this study
| ATC code | Therapeutic Area | INN | Product | Strength | Pharmaceutical form | Company | EMA Authorisation [18] |
| A02BC04 | Proton pump inhibitors | Rabeprazole | Pariet | 10 mg | Tabs | Janssen-Cilag | November 1998 |
| A08AA10 | Obesity | Sibutramine | Reductil | 10 mg | Caps | Abbott | April 2001 |
| A08AB01 | Obesity | Orlistat | Xenical | 120 mg | Tabs | Roche | July 1998 |
| A10BG02 | Diabetes | Rosiglitazone maleate | Avandia | 4 mg | Tabs | GSK | July 2000 |
| A10BG03 | Diabetes | Pioglitazone hydrochloride | Actos | 30 mg | Tabs | Eli Lilly | October 2000 |
| B01AB05 | Acute coronary syndrome | Enoxaparin | Lovenox | 100 mg | Prefilled syringe | Sanofi-Aventis | November 2000 |
| B01AX05 | Other antithrombotic agents | Fondaparinux | Arixtra | 2.5 mg/0.5 ml | Prefilled syringe | GSK | March 2002 |
| J05AE07 | Protease inhibitors | Fosamprenavir calcium | Telzir | 700 mg | f/c tabs | GSK | July 2004 |
| J05AE10 | Protease inhibitors | Darunavir | Prezista | 300 mg | Tabs | Janssen-Cilag | February 2007 |
| J05AF06 | HIV | Abacavir sulfate | Ziagen | 300 mg | Tabs | GSK | July 1999 |
| J05AF07 | HIV | Tenofovir disoproxil fumarate | Viread | 245 mg | Tabs | Gilead | February 2002 |
| J05AG03 | Non-nucleoside reverse transcriptase inhibitors | Efavirenz | Stocrin | 600 mg | f/c tab | MSD | May 1999 |
| J05AX09 | Other antiviral | Maraviroc | Celsentri | 150 mg | Tabs | Pfizer | September 2007 |
| L01BC06 | Oncology | Capecitabine | Xeloda | 500 mg | Tabs | Roche | February 2001 |
ATC = Anatomical Therapeutic Chemical, INN = International Non-Proprietary Name f/c=film coated
Statistical analysis
The unit ex-factory prices in EURO of all products, all countries and of both years were adjusted to a fixed exchange rate for 2007/2008, as some exchange rates (e.g. Norwegian Krone) fluctuated more than the price differences. The prices were converted to scaled ranks so that different price levels were ineffectual as well as it guaranteed a robust data set.
For the regression analysis the scaled ranks per country and product were weighted. Each country had the same sum of weights. Within a country the weights were proportional to its sales volume in the year. Based on the scaled ranks several analyses were performed by using the Program “R” version 2.11.1 [24]:
An analysis was undertaken to assess how homogeneous the price level in a country was by looking at each product. It also showed whether countries with EPR had lower prices than countries that do not apply EPR.
The relationship between the scaled ranks and several explanatory variables was modelled by a linear regression model. The following variables were considered as predictors in the model: EPR, TPE per capita and GDP per capita.
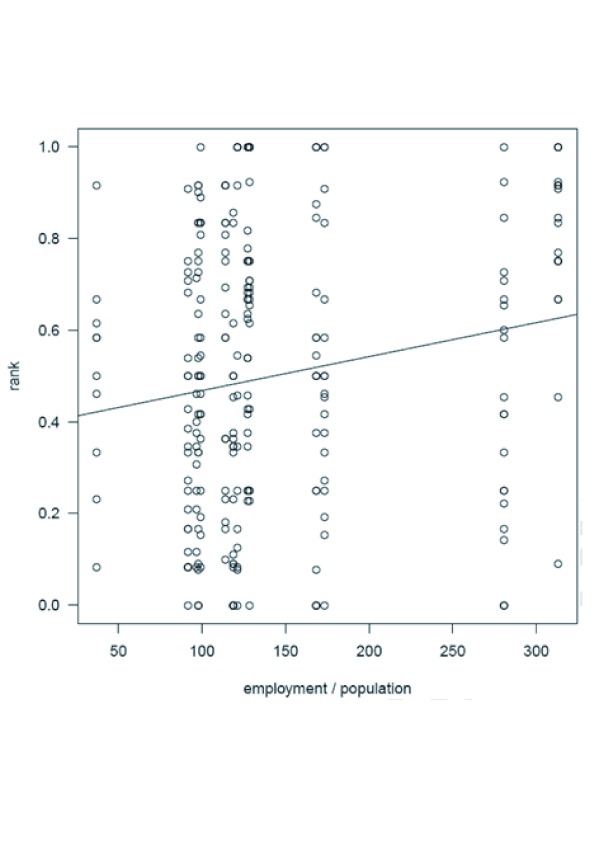
The correlation between EPR and pharmaceutical employment per 100,000 inhabitants was plotted separately as there is interaction between the two variables (cf. figure 4). It is unknown in which direction the interaction of the two variable goes.
Results
Out of the fourteen analysed countries, three did not apply EPR (Germany, Denmark, Sweden) at the time of investment. As shown in table 2, large variations in GDP per capita (ranging from Portugal EURO 14,684 to Norway EURO 41,346) and TPE per capita (ranging from Denmark EURO PPPa 246 to Greece EURO PPPa 503) existed among the included countries. Employment in pharmaceutical industry also showed large variations within Europe (10-fold difference; Slovak Republic 37 employees/100,000 inhabitants and Denmark 313 employees/100,000 inhabitants).
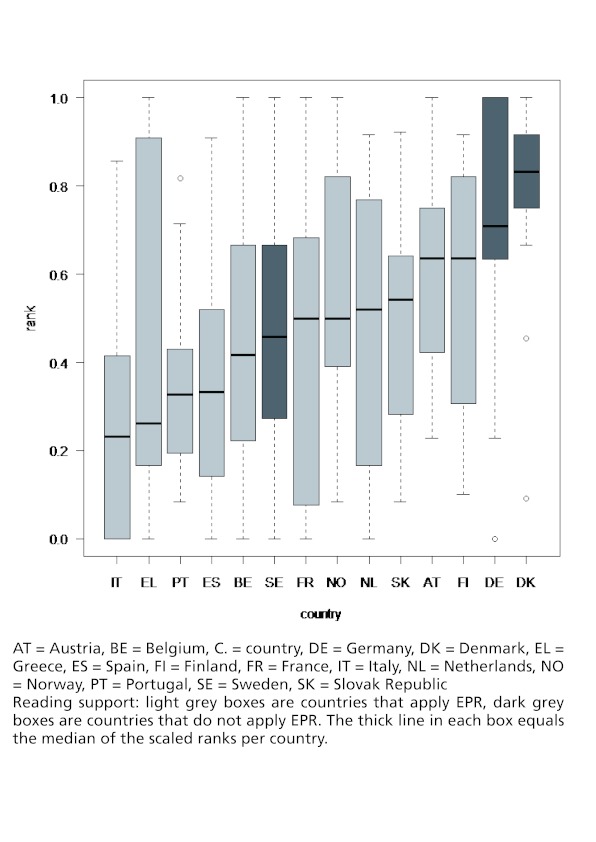
Figure 1 shows the distribution of the scaled ranks in a country as well as across countries. The median of the scaled ranks (incl. both years) of the countries varies from as low as 0.23 in Italy to 0.83 in Denmark. It is visible that two of the countries that do not apply EPR (Germany and Denmark) have the highest scaled ranks. But it is also visible that the scaled ranks vary to a great extent among the countries that apply EPR.
Figure 1: Impact of EPR on scaled ranks (incl. both years).
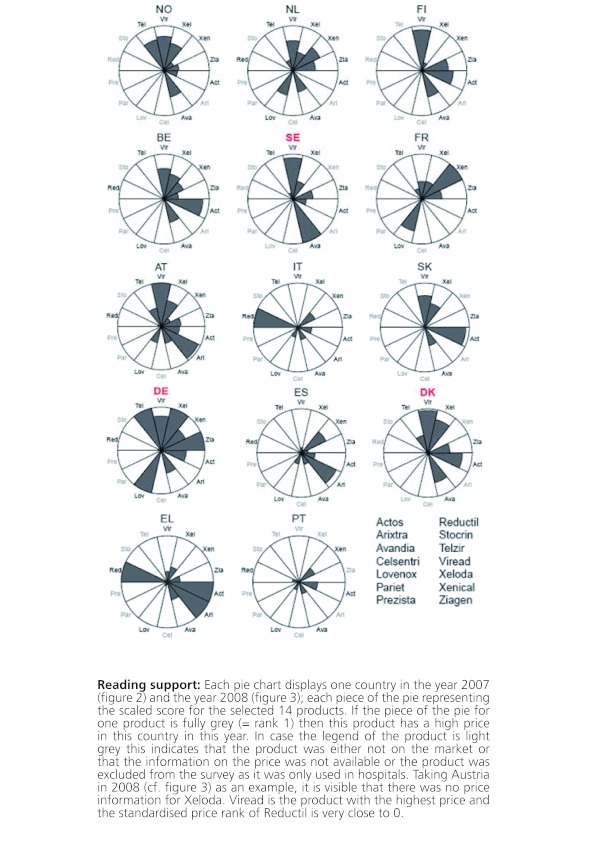
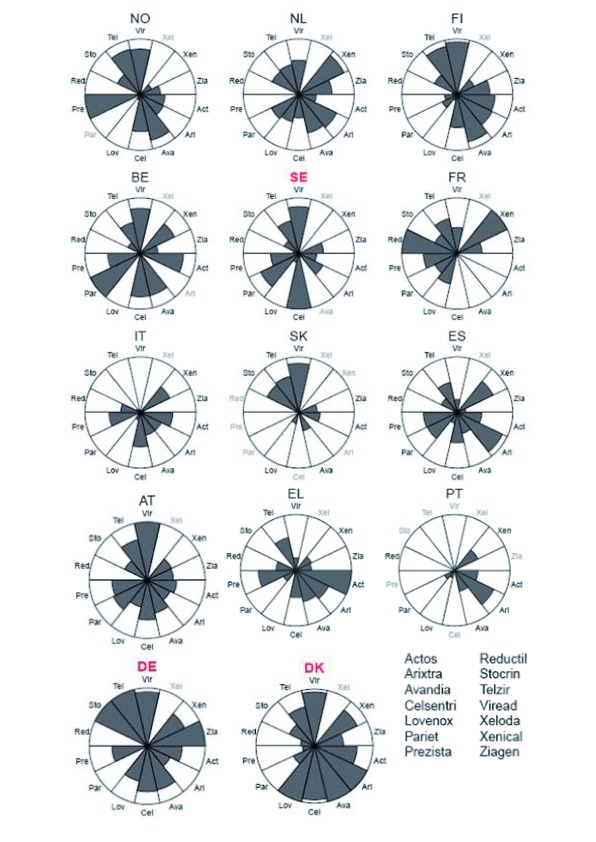
Figures 2 and 3 give a more detailed picture on the variances of the scaled ranks among products within a country and over time. These segment plots confirm that those countries without EPR (Denmark, Germany, Sweden) had in general higher price levels. This result is especially true for Germany in both years (2007: 8 out of 14 products for which price data were available were among the most expensive products of the products under observation, 2008: 11 out of 14 products were among the most expensive ones of the products included in the study) but also Denmark and Sweden had in each year around two or three products with high price levels but also had some products where the price level was relatively low.
Figure 2: Price variances among the selected products within individual countries; countries are displayed in order of GDP per capita in 2007.
Figure 3: Price variances among the selected products within individual countries; countries are displayed in order of GDP per capita in 2008.
As mentioned before, those countries who apply EPR still show different price levels. Figures 2 and 3 show that countries with a high GDP per capita (Norway, the Netherlands, Finland, Austria and Belgium) have higher price scores than countries with a lower GDP per capita such as Spain, Greece and Portugal. The Netherlands, a country that applies EPR, is an example of a country where the price level of the different products was all around the average. None of the products showed a very high price nor a very low price.
The unadjusted linear regression model confirms that applying EPR in a country is associated with a lower scaled rank (p=0.002). This association persisted after inclusion of total pharmaceutical expenditure per capita and GDP per capita in the final model.
Looking at the relationship of pharmaceutical employment per 100,000 inhabitants and the scaled ranks per country and product in figure 4, it is visible that there is a significant correlation (p-value: 0.0063). This means the higher the scaled ranks the higher the employment in pharmaceutical industry. However it is unknown in which direction the causality of the two variables goes: do companies (thus pharmaceutical employment) decide to produce in countries with high prices or do countries with high prices attract investments by companies?
Figure 4: Scatter plat of pharmaceutical employment per 100,000 inhabitants vs. the scaled ranks.
Table 2: Overview of EPR, economic variables and the median of scaled ranks
| C. | EPR (Y/N)a | GDP per capita in EURO 2006b | TPE per capita in EURO PPPa 20062, b | Pharmaceutical empl. Industry per 100,000c | Median of scaled ranks |
| AT | Y | 31,067 | 413 | 127 | 0.64 |
| BE | Y | 30,187 | 469 | 281 | 0.42 |
| DE | N | 28,184 | 416 | 129 | 0.71 |
| DK | N | 21,145 | 246 | 313 | 0.84 |
| EL | Y | 19,123 | 503 | 121 | 0.26 |
| ES | Y | 22,291 | 453 | 92 | 0.33 |
| FI | Y | 31,709 | 327 | 114 | 0.64 |
| FR | Y | 28,601 | 475 | 169 | 0.50 |
| IT1 | Y | 25,419 | 443 | 119 | 0.23 |
| NL | Y | 33,031 | 351 | 98 | 0.52 |
| NO | Y | 41,346 | 327 | 99 | 0.50 |
| PT | Y | 14,684 | 391 | 97 | 0.33 |
| SE | N | 29,025 | 365 | 173 | 0.46 |
| SK | Y | 23,797 | 330 | 37 | 0.54 |
AT = Austria, BE = Belgium, C. = country, DE = Germany, DK = Denmark, empl. = employment, EPR = External price referencing, EL = Greece, ES = Spain, FI = Finland, FR = France, GDP = Gross Domestic Product, IT = Italy, n.a. = not available, NL = Netherlands, NO = Norway, PE = Pharmaceutical Expenditure, PPPa = Purchasing Power Parities adjusted, PT = Portugal, SE = Sweden, SK = Slovak Republic, TPE = Total Pharmaceutical Expenditure
1 Italy: EPR is only an additional pricing policy complementing negotiations between the manufacturer and the Medicines Agency
2 TPE for the Netherlands and Norway as of 2007
Table 3: Linear multivariable regression
| Estimate | Std. Error | t-value | P-value | |
| (Intercept) | 0.5362546 | 0.0905139 | 5.925 | 0.00000 |
| EPR | -0.1427916 | 0.0458698 | -3.113 | 0.00206 |
| Total pharmaceutical expenditure per capita | 0.0000019 | 0.0000012 | 1.614 | 0.10781 |
| GDP per capita | 0.0000004 | 0.0000029 | 0.142 | 0.88718 |
| Dependent variable: scaled ranks per country and product; N = 262 | ||||
Discussion
This study showed that in general the use of EPR as a pricing policy is associated with lower prices. However, the large price variation among countries confirms that prices are not only driven by EPR policies but also by other unobserved factors (such as other pricing policies).
One of the results of the study was that price differences among countries could be observed. This was especially the case in countries which apply EPR. One reason could be the fact that EPR is very differently applied in the countries in terms of the country basket, frequency of price updates and the price calculation method [3-5]. Another confounding factor is that EPR is only one of many pharmaceutical price regulation policies applied in each country. For instance Italy uses EPR only as an additional pricing policy complementing price negotiations between the manufacturer and the Medicine Agency. While Italy states this supplementary role of EPR very openly, the relevance of EPR versus (confidential) negotiation is not so clear for other countries. Another fact is the time lag between the variables; referencing pricing in countries does not happen instantaneously, so the current price factor may be explained by the previous time periods’ independent variables.
An additional explanation for the price variances can be found in the selection of the products included in this study: Most products were reimbursable medicines in the out-patient sector. We observed that in a few countries e.g. Portugal, the prices of several products were not available. The reasons were that either the products were not on the market or some of the products (e.g. Telzir / Fosamprenavir calcium, Viread / Tenofovir disoproxil fumarat or Ziagen / Abacavir sulfat) were only used in a hospital setting for which the price was not available.
The fact that some products are hospital medicines can be seen as a limitation of this study. It was decided to exclude the prices of products exclusively applied in hospitals as firstly EPR is commonly not applied for hospital products and secondly actual hospital prices are often the outcome of negotiations which are not made public [25]. However, as this pertained to only a limited number of products and countries, we do not feel that this will have affected our overall results.
Another point for discussion is whether countries take into account possible discounts and rebates when they are applying EPR. Very often publicly available prices for reimbursable medicines do not reflect discounts from price negotiations between third party payers and companies as they are confidential. This limits the positive effects of EPR by not taking into account the lower discounted prices when referencing to other countries.
Even though the selected products are not primarily subject to parallel trade, it needs to be mentioned that parallel trade plays an important role also with respect to EPR. Parallel trade effectively arbitrage price differences across countries and therefore has a similar effect to EPR in terms of compressing price differences and inducing strategic launch behaviour by firms. The presence of parallel trade reduces the incentive of countries with high prices to adopt EPR [26, 27].
With regard to the methodology chosen in this study it was decided to take the unit ex-factory price as the majority of the countries that apply EPR also use the ex-factory price level for the price comparison (exceptions are Denmark, Finland, the Netherlands and Sweden which set their prices at pharmacy purchasing level). Furthermore this methodological approach is supported by literature on price comparisons [2, 28]. The decision to use the unit ex-factory price is supported by the argument that price comparison on pharmacy purchasing or pharmacy retail price level is difficult due to different remuneration schemes ( and distribution margins) and VAT on medicines.
With respect to price comparisons, either with prices in other countries or with similar existing treatments, numerous studies have discussed different methodological approaches.
Danzon [29] argued that “valid measures of average price levels can only be obtained from comparisons based on a comprehensive or representative sample of products, appropriately weighted, following standard index number methods”. Following these considerations the methodology chosen in this study takes into account an indicative sample of 262 observations (including the 14 products and volume data from two years).
Medicine prices are the results of many different policy effects. Hence the price of a medicine may change as soon as it is included in the reimbursement schemes or as soon as a generic equivalent enters the market. Therefore, it is difficult to separate the effects of one pricing policy on medicine prices. This was the reason why only medicines that were on-patent in the observed countries in 2007 and 2008 were included in the study. In addition, it should be acknowledged that other factors (e.g. other national pricing policies) were not considered which may account for variation in price levels as well. For example by barring endogenous responses by firms (changing their launch strategies) EPR would be expected to compress the distribution of prices across countries, which makes finding any difference in the cross-section more of a challenge.
Our results support the assumption that EPR places greater pressure on countries that are selected by others as a reference country to keep prices high, especially if they want early market entry of new products or in order to support a national pharmaceutical industry. A consequence of EPR is illustrated by a tendency for pharmaceutical industries to set high entry prices for new products in countries without basic regulations. These pricing levels then become indicative for the other countries that use EPR for regulating prices on their markets [30].
This argument is supported by a report by the European Commission (Sector Inquiry), which stated that companies preferred to initiate their product launch in countries with no direct price control (Germany, Sweden and United Kingdom) or in countries that are used as references by others and have received approval from the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (Italy and Sweden) [31]. The results of our study showed the same trend. For example, Germany - a country with the tradition of free pricing at ex-factory price level - was not only the country with the highest prices in both years but also with the highest availability of products. Hence, it can be concluded that ERP may have negative impacts on individual country prices and unexpected consequences in countries applying such policies.
Conclusions
This study demonstrated that for patented products prices were generally lower in the countries which applied external reference pricing. Possible explanations could be found through an association of the scaled ranks with the pharmaceutical industry size and scaled weighted price ranks. However, it needs to be acknowledged that huge price difference could be found between countries which apply external price referencing. This could be explained by different methodologies with respect to the selection of countries in a reference basket or the method for calculating the price.
Author Contributions
All authors contributed to the paper’s conception, design and production. CL wrote major parts of the article and revised the article following contributions from the other co-authors. SL performed the statistical analysis. Numerous times the results of the study were circulated among the co-authors, who then gave critical comments. All authors have approved the final version for submission.
Acknowledgments
Deep acknowledgements are given to Peter Stephens from IMS Health for providing sales volume data for 2007 and 2008 for the 14 products. We would like to express sincere gratitude to the Austrian Health Institute for providing price data and to the PPRI network members for providing data on implementation of EPR in their respective countries.
Funding Statement
Funding of this study was provided by Christine Leopold as part of her PhD program at the WHO Collaborating Centre for Pharmacoepidemiology and Pharmaceutical Policy, Division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht in the Netherlands.
Footnotes
The department of Pharmacoepidemiology and Clinical Pharmacology at the Utrecht Institute for Pharmaceutical Sciences, employing AKM and HGML, has received unrestricted research funding from the Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), the Royal Dutch Pharmacists Association (KNMP), the private-public funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government, and industry), the EU Innovative Medicines Initiative (IMI), EU 7th Framework Program (FP7), the Dutch Medicines Evaluation Board, the Dutch Ministry of Health and industry (including GlaxoSmithKline, Pfizer, and others).
The other authors declare no conflict of interest.
References
- 1.Marquis M S, Phelps C E. Price elasticity and adverse selection in the demand for supplementary health insurance. Econ Inq. 1987 Apr;25(2):299–313. doi: 10.1111/j.1465-7295.1987.tb00741.x. http://www.scholaruniverse.com/ncbi-linkout?id=10281607. [DOI] [PubMed] [Google Scholar]
- 2.Brekke K, Holmas TH, Straume OR. Are pharmaceuticals inexpensive in Norway? A comparison of prices of prescription pharmaceuticals between Norway and nine west European countries. Institute for Research in Economics and Business Administration. 2008 [Google Scholar]
- 3.Docteur E, Paris V. Pharmaceutical Pricing Policies in a global market. Paris: OECD Health Policy Studies; 2007. http://www.oecd.org/document/44/0,3343,en_2649_37407_41382764_1_1_1_1,00.html. [Google Scholar]
- 4.Kanavos P, Vandoros S, Irwin R, Nicod E, Casson M. Differences in costs of and access to pharmaceutical products in the EU (IP/A/ENVI/ST/2010-12) Brussels: European Parliament; 2011. [Google Scholar]
- 5.Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Report. Vienna: Gesundheit Österreich GmbH / Geschäftsbereich ÖBIG; 2008. [Google Scholar]
- 6.Vogler S. Preisbildung und Erstattung von Arzneimitteln in der EU - Gemeinsamkeiten, Unterschiede und Trends. Pharmazeutische Medizin. 2012;14(1):48–56. [Google Scholar]
- 7.Leopold C, Vogler S, Mantel-Teeuwisse AK, de_Joncheere K, Leufkens HG, Laing R. External Price Referencing in Europe – a descriptive overview of one policy with many national characteristics. Health Policy. 2012;104(1):60. doi: 10.1016/j.healthpol.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Espin J, Rovira J, De Labry AO. External Reference Pricing, WHO/HAI Project on Medicine Prices and Availability. WHO review series on Pharmaceutical Pricing Policies and Interventions, Working Paper. 2011.
- 9.Heuer A, Mejer M, Neuhaus J. The National Regulation of Pharmaceutical Markets and the Timing of New Drug Launches in Europe, Institute for the World Economy Working Paper No. 437. 2007 http://www.ifw-kiel.de/ausbildung/asp/asp-wp/2007/aspwp437.pdf.
- 10.Mariñoso BG, Jelovac I, Olivella P. External referencing and pharmaceutical price negotiation. GATE Groupe d’Analyse et de Théorie Économique. Working paper 08-15. 2008. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=1168822.
- 11.Stargardt Tom, Schreyögg Jonas. Impact of cross-reference pricing on pharmaceutical prices: manufacturers' pricing strategies and price regulation. Appl Health Econ Health Policy. 2006;5(4):235–47. doi: 10.2165/00148365-200605040-00005. http://www.scholaruniverse.com/ncbi-linkout?id=17249840. [DOI] [PubMed] [Google Scholar]
- 12.Richter Anke. Assessing the impact of global price interdependencies. Pharmacoeconomics. 2008;26(8):649–59. doi: 10.2165/00019053-200826080-00003. http://www.scholaruniverse.com/ncbi-linkout?id=18620459. [DOI] [PubMed] [Google Scholar]
- 13.Windmeijer Frank, de Laat Eric, Douven Rudy, Mot Esther. Pharmaceutical promotion and GP prescription behaviour. Health Econ. 2006;15(1):5–18. doi: 10.1002/hec.1007. [DOI] [PubMed] [Google Scholar]
- 14.Merkur Sherry, Mossialos Elias. A pricing policy towards the sourcing of cheaper drugs in Cyprus. Health Policy. 2006 Sep 01;81(2-3):368–75. doi: 10.1016/j.healthpol.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Filko M, Szilagyiova P. The Slovak experience in the international price benchmarking for prescription drugs. Value in Health. 2009;12(7):229–30. [Google Scholar]
- 16.Kaiser U, Mendez SJ, Ronde T. Regulation of pharmaceutical prices: evidence from a reference price reform in Denmark. Centre for European Economic Research. Discussion Paper No. 10-062. doi: 10.1016/j.jhealeco.2014.04.003. ftp://ftp.zew.de/pub/zew-docs/dp/dp10062.pdf. [DOI] [PubMed]
- 17.Kyle MK. The Role of Firm Characteristics in Pharmaceutical Product Launches. The RAND Journal of Economics. 2006;37(3):602–618. [Google Scholar]
- 18.European Medicine Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124.
- 19.PPRI network members. PPRI / PHIS Pharma Profiles. PPRI (Pharmaceutical Pricing and Reimbursement Information); 2007-2011. http://whocc.goeg.at/Publications/CountryReports. [Google Scholar]
- 20.Pharmaceutical Price Information (PPI), Austrian Health Institute. http://www.goeg.at/en/Reports-Service.html.
- 21.IMS Health Institute.
- 22.OECD Health database. OECD Health database. http://www.oecd.org/document/19/0,3746,en_21571361_33915056_39091923_1_1_1_1,00.htm.
- 23.European Federation of Pharmaceutical Industry Association (EFPIA) http://www.efpia.eu/content/default.asp?PageID=559&DocID=9158.
- 24.R: A language and environment for statistical computing (ISBN 3-900051-07-0) Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.R-project.org. [Google Scholar]
- 25.Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Pharmaceutical Health Information System; Gesundheit Österreich GmbH / Geschäftsbereich ÖBIG; 2010. [Google Scholar]
- 26.Costa-i-Font J, Kanavos P. LSE Health working papers. London, UK: London School of Economics and Political Science; 2007. Medicines in parallel trade in the European Union: a gravity specification. [Google Scholar]
- 27.Glynn D. The effects of parallel trade on affordable access to medicines. Eurohealth. 2009;15(2):1–4. [Google Scholar]
- 28.Rosian I, Vogler S. Internationale Arzneimittelpreisvergleiche – Meta-Analyse. ÖBIG; 2004. [Google Scholar]
- 29.Danzon PM, Chao LW. Cross-national price differences for pharmaceuticals: how large, and why. Journal of Health Economics. 2000;19(2):159–95. doi: 10.1016/s0167-6296(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 30.Danzon Patricia M, Wang Y Richard, Wang Liang. Health Econ. 3. Vol. 14. Health Economics; 2005. The impact of price regulation on the launch delay of new drugs--evidence from twenty-five major markets in the 1990s; pp. 269–92. [DOI] [PubMed] [Google Scholar]
- 31.Pharmaceutical Sector Inquiry. European Commission; 2008. http://ec.europa.eu/competition/sectors/pharmaceuticals/inquiry/index.html. [Google Scholar]