Abstract
Objectives
Losses to follow-up after initiation of antiretroviral therapy (ART) are common in Africa and are a considerable obstacle to understanding the effectiveness of nascent treatment programs. We sought to characterize, through a sampling-based approach, reasons for and outcomes of patients who become lost to follow-up.
Design
Cohort study.
Methods
We searched for and interviewed a representative sample of lost patients or close informants in the community to determine reasons for and outcomes among lost patients.
Results
Three thousand six hundred twenty-eight HIV-infected adults initiated ART between January 1, 2004 and September 30, 2007 in Mbarara, Uganda. Eight hundred twenty-nine became lost to follow-up (cumulative incidence at 1, 2, and 3 years of 16%, 30%, and 39%). We sought a representative sample of 128 lost patients in the community and ascertained vital status in 111 (87%). Top reasons for loss included lack of transportation or money and work/child care responsibilities. Among the 111 lost patients who had their vital status ascertained through tracking, 32 deaths occurred (cumulative 1-year incidence 36%); mortality was highest shortly after the last clinic visit. Lower pre-ART CD4+ T-cell count, older age, low blood pressure, and a central nervous system syndrome at the last clinic visit predicted deaths. Of patients directly interviewed, 83% were in care at another clinic and 71% were still using ART.
Conclusions
Sociostructural factors are the primary reasons for loss to follow-up. Outcomes among the lost are heterogeneous: both deaths and transfers to other clinics were common. Tracking a sample of lost patients is an efficient means for programs to understand site-specific reasons for and outcomes among patients lost to follow-up.
Keywords: Africa, antiretroviral scale-up, losses to follow-up, monitoring and evaluation, sampling studies
The roll out of antiretroviral therapy (ART) to the millions of persons with HIV/AIDS in resource-limited settings represents one of the largest public health interventions ever undertaken.1–4 As the process accelerates, evaluating outcomes among treated patients is vital to understanding the impact of treatment programs and to acquire evidence to improve future strategies. A critical barrier, however, has emerged in the evaluation of outcomes in the form of the substantial fraction of patients defaulting, or becoming lost, from care. Specifically, between 15% and 40% are lost within the first year of ART.1,3,5,6 Without an understanding of why patients become lost or what their subsequent outcomes are, an accurate picture of the impact of the global ART scale-up is impossible.
Whereas determining mortality among those lost to follow-up in ART programs has received most of the immediate attention,7–9 a number of other aspects of these patients also require better understanding. First, knowledge of the reasons for loss is critical to design retention interventions. To date, reasons have been ascertained mostly by epidemiologic analyses evaluating factors associated with absence from clinic10; direct interview of lost patients for their actual reasons has rarely been employed.11 Second, the full spectrum of outcomes among patients who do not return to care has not been described. It has become increasingly common to combine losses with deaths as an end point in research and program evaluation under the assumption that all losses represent adverse events (i.e., deaths or patients who stop ART and are at high risk for death).12–14 This neglects potentially favorable outcomes such as transfers to closer treatment centers that are inevitable in the setting of rapid scale-up. Third, determinants of deaths among those lost to follow-up have not been identified. Understanding which patients have the most precipitous clinical declines after becoming lost can inform the timing and targeting of finding those at greatest risk.
To better account for mortality among losses to follow-up, we previously presented a strategy based on searching in the community for a representative sample of lost patients to ascertain their updated vital status.8 Incorporating updated vital status from the sample of lost patients increased mortality estimates for the underlying clinic population 5-fold. To deepen our understanding of patients lost to follow-up, we have now extended this sampling approach to address several other questions. Specifically, we determined reasons for loss to follow-up, outcomes among patients who did not return for care, and what factors predict poor outcomes once a patient has become lost.
METHODS
Patients
We evaluated all HIV-infected adults attending the Immune Suppression Syndrome (ISS) clinic in Mbarara, Uganda, who initiated ART between January 1, 2004, and September 30, 2007. Mbarara is 4.5 hours by automobile from Kampala, the capital city of Uganda. The ISS clinic serves the surrounding Mbarara district (population 1.1 million, 92% in rural areas) and portions of adjacent districts. Patients were followed from the time of ART initiation to either death or administrative closure on September 30, 2007. An estimate of the mortality in this population has been reported previously.8
Tracking a Sample of the Patients Who Become Lost to Follow-Up
In July 2006, the ISS clinic began a program in which a tracker was sent into the community to determine the outcomes of an unselected and consecutive sample of patients who had become lost to follow-up. Each month, the clinic’s electronic medical record system, which captures all patient visits, generated a list of patients who were lost to follow-up, defined as not being seen at the clinic for at least 6 months. This is performed by the data manager who does not interact with the clinic patients and who uses structured query language within the OpenMRS platform15 to identify all patients who are lost to follow-up. Monthly quality assurance assessment of the completeness of the electronic medical record system in capturing patient visits by a comparison with handwritten log books at the reception desk reveals this to be consistently above 99.0% (data not shown). As the cumulative number of patients lost each month exceeds the number that can be traced, patients can appear on the list of lost patients in multiple months as long as they are not sought by the tracker. Patients who have appeared on the list and are sought are excluded from the list in future months whether their vital status is ascertained or not.
The patient tracker, a health educator at the clinic, was chosen because of his experience with HIV counseling, education, issues of confidentiality and familiarity with local geography and social norms. The tracker was given information about the identity (name, sex, age, and occupation) and residence (district, county, subcounty, parish, and village) of each lost patient and sought to locate and directly speak to the missing patient. Failing that, he would record vital status information from close informants (e.g., family members, neighbors, or friends). Because the district is mostly rural, tracking was done on motorcycle or on foot. A typical tracking episode involves first traveling paved roads on motorcycle in the general direction of the patient’s residence. As there are few paved roads, however, the tracker typically spends most of the search on unpaved roads between villages. Once in the vicinity of the parish or village he seeks, he asks local residents for further geographic directions. Most villages are composed of small clusters of homes separated by fields and linked by dirt footpaths. Although the area is rural, the population density is relatively high and informants are common. Within the village, he begins to ask for the patient by name. Once at the home, the tracker calls from a short distance to greet and initiate conversation with the residents. During this process, the tracker does not reveal that he is from the clinic until he has positively identified the patient and confidentiality can be maintained.
The tracker sought each missing patient for 1 afternoon and if unable to locate could either continue for 1 more afternoon or move on to the next lost patient at his discretion. He was instructed to attempt to locate as many patients on the list as time permitted each month. Given that it was acknowledged that time was limited relative to the large number of lost patients, each month, only a sample of patients was sought.
Measurements
Demographic and clinical characteristics before ART initiation and, for selected variables, at the last clinic visit before becoming lost were obtained from the clinic’s electronic medical record system. Actual distance between village of residence and clinic in the lost to follow-up patients who were sought after was calculated using ARCVIEW, version 9.2 (CMC International, Dallas, TX). Among the patients who were lost and then sought after, further medical record abstraction was performed for any mention of organ-specific signs and symptoms at the last available clinic visit. Using these organ-specific findings, 3 clinical syndromes were defined. A chronic pulmonary syndrome was defined as the presence of complaints of cough, weight loss, and fever for at least 1 month. An acute pulmonary syndrome was defined by complaints of cough and fever for <1 month. A central nervous system syndrome was defined as (a) signs or symptoms of a focal neurological deficit, (b) altered mental status, or (c) nausea and vomiting in the absence of any other abnormal gastrointestinal signs or symptoms.
Among those lost patients who were sought after, the tracker also administered a short structured questionnaire regarding the relationship of the informant to the patient (in cases when the patient could not be found), reasons for not returning to clinic, and whether the patient had transferred to another clinic and was using ART. Among those found to be dead, the tracker inquired whether the death was because of childbirth, accident/trauma, or illness.
Statistical Analyses
The incidence of becoming lost to follow-up was calculated using the Kaplan–Meier method with ART initiation as time 0 and lost to follow-up (the event) at the time a patient had not returned to clinic for 6 months. By definition, this resulted in no losses to follow-up in the first 6 months after ART initiation. For those who did not become lost to follow-up, censoring occurred at the last clinic visit. Because death could not be considered as noninformative censoring as it relates to becoming lost to follow-up, deaths that were known to the clinic and occurred within 6 months of the last clinic visit were managed in a competing risk framework.16 Deaths that occurred after 6 months of the last clinic visit were ignored in this calculation as the patient was recorded as being lost to follow-up at 6 months. Among the sample of lost patients who were sought after, we used logistic regression to evaluate factors associated with the ability to ascertain vital status (via interviewing the patient or an informant). Among this sample, we also evaluated factors associated with death after the last known clinic visit using proportional hazards regression. The proportional hazards assumption was evaluated using plotted scaled Schoenfeld residuals.17 In both these regression analyses, potential predictor variables were first examined in unadjusted models. Factors associated at a P value <0.05 level with the outcome were then evaluated in multivariable models. In the case of the proportional hazards analysis, where more events allowed for evaluation of more predictor variables, both gender and age were fixed in the final model, given their a priori likelihood of importance. The study was approved by the Institutional Review Boards of University of California, San Francisco, and Mbarara University of Science and Technology.
RESULTS
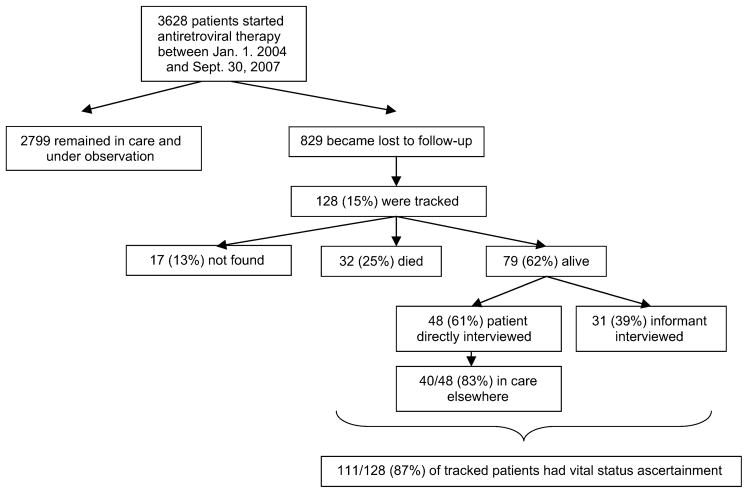
A total of 3628 HIV-infected adults newly initiating ART were evaluated (Fig. 1). The median age was 35 years [interquartile range (IQR) 30–42], and 61% were women. The median CD4+ T-cell count before ART in 1674 patients in whom it was available was 95/mm3 (IQR 36–172). The number of new patients starting therapy was 522 in 2004, 1465 in 2005, 850 in 2006, and 796 in 2007.
FIGURE 1.
Flow chart of HIV-infected patients initiating ART at the ISS clinic in Mbarara, Uganda, between January 1, 2004, and September 30, 2007.
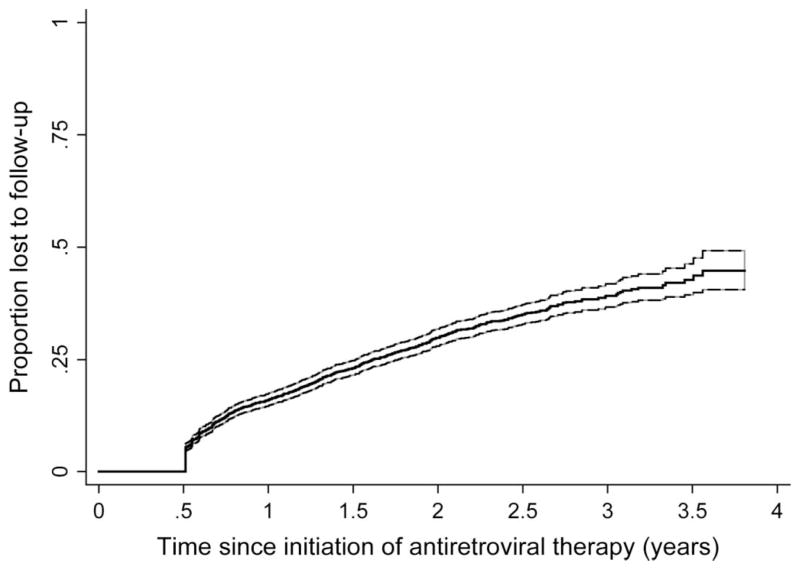
Losses to Follow-Up
Over a maximum of 3.75 years of observation, 829 patients became lost to follow-up as defined by 6 months of absence from clinic (Fig. 2). This corresponds to a cumulative incidence of loss to follow-up at 1, 2, and 3 years of 16% [95% confidence interval (CI): 15 to 17], 30% (95% CI: 28 to 32), and 39% (95% CI: 37 to 42), respectively. Of the 829 patients deemed lost, a sample of 128 (15%) was sought after in the community. The median interval between last clinic visit and tracking of the patient was 11.6 months (IQR 9.4–14.3); the median distance of residence from clinic was 33 km (IQR 4–66). Of the 128 patients sought after, we were able to ascertain vital status in 111 (87%, 95% CI: 80 to 92). In 48 cases (43%), the patient was directly found, and in 63 cases (57%), an informant was found. Informants included parents (15%), children (9%), friends (5%), neighbors (8%), siblings (5%), spouse (4%), and others (11%). Patients who were lost and subsequently had vital status ascertained by tracking (n = 111) were similar in age (median 35 vs. 36 years), sex (59% vs. 58% women), pretherapy CD4+ T-cell count (median 75 vs. 72 cells/mm3), and pretherapy World Health Organization stage (76.6% vs. 79.9% were stage 3 or 4) to those who were lost and did not have ultimate vital status ascertained (n = 718) (Table 1).
FIGURE 2.
Time to loss to follow-up among patients starting ART between January 1, 2004, and September 30, 2007. The 95% confidence bands are shown with dashed lines. Because definition of loss to follow-up required 6 months of absence, there are no events in the first 6 months.
TABLE 1.
Patient Characteristics at the Time of ART Initiation at the ISS Clinic in Mbarara, Uganda
| All Patients (Group A) | Patients Lost to Follow-Up (Group B) | Patients Lost to Follow-Up and Tracked (Group C) | Patients Tracked and Vital Status Ascertained (Group D) | All Lost Patients Without Vital Status Ascertainment (Groups E or B–D) | |
|---|---|---|---|---|---|
| Total No. | 3628 | 829 | 128 | 111 | 718 |
| Female (%) | 61 | 58 | 59 | 59 | 58 |
| Age (years), median (IQR)* | 35 (30–42) | 36 (30–42) | 35 (29–42) | 35 (29–42) | 36 (31–42) |
| CD4+ T-cell count, median (IQR)† | 95 (36–172) | 72 (19–150) | 90 (20–187) | 75 (20–191) | 72 (19–144) |
| WHO stage 3 or 4 (%)‡ | 71.9 | 79.5 | 77.3 | 76.6 | 79.9 |
Age was missing in 30, 9, 1, 1, and 8 in groups A–E, respectively.
The last CD4+ T-cell count value obtained before initiation of ART in the 6 months before starting therapy. The data were missing in 1954, 358, 48, 41, and 317 patients in groups A–E, respectively.
Refers to World Health Organization (WHO) stage at the time of initiation of ART. These data were missing in 273, 92, 14, 12, and 80 in groups A–E, respectively.
In an attempt to identify factors related to successful ascertainment of vital status among those sought after, a logistic regression model evaluated a variety of factors (age, sex, pre-ART CD4+ T-cell count, and distance of residence from clinic) but none were significantly associated at a P< 0.05 level.
Reasons for Absence From Clinic
Of the 79 sampled patients found to be alive, 48 were directly interviewed, and in the remaining 31, an informant was interviewed. The most common reasons the directly interviewed patients stated for not returning to the ISS clinic were lack of transportation in 50% (95% CI: 35 to 65), distance in 42% (95% CI: 28 to 57), lack of money in 35% (95% CI: 22 to 51), work responsibilities in 27% (95% CI: 15 to 42), child care commitments in 22% (95% CI: 12 to 37), feeling that the clinic was not helping in 12.5% (95% CI: 5 to 25), feeling too sick in 6.3% (95% CI: 1.3 to 17), family members advised against returning in 4.2% (95% CI: 0.5 to 14), and religious beliefs in 2.1% (95% CI: 0.05 to 11). Despite their failure to return to the ISS clinic, 40 of 48 patients (83%, 95%, CI: 70 to 93) reported that they had seen a health care provider at a different facility in the previous 3 months, and 34 of 48 (71%, 95% CI: 56 to 83) responded that they had taken ART in the last 30 days.
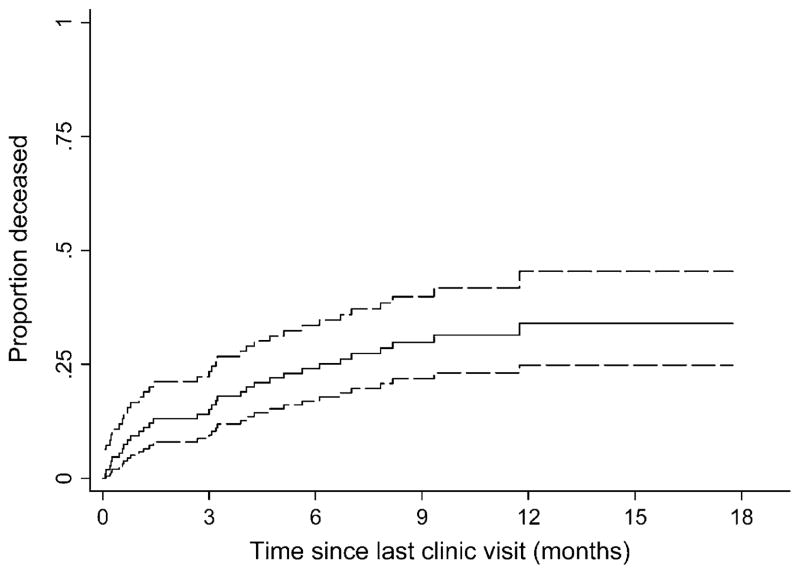
Survival in Patients Lost to Follow-Up
In the sample of 111 patients who were lost to follow-up and who had their vital status ascertained, 32 were determined to have died. Most of the deaths (30) were reported to be because of illness, but 2 deaths were said to be because of accident or trauma. From the time of the last clinic visit, the cumulative incidence of death in these lost patients at 1, 3, 6, and 12 months was 9.1% (95% CI: 5.0 to 16), 15% (95% CI: 9.3 to 23), 23% (95% CI: 16 to 33), and 36% (95% CI: 27 to 47), respectively (Fig. 3). Death rates were higher early after last clinic visit: in month 1, months 2 through 6, and months greater than 6, the death rates were 115, 42, and 21 per 100 person-years, respectively.
FIGURE 3.
Time to death in a sample of 111 patients lost to follow-up whose vital status was ascertained through tracking in the community. Time 0 is defined as the date of the last recorded clinic visit. The 95% CIs are shown with dashed lines.
Predictors of Survival Among Those Lost to Follow-Up
Among the 111 patients who were lost to follow-up and had their vital status ascertained, 70% had expressed a symptom at the time of last clinic visit, with cough (21%) and fever (21%) being the most common (Table 2). In terms of clinical syndromes at the last clinic visit, 22% were deemed to have an acute pulmonary syndrome, 24% a chronic pulmonary syndrome, and 11% a central nervous system syndrome.
TABLE 2.
Relationship Between Demographic Characteristics and Clinical Factors at Last Clinic Visit and Subsequent Death Among Patients Who Became Lost to Follow-Up. Sample Is Restricted to 111 Patients Who Were Sought After in the Community and Had Their Vital Status Ascertained
| Factor | N (%) With Finding | Unadjusted
|
Adjusted*
|
||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | ||
| Demographic characteristics | |||||
| Male | 46 (41.0) | 0.85 (0.42 to 1.75) | 0.67 | 1.54 (0.53 to 4.12) | 0.42 |
| Age (per 10 years)† | — | 1.30 (0.95 to 1.77) | 0.10 | 2.04 (1.08 to 3.82) | 0.03 |
| Distance from residence to clinic (per 10 km) | — | 1.03 (0.94 to 1.12) | 0.53 | — | — |
| Symptoms | |||||
| Any complaints | 78 (70.3) | 1.17 (0.53 to 2.61) | 0.70 | — | — |
| Fever | 23 (20.7) | 0.67 (0.26 to 1.74) | 0.41 | — | — |
| Headache | 15 (13.5) | 0.88 (0.31 to 2.51) | 0.81 | — | — |
| Cough | 23 (20.7) | 0.71 (0.27 to 1.84) | 0.49 | — | — |
| Shortness of breath | 4 (3.6) | 3.71 (1.12 to 12.3) | 0.03 | — | — |
| Nausea and vomiting | 13 (11.7) | 1.87 (0.77 to 4.57) | 0.16 | — | — |
| Abdominal pain | 9 (8.1) | 0.78 (0.19 to 3.28) | 0.74 | — | — |
| Diarrhea | 11 (9.9) | 1.27 (0.44 to 3.62) | 0.66 | — | — |
| Weight loss (selfreported) | 22 (19.8) | 1.27 (0.57 to 2.84) | 0.60 | — | — |
| Complaints present for >1 month | 38 (34.2) | 1.06 (0.52 to 2.16) | 0.88 | — | — |
| Signs | |||||
| Temperature >37. 8°C | 4 (3.6) | 1.50 (0.36 to 6.29) | 0.56 | — | — |
| Mean arterial blood pressure, <75 mm Hg | 12 (10.8) | 2.41 (1.18 to 4.96) | 0.02 | 2.97 (1.15 to 7.69) | 0.02 |
| Functional impairment | 38 (34.2) | 1.46 (0.72 to 2.96) | 0.29 | — | — |
| Abnormal pulmonary examination | 14 (12.6) | 0.69 (0.21 to 2.27) | 0.54 | — | — |
| Rash | 17 (15.3) | 1.35 (0.56 to 3.27) | 0.65 | — | — |
| Thrush | 8 (7.2) | 0.80 (0.19 to 3.35) | 0.76 | — | — |
| Clinical syndrome | |||||
| Chronic pulmonary syndrome | 27 (24.3) | 1.14 (0.51 to 2.52) | 0.75 | — | — |
| Acute pulmonary syndrome | 24 (21.6) | 1.13 (0.52 to 2.44) | 0.98 | — | — |
| Central nervous system syndrome | 12 (10.8) | 2.77 (1.19 to 6.40) | 0.02 | 2.86 (1.11 to 7.40) | 0.03 |
| Laboratory results | |||||
| Pre-ART CD4+ T-cell count (per 50 cells/mm3)‡ | — | 0.65 (0.47 to 0.91) | 0.01 | 0.62 (0.42 to 0.94) | 0.02 |
Each factor is adjusted for all other factors in the column.
Missing in 1 patient.
Missing in 41 patients.
In unadjusted analyses, factors identified at the last available clinic visit among the lost to follow-up that were found to be significantly related to subsequent death included complaint of shortness of breath, mean arterial blood pressure <75 mm Hg, and presence of a central nervous system syndrome. Among the pre-ART factors, only low CD4+ T-cell count was associated. The multivariable analysis identified 4 independent risk factors for death. The presence of mean arterial blood pressure <75 mm Hg at the last visit conferred a 2.97-fold increased rate of death (P = 0.02), and a central nervous syndrome raised the rate by 2.86-fold (P = 0.03). Each increase of pre-ART CD4+ T-cell count by 50 cells/mm3 reduced the rate of death by 38% (P = 0.02), and each 10-year increase in age increased the rate by 2.04-fold (P = 0.03) (Table 2).
DISCUSSION
The reasons for and outcomes in patients lost to follow-up from African ART programs are not well understood. In a prototypical scale-up program in Mbarara, Uganda, we found that the incidence of losses to follow-up was high (39% by 3 years after ART initiation) and comparable to prior reports.3,5,18 By tracking a representative sample of patients in the community after loss, we achieved a clearer understanding of this important group. Common reasons for loss to follow-up were social or structural. These included problems with transportation, finances, and work/child care responsibilities. Among those lost to follow-up, subsequent outcomes were heterogeneous. An important fraction of patients had died, but, in fact, the majority of patients had transferred to other care facilities—a favorable outcome. Finally, we identified several clinical factors at the time of last clinic visit associated with deaths after patients become lost.
Direct interviews of lost patients provide among the most direct evidence to date as to the reasons for loss in rural Africa. Lack of transportation and distance to clinic were the most common reasons for not returning to clinic. This is not surprising because Mbarara district is mainly rural and distances between home and clinic can be great. More than 70% of sub-Saharan Africans reside in rural settings; therefore, transportation is likely to be important throughout the continent. This finding suggests that alternative models to delivering care such as more dispersed satellite clinics or home-based programs19 are needed to ensure continuous care. Lack of money was the second most common reason for absence. Although poverty likely exacerbates other barriers such as distance and transportation, 30% fewer people cited lack of money compared with transportation. We believe that this may reflect the fact that transportation is a structural barrier that cannot always be overcome by individual-level financial assistance. For example, if a road does not exist, individual financial resources cannot ensure continued attendance in clinic.20 Work and child care were also common reasons for failure to return to clinic. In resource-limited settings, accessing health care is often only one competing need in a nexus of urgent priorities, and social responsibilities to work and children may take precedent over personal health. These factors, furthermore, may affect women more than men. These reasons for failure to return to clinic argue that in Africa, socio-structural factors are more important than individual-based psychosocial factors, as put forth by prevailing Western information–motivation–behavior models.21
Our findings confirm previous observations that a substantial fraction of lost patients had died9,22,23 and extend these observations by demonstrating that a large portion of the deaths occurred shortly after the last clinic visit. The patients who died in the first months after their last clinic visit likely died from conditions that evolved while in care rather than from clinical deterioration after cessation of care. In other words, not all deaths in patients lost to follow-up occurred because of loss to follow-up. Prevention of these early deaths will likely only be achieved via strengthening of the clinical infrastructure including improved point-of-care diagnostic testing and availability of effective therapies.
Our study also provides further information on the predictors of deaths, which has implications for outreach interventions. We found that several clinical characteristics present at the last available clinic visit were associated with subsequent deaths. These included older age, low blood pressure, a central nervous syndrome, and a low pre-ART CD4+ T-cell count. Whether or not these specific factors will be operative in other settings with other care paradigms requires further study, but these data provide proof-of-concept that discrete factors can be identified at the time of last clinic visit that are predictive of subsequent death. This suggests that programs may be able to efficiently target which lost patients to seek after they fail to return and how urgently to do so.
We found important heterogeneity in outcomes that challenge prevailing notions. Extrapolating the experience of the 48 lost patients we directly interviewed to all patients still alive, 66 patients (59% of the total 111 lost) had attended a different care facility in the prior 3 months. The same extrapolation would yield 56 patients (50%) having taken ART in the prior 30 days. These transfers should be considered favorable outcomes because patients are still in care and likely transferred to a more accessible clinic. The heterogeneity may mean that using losses to follow-up as an end point in epidemiologic studies or combining them together with death may be aggregating very different outcomes. Novel approaches, such as sampling, may better distinguish heterogeneous outcomes among the lost.
The validity of this sampling-based approach depends on the proportion of patients sought whose vital status is ascertained. The higher proportion found, the less potential bias. As proof that a high percentage of vital status ascertainment is possible, we determined vital status in 87% of patients sought. Interestingly, the distance a lost patient resided from clinic did not influence our ability to find him, although our analysis was admittedly underpowered to detect anything but large effects. We attribute high ascertainment of vital status to several factors. Our tracker had intimate knowledge of the community’s terrain and understanding and sensitivity to issues involving searching for persons with HIV/AIDS. Ample time (up to several days) was allowed to search for lost patients.
This study does have limitations. One is that with substantial socioeconomic, geographic, and programmatic differences across African-based treatment settings, our findings may not necessarily generalize to other clinics. Specifically, just as how our previously reported 5-fold difference in mortality after sampling-based correction for those lost to follow-up8 may not necessarily be the right correction factor for all other settings, the reasons for not returning to clinic and the diversity of outcomes after becoming lost may differ as well. For example, our finding that transportation was a major reason for loss to follow-up is in contrast with a report from urban South Africa.11 This suggests that reasons for loss likely differ across treatment delivery settings. We therefore believe that diverse treatment programs, as part of routine monitoring and evaluation, need to conduct their own analyses of losses to follow-up. Another limitation is that although our sample was unselected and consecutive, it was not a formal random sample. Hence, our findings could theoretically be biased in directions that are difficult to predict. However, our sample was objectively identified by an electronic medical record system that captures all visits and assembled by a data manager with no prior knowledge of the patients and hence no predilection for whom to track or not. Furthermore, the lack of substantial differences in characteristics between the lost patients whom we tracked in the community and all other lost patients suggests that the sample was representative. A third limitation is that although we have no reason to doubt the truthfulness of the responses given for failure to report to clinic, it is conceivable that patients may have withheld less socially desirable responses. Because only extensive in-depth interviews (which were beyond the scope of our cost-efficient approach) may have brought out these responses, our findings should not be considered to be definitive. Furthermore, a comparison group, which we did not have, would allow for a quantitative estimate of the effect of each factor reported on actual loss to follow-up. The final limitation is that working with data collected during routine clinical care in a resource-limited setting did result in our having a substantial number of missing CD4+ T-cell values for the analysis of predictors of death among the lost patients. It is theoretically possible that these missing determinations may not have been missing at random and hence could lead to bias.
Although not a threat to the validity of our findings, one potential criticism of our sampling approach is that it does not offer all lost patients the extra encouragement to return to care that might be conferred by a visit from a tracker. This underscores the fact that this sampling-based strategy is a tool for understanding outcomes and not a retention intervention per se. Clearly, if resources permitted, it would be preferable to search for all lost patients, but because this is rarely the case in the African setting, a sampling-based approach offers a resource-efficient means for individual clinics to understand the local circumstances regarding their losses to follow-up and can be used to target outreach efforts to the right patients at the right time. Interestingly, recent data from a resource-rich setting found a rate of loss to follow-up comparable to resource-limited settings,24 suggesting that a sampling-based approach to understand losses to follow-up would be appropriate there as well.
In summary, we used a sampling-based approach to understand the reasons for and outcomes of patients who become lost to follow-up after initiation in Africa. Among those deemed lost, we found a high percentage of both adverse and favorable outcomes. Deaths often occur shortly after interaction with the clinic, suggesting that they are caused by conditions that evolved while in care. Major reasons for not returning to the original clinic were transportation, money, and employment/child care responsibilities. The reasons for and outcomes of losses may not be the same across varied national, cultural, and program settings in Africa, and hence, different regions will need to study their own lost patients. To do this, we believe that a sampling-based approach is both cost efficient and feasible.
Acknowledgments
Supported by grants U01 AI069911, T32 AI06530, P30 AI027763, and R01 MH054907 from the National Institutes of Health, the East Africa International Epidemiologic Databases to Evaluate AIDS Consortium, the United States President’s Emergency Plan for AIDS Relief, and the Antiretroviral Treatment in Lower Income Countries Collaboration.
We are grateful to Hassan Baryahikwa; Adam Pepper; Larry Pepper, MD; John Bennett, MPH; Torsten Neilands, PhD; Este Hudes, PhD; and Mark and Lisa Schwartz.
Footnotes
Presented in part at the 15th Conference on Opportunistic Infections and Retroviruses, February 3–6, 2008, Boston, MA.
References
- 1.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 2.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 3.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20:41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Unites States Global AIDS Coordinator. [Accessed 30 March 2009];The Power of Partnerships: The President’s Emergency Plan for AIDS Relief Fifth Annual Report to Congress. Available at: http://www.pepfar.gov/press/fifth_annual_report/index.htm.
- 5.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS ONE. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 12.Maskew M. Gender Differences in HIV Outcomes in South African Patients Receiving Highly Active Antiretroviral Therapy. HIV/AIDS Implementer’s Meeting; Kampala, Uganda. 2008. p. Abstract no. 1511. [Google Scholar]
- 13.Lewden C, Balestre E, Thiebaut R, et al. Effectiveness of Cotrimoxazole (CTX) Prophylaxis on Survival and Program Retention in African HAART-Treated Adults with Baseline CD4.>= 200/mm3. HIV/AIDS Implementer’s Meeting; Kampala, Uganda. 2008. p. Abstract no. 1621. [Google Scholar]
- 14.Pujades-Rodriguez M, O’Brien D, Humblet P, et al. Second-line anti-retroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008;22:1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- 15.Mamlin BW, Biondich PG, Wolfe BA, et al. Cooking up an open source EMR for developing countries: OpenMRS - a recipe for successful collaboration. AMIA Annu Symp Proc. 2006;2006:529–533. [PMC free article] [PubMed] [Google Scholar]
- 16.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. [Google Scholar]
- 17.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 18.van Oosterhout JJ, Bodasing N, Kumwenda JJ, et al. Evaluation of antiretroviral therapy results in a resource-poor setting in Blantyre, Malawi. Trop Med Int Health. 2005;10:464–470. doi: 10.1111/j.1365-3156.2005.01409.x. [DOI] [PubMed] [Google Scholar]
- 19.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-unin-fected children: a prospective cohort study. Lancet. 2008;371:752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 20.Bangsberg DR, Ware N, Simoni JM. Adherence without access to anti-retroviral therapy in sub-Saharan Africa? AIDS. 2006;20:140–1. doi: 10.1097/01.aids.0000196168.50303.31. author reply 141–142. [DOI] [PubMed] [Google Scholar]
- 21.Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17:661–673. doi: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- 22.Anglaret X, Toure S, Gourvellec G, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 23.Brinkhof MW, Pujades-Rodriguez M, et al. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerver S, Chadborn T, Ibrahim F, et al. HIV-Positive Patient Retention in the UK: High Rate of Loss to Clinical Follow-Up Among Patients From a London Clinic. IAS 2008; Mexico City, Mexico. p. Abstract no. TUAB0205. [Google Scholar]