Abstract
Objective
To characterize the impact of longitudinal adherence on survival in drug-naive individuals starting currently recommended highly active antiretroviral therapy (HAART) regimens.
Methods
Eligible study participants initiated HAART between January 2000 and November 2004 and were followed until November 2005 (N = 903). HAART regimens contained efavirenz, nevirapine, or ritonavir-boosted atazanavir or lopinavir. Marginal structural modeling was used to address our objective.
Results
The all-cause mortality was 11%. Individual adherence decreased significantly over time, with the mean adherence shifting from 79% within the first 6 months of starting HAART to 72% within the 24- to 30-month period (P value < 0.01). Nonadherence over time (<95%) was strongly associated with higher risk of mortality (hazard ratio: 3.13; 95% confidence interval (CI): 1.95 to 5.05). Nonadherent (<95%) patients on nonnucleoside reverse transcriptase inhibitor (NNRTI)–based and boosted protease inhibitor–based regimens were, respectively, 3.61 times (95% CI: 2.15 to 6.06) and 3.25 times (95% CI: 1.63 to 6.49) more likely to die than adherent patients. Within the NNRTI-based regimens, nonadherent individuals on efavirenz were at a higher risk of mortality.
Conclusions
Incomplete adherence to modern HAART over time was strongly associated with increased mortality, and patients on efavirenz-based NNRTI therapies were particularly at a higher risk if nonadherent. These results highlight the need to develop further strategies to help sustain high levels of adherence on a long-term basis.
Keywords: adherence, boosted PI, HAART, marginal structural models, mortality, NNRTI
INTRODUCTION
Highly active antiretroviral therapy (HAART) has led to a dramatic decrease in HIV morbidity and mortality over the last decade. Essentially, all patients starting HAART are now very likely to achieve sustained HIV RNA plasma viral loads below 50 copies per milliliter and substantial CD4 cell count increases.1–8 The success of HAART has not been without challenges.9 HAART requires daily dosing and sustained adherence to fully benefit from therapy.10–13 Incomplete adherence (often due to adverse events or toxicity) has been an important driver of virological failure, the emergence of drug resistance, and ultimately progression to AIDS and death.
Studying the relation between antiretroviral therapy adherence and mortality is difficult. There are risk factors that are associated with both adherence and mortality resulting in complex causal relationships that must be accounted for in these types of analyses. These factors include clinical information such as drug toxicity, side effects, resistance, complexity of the drug regimen and patient’s disease stage, sociodemographic, socioeconomic, and psychosocial characteristics.10,14–24
The few prospective studies available suggest that adherence declines with time on therapy.20,25–33 Most of these studies, however, have either a short-term follow-up time (≤1 year) or a small sample size or they rely on patient-reported adherence, which often overestimates the actual adherence.33 As importantly, some of these studies have included initial HAART regimens no longer recommended. We therefore undertook this study to characterize the impact of longitudinal adherence on survival in drug-naive individuals starting currently recommended HAART regimens.34,35 We based our analysis on data from a large population-based cohort of HIV-1–infected antiretroviral-naive adults initiating HAART in British Columbia, Canada, between January 1, 2000, and November 30, 2004.
METHODS
Data and Study Population
This study was conducted using data from the British Columbia Centre for Excellence in HIV/AIDS (the Centre), which distributes antiretroviral agents at no cost to all eligible HIV-infected individuals through its HIV/AIDS drug treatment program. The details of this program have been described elsewhere.1,36 Antiretroviral drugs are distributed at no cost to all HIV-infected British Columbia residents according to specific guidelines generated by the Therapeutic Guidelines Committee. These have remained consistent with those put forward by the International AIDS Society-United States since the summer of 1996 and until the most recent guidelines released in 2006.34 The Centre’s drug treatment program has received ethical approval from the St. Paul’s Hospital, University of British Columbia Ethics Review Committee.
The Centre’s guidelines recommend that plasma HIV-1 RNA levels (pVL) and CD4 cell count be monitored at baseline, at 4 weeks after starting antiretroviral therapy, and every 3 months thereafter. The pVLs are determined using the Roche Amplicor Monitor assay (Roche Diagnostics, Laval, Quebec, Canada) using the ultrasensitive adaptation. CD4 cell counts are measured by flow cytometry, followed by fluorescent monoclonal antibody analysis (Beckman Coulter, Inc, Mississauga, Ontario, Canada).
Eligible study participants were 18 years or older, naïve to antiretroviral therapy when they started HAART consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), or a NRTI and a nucleotide reverse transcriptase inhibitor (NtRTI) as backbone, plus either (1) the nonnucleoside reverse transcriptase inhibitors (NNRTIs), efavirenz or nevirapine, or (2) the protease inhibitors (PIs), atazanavir or lopinavir, each boosted with ≤200 mg/d ritonavir (boosted PI). Participants started treatment between January 01, 2000 and November 30, 2004 and were followed until November 30, 2005. Finally, to be eligible for analysis, participants were required to have at least 1 baseline CD4 cell count and a pVL measurement available within 6 months before the first antiretroviral starting date. Study data from eligible participants were extracted from the Centre’s monitoring and evaluation system to form the HAART Observational Medical Evaluation and Research cohort.
Outcome Measure and Potential Confounder Variables
The primary end point in this analysis was all-cause mortality. Deaths occurring during the follow-up period were identified on a continuous basis from physician reports and through annual record linkages carried out with the British Columbia Division of Vital Statistics. Event-free subjects in this study were not followed after November 30, 2005.
The following baseline potential confounder variables were investigated: age, gender, CD4 cell count, pVL (log10 transformed), HAART regimen class (boosted PI or NNRTI), AIDS diagnosis, and history of injection drug use (IDU).
Time-Varying Adherence to Therapy
Estimates of adherence to antiretroviral therapy were based on regimen exposure of each patient. Although patients with undetectable pVL less commonly have medications changed, the regimens can change due to adverse effects or other medical and nonmedical reasons. Measuring adherence to any specific antiretroviral drug as index medication when it can be changed at any point in time makes it difficult for accurate monitoring. For this reason, the measure of adherence that we chose to adopt does not use any specific index medication, and it thus only measures the overall exposure to any antiretroviral regimen.11
As previously described, adherence was defined as the number of days of antiretrovirals dispensed divided by the number of days of follow-up (expressed as percent).8,11,22 Adherence was assessed for each 6-month interval from antiretroviral starting date until the death date, the last contact date, or until November 30, 2005 (end of follow-up). The quantity of antiretroviral drugs dispensed (or number of days of therapy supplied) varied from patient to patient and also within patient over time. Prescriptions dispensed early in therapy are generally for a month supply. Later, prescription refills dispensed are generally extended to 2–3 months if patients are deemed to be clinically stable by the treating physicians. We used a 6-month time interval to measure adherence to accommodate heterogeneous drug refill patterns. Adherence to therapy was measured, used as a continuous measure for descriptive purposes and also as a dichotomous measure (<95% versus ≥95%), in our statistical models.
Statistical Analyses
Adherence to therapy was the primary time-dependent exposure. CD4 cell count, pVL, HAART regimen, and AIDS diagnosis were considered as potential time-dependent confounders of the relation between adherence and mortality. In brief, a confounder can be 1 or more variables associated with the variable of main interest, and these associations have the potential to mask the true association of the main variable and the study outcome. If confounding factors are not measured and controlled for, their effects have the potential to bias our results and conclusions. To perform this study, we monitored all time-varying factors synchronized with the 6-month interval start time used to obtain the adherence measurements.
In our study, CD4 cell count, pVL, HAART regimen, and AIDS diagnosis are independent prognostic factors for mortality (condition 1). Additionally, they may be also predictors of subsequent adherence (condition 2) and predicted by past adherence to therapy (condition 3). Measured risk factors satisfying conditions 1–3 are called time-dependent confounders. In cross-sectional studies, the effect of potential confounders can be easily dealt with by adjusting for them in regression models. However, this adjustment produce biased estimates of the effect of the main exposure on the outcome when both the main exposure and several potential confounders are assessed in multiple time points. Therefore, marginal structural models were used to control for the potential effect of time-dependent confounders on the association of adherence and mortality present when fitting a Cox proportional hazard regression model.37–40 Using the theory behind marginal structural models, unbiased estimates of the causal effect of exposure on the outcome can then be computed using estimated weights (allowing for appropriate adjustment for time-varying confounders) for each observation, and baseline predictors provided that all important confounders have been considered and that the models used to compute these weights and the effects are properly specified. Categorical variables were compared using the χ2 test or Fisher exact test, and continuous variables were compared using the Wilcoxon rank sum test. Analyses were performed using SAS software version 9.1.3 Service Pack 3 (SAS, Cary, NC).
RESULTS
Overall Cohort Characteristics
Between January 1, 2000 and November 30, 2004, 903 antiretroviral-naive adults (79% males) who initiated HAART were eligible to participate in this study (Table 1). At baseline, the median age was 41 years [interquartile range (IQR): 35–47 years], CD4 cell count was 170 cells per cubic millimeter (IQR: 80–250 cells/mm3), and pVL was 5.0 log10 copies per milliliter (IQR: 4.7–5.0 log10 copies/mL). Of these, 25% had a history of IDU, 15% had AIDS at baseline, 36% were first prescribed therapies containing boosted PIs, and 40% of participants were less than 95% adherent during the first year of follow-up. The overall median time of follow-up was 33 months (IQR: 22–48 months). In our cohort, IDU status only captures history of IDU and not active IDU status, and it is highly correlated with having hepatitis C (P < 0.01).
TABLE 1.
Baseline Characteristics Associated With Mortality for Patients Initiating Triple Combination ARV Therapy
| Variable | Overall N = 903 | Alive n = 807 | Deceased n = % | P |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 190 (21.0) | 172 (21.3) | 18 (18.8) | 0.5602 |
| Male | 713 (79.0) | 635 (78.7) | 78 (81.3) | |
| IDU history, n (%) | ||||
| No | 680 (75.3) | 615 (76.2) | 65 (67.7) | 0.0679 |
| Yes | 223 (24.7) | 192 (23.8) | 31 (32.3) | |
| AIDS diagnosis, n (%) | ||||
| No | 772 (85.5) | 696 (86.2) | 76 (79.2) | 0.0626 |
| Yes | 131 (14.5) | 111 (13.8) | 20 (20.8) | |
| Year of first ARV, n (%) | ||||
| 2000 | 140 (15.5) | 107 (13.3) | 33 (34.4) | <0.0001 |
| 2001–2002 | 308 (34.1) | 268 (33.2) | 40 (41.7) | |
| 2003–2004 | 455 (50.4) | 432 (53.5) | 23 (24.0) | |
| Initial regimen, n (%) | ||||
| Boosted PI | 232 (35.8) | 295 (36.6) | 28 (29.2) | 0.1534 |
| NNRTI | 580 (64.2) | 512 (63.4) | 68 (70.8) | |
| Adherence (first year) | ||||
| ≥95% | 541 (59.9) | 506 (62.7) | 35 (36.5) | <0.0001 |
| <95% | 362 (40.1) | 301 (37.3) | 61 (63.5) | |
| Hepatitis C status | ||||
| Negative | 310 (34.3) | 290 (35.9) | 20 (20.8) | 0.0002 |
| Positive | 273 (30.2) | 227 (28.1) | 46 (47.9) | |
| Unknown (not tested) | 320 (35.4) | 290 (35.9) | 30 (31.3) | |
| Age (yrs) | ||||
| Median | 41.0 | 40.7 | 42.1 | 0.0753 |
| Interquartile range | 34.8–47.4 | 34.7–47.1 | 35.5–49.2 | |
| CD4 cell count (cells/mm3) | ||||
| Median | 170 | 170 | 110 | <0.0001 |
| Interquartile range | 80–250 | 80–260 | 35–205 | |
| Plasma HIV-1 RNA level (copies/mL) | ||||
| Median | 100,010 | 100,010 | 100,010 | 0.0023 |
| Interquartile range | 45,400–100,010 | 40,200–100,010 | 79,300–100,010 | |
| Follow HIV-1 RNA level (copies/mL) | ||||
| Median | 33 | 33 | 25 | <0.0001 |
| Interquartile range | 22–48 | 22–49 | 13–38 |
ARV, antiretroviral.
As of November 30, 2005, 96 deaths were identified, yielding an overall all-cause mortality of 11%. The product limit estimates of the cumulative mortality rate at 12, 18, 24, and 30 months of follow-up were, respectively, 2.5% (±0.5%), 3.8% (±0.6%), 4.9% (±0.7%), and 6.6% (±0.8%). Table 1 also presents the association between baseline characteristics and mortality. Persons who died were more likely to have started therapy during 2000–2002, to have incomplete adherence, to have lower CD4 cell counts, and to have higher pVL (all P values < 0.01).
For those receiving boosted PIs or NNRTIs, the most common NRTI pairs were stavudine/lamivudine (43% and 32%, respectively) and azidothymidine/lamivudine (23% and 40%, respectively). The most common third drug in a combination was nevirapine (79%; follow-up time: 41 months, IQR: 27–56 months) for those receiving NNRTIs and lopinavir boosted with ritonavir (77%; follow-up time: 29 months, IQR: 21–36 months) for those receiving boosted PIs (Table 2). During follow-up, a large proportion of people continued with the drug class regimen initially prescribed (32% for boosted PI; 49% for NNRTI). Note that 16% of patients who started on NNRTIs changed therapy to either boosted PIs or to a nonboosted PI plus an NNRTI, whereas those first prescribed boosted PIs were less likely to change therapy to an NNRTI or to a nonboosted PI plus an NNRTI (4%; P < 0.01) (Table 2). We observed no association between prescribed third drug and mortality within each of the HAART classes (Table 2).
TABLE 2.
Initial Triple Combination ARV Therapy, by NRTI Pair and Third Drug; Distribution of Initial Triple Combination ARV Therapy, by Initial Therapy; Distribution of Initial Triple Combination ARV Therapy by Third Drug and Mortality Status
| First regimen |
||||
|---|---|---|---|---|
| NRTI pair | Overall frequency |
Boosted PI (n = 323) |
NNRTI (n = 580) |
Follow-up* (mo) |
| AZT/3TC, n (%) | 307 (34.0) | 75 (23.2) | 232 (40.0) | 32.3 (22.6–44.0) |
| d4T/3TC, n (%) | 323 (35.8) | 138 (42.7) | 185 (31.9) | 38.2 (26.7–55.3) |
| ddI/3TC, n (%) | 167 (18.5) | 18 (5.6) | 149 (25.7) | 32.7 (21.1–48.1) |
| Other, n (%) | 106 (11.7) | 92 (28.5) | 14 (2.4) | 17.7 (13.8–28.7) |
| First regimen |
||||
| Third drug | Boosted PI (n = 323) |
NNRTI (n = 580) |
Follow-up† (mo) |
|
| Efavirenz, n (%) | — | 120 (20.7) | 32.5 (20.8–43.8) | |
| Nevirapine, n (%) | — | 460 (79.3) | 40.6 (27.0–56.4) | |
| Atazanavir + ritonavir, n (%)† | 75 (23.2) | — | 17.2 (13.5–19.3) | |
| Lopinavir + ritonavir, n (%)† | 248 (76.8) | — | 28.9 (21.3–36.4) | |
| Changed regimen to |
||||
| First regimen | Boosted PI | NNRTI | Nonboosted PI plus NNRTI |
|
| Boosted PI, n (%) | 285 (31.6) | 33 (3.7) | 6 (0.7) | |
| NNRTI, n (%) | 133 (14.7) | 441 (48.8) | 7 (0.8) | |
| Regimens | Alive total = 807 | Deceased total = 96 | P | |
| NNRTI-based regimens | n = 512 | n = 68 | 0.5264 | |
| Efavirenz, n (%) | 104 (20.3) | 16 (23.5) | ||
| Nevirapine, n (%) | 408 (79.7) | 52 (76.5) | ||
| Boosted PI–based regimens | n = 295 | n = 28 | 0.1571 | |
| Atazanavir + ritonavir, n (%) | 72 (24.4) | 3 (10.7) | ||
| Lopinavir + ritonavir, n (%) | 223 (75.6) | 25 (89.3) | ||
The numbers of this table do not sum to 903 patients or 100% given that during the follow-up of some individuals, their therapy had been changed to all categories displayed.
ARV, antiretroviral; AZT, zidovudine; 3TC, lamivudine; d4T, stavudine; ddI, didanosine.
The values are reported in median years and the interquartile range is presented in parenthesis.
Protease inhibitors boosted with <200 mg/d ritonavir;
Adherence Trends Based on the Continuous Measure of Adherence
The follow-up time was divided into 5 consecutive 6-month intervals (results not shown). We observed a decreasing trend in the overall adherence over time. Mean adherence decreased from 79% [95% confidence interval (CI) mean: 77%–81%] in the first 6 months after starting HAART to 72% (95% CI mean: 68% to 76%) at the 24–30 months period (P value for trend <0.01). When stratified for those alive or deceased, the P values for the decreasing trend in adherence was <0.01 for those remaining alive and 0.3 for those deceased, possibly due to differences in follow-up time.
Effect of Adherence to Therapy on Mortality
In all analyses, the time-dependent confounders were AIDS diagnosis, CD4 cell count, HAART regimen, and pVL. The primary exposure and outcome of main interest in the marginal structural model analysis were, respectively, time-dependent therapy adherence and all-cause mortality. Those individuals with incomplete adherence over time were 3.13 times more likely to die than those highly adherent (95% CI: 1.95 to 5.05) (Table 3). In addition, those who started therapy during 2000 were 2.96 (95% CI: 1.46 to 6.00) times more likely to die than those who started during 2003–2004; this effect was of borderline significance for those starting therapy in the year 2001–2002 [hazard ratio (HR): 1.91; 95% CI: 0.99 to 3.72]. Older patients also experienced a high mortality risk (HR: 1.04; 95% CI: 1.02 to 1.06). In this population, the effect of HAART regimen, history of IDU, pVL, and gender did not influence the mortality risk.
TABLE 3.
Univariate and Multivariate Analyses of the Baseline and Time-Dependent Factors Associated With Survival Among 903 Persons First Prescribed Any Triple Combination ARV Therapy
| Variable | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|---|---|---|
| Gender | ||
| Female | 1.00 (—) | 1.00 (—) |
| Male | 1.24 (0.74 to 2.07) | 0.99 (0.55 to 1.79) |
| Age (yrs) | 1.02 (1.00 to 1.04) | 1.04 (1.02 to 1.06) |
| Baseline CD4 cell count (per 100 cells/mm3 increment) |
0.68 (0.57 to 0.81) | 0.73 (0.58 to 0.93) |
| Baseline Plasma HIV-1 RNA level (log10 copies/mL) |
2.58 (1.34 to 4.95) | 2.14 (0.85 to 5.41) |
| Baseline AIDS diagnosis | ||
| No | 1.00 (—) | 1.00 (—) |
| Yes | 1.94 (1.18 to 3.18) | 1.48 (0.81 to 2.74) |
| IDU | ||
| No | 1.00 (—) | 1.00 (—) |
| Yes | 1.35 (0.88 to 2.07) | 1.34 (0.82 to 2.21) |
| Baseline regimen | ||
| NNRTI | 1.00 (—) | 1.00 (—) |
| PI boosted | 1.38 (0.87 to 2.18) | 0.91 (0.47 to 1.75) |
| Adherence level (time varying) | ||
| ≥95% | 1.00 (—) | 1.00 (—) |
| <95% | 2.79 (1.84 to 4.23) | 3.13 (1.95 to 5.05) |
| Year of first therapy | ||
| 2003–2004 | 1.00 (—) | 1.00 (—) |
| 2001–2002 | 1.08 (0.62 to 1.88) | 1.91 (0.99 to 3.72) |
| 2000 | 1.28 (0.69 to 2.39) | 2.96 (1.46 to 6.00) |
The univariate model used Cox proportional models. The multivariate model, using marginal structural models, considered as time-dependent confounders AIDS diagnosis, CD4 cell count, HAART therapy, and plasma HIV-1 RNA level. The exposure of main interest in this model was time-dependent adherence to therapy (<95% versus ≥95%).
ARV, antiretroviral.
Sensitivity Analyses
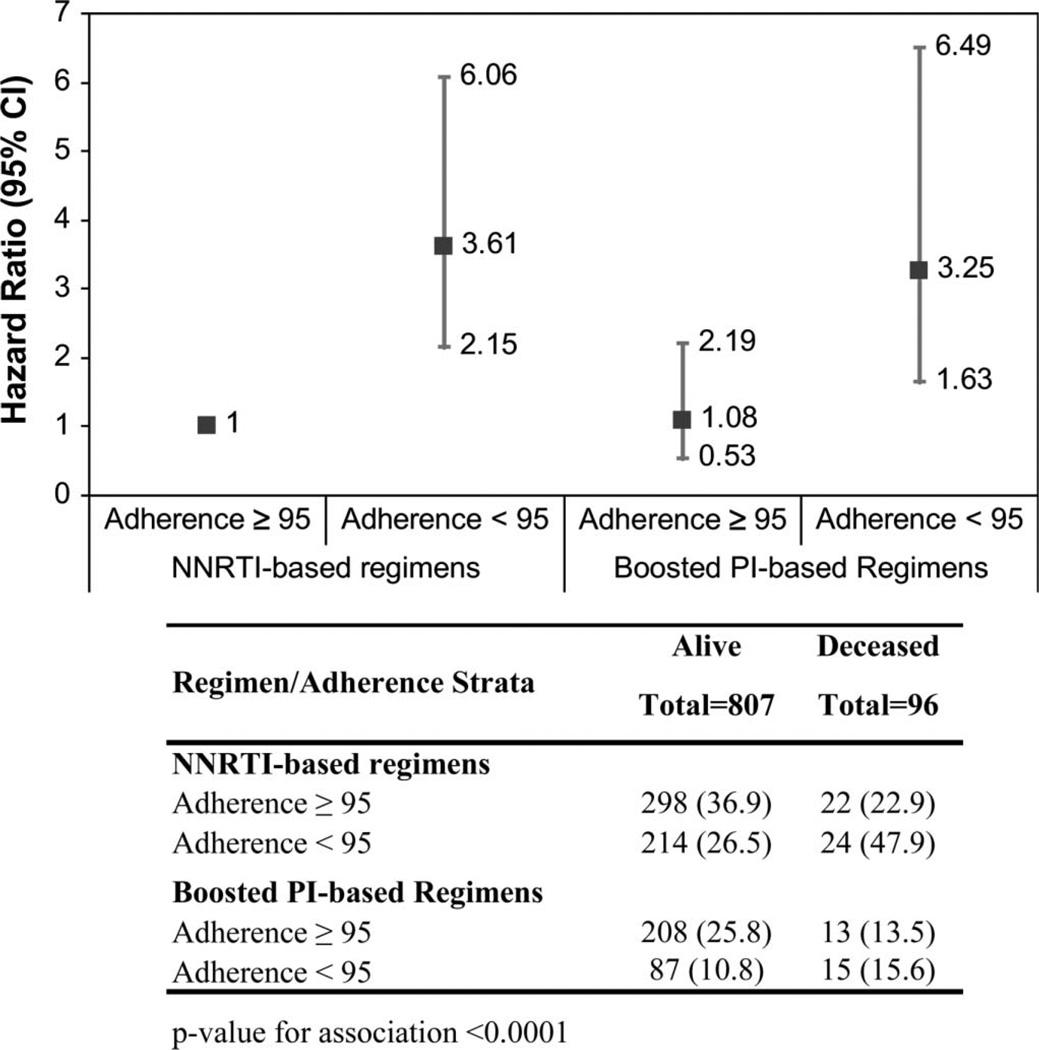
In the main analysis, we observed no association between different HAART regimens and mortality. However, we suspect that adherence is in the causal pathway of the effect of regimen type on mortality, as different regimen potencies are associated with different minimal levels of adherence to prevent viral evolution, and therefore disease progression. To address this hypothesis, we first created a new 4-level categorical variable combining the levels of adherence (<95% and ≥95%) and regimen type (NNRTI and boosted PI). The multivariate survival analysis (Fig. 1), after controlling for several baseline factors, showed that those patients on NNRTI-based regimens and with adherence <95% were 3.61 times (95% CI: 2.15 to 6.06) more likely to die than those on NNRTI-based regimens with adherence ≥95%. As also shown by this model, those on boosted PI–based regimens with adherence <95% were 3.25 times (95% CI: 1.63 to 6.49) more likely to die than those on NNRTI-based regimens with adherence ≥95%. There was no difference on the risk of mortality of individuals on boosted PI–based versus NNRTI-based regimens when the level of adherence was ≥95% (HR: 1.08; 95% CI: 0.53 to 2.19).
FIGURE 1.
Hazard ratio for mortality of 903 patients with HIV, stratified by adherence initial regimen. Vertical bars, 95% CI.
Second, given the high number of mortality events among individuals on NNRTI-based regimens, we investigated whether the adherence of individuals on nevirapine-based or efavirenz-based NNRTI regimens confer different risks of mortality. We observed that those individuals on nevirapine-based NNRTI regimens with incomplete adherence over time were 2.89 times more likely to die than those highly adherent (95% CI: 1.59 to 5.18); and those on efavirenz-based NNRTI regimens with incomplete adherence over time were 7.11 times more likely to die than those highly adherent (95% CI: 2.01 to 25.19).
Last, in all previous analysis, we used adherence as a time-dependent exposure on the risk of mortality. We observed that when adherence and pVL change over time, the effect of pVL on mortality disappears. This finding is interesting because these time-varying factors are highly collinear, and we in no way are suggesting that pVL is an unimportant predictor of mortality. To understand this issue, we assessed how the slope of adherence (measured over time) predicts the risk of mortality. We categorized the adherence slope into <0 versus ≥0 (reference category). In a multivariate survival analysis, patients with decreasing adherence over time were 2.25 times (95% CI: 1.40 to 3.60) more likely to die than those with a nonnegative slope. We then assessed the degree of decline in adherence slope in a multivariate survival model. For each 1% decline in the slope of adherence, the risk of mortality increased 1.63 times (95% CI: 1.45 to 1.84). It is important to note that in both analyses, high viral load significantly confers a higher risk of mortality.
DISCUSSION
Our results demonstrate that adherence of individuals decreased during chronic HIV therapy. Furthermore, we showed that the degree of incomplete adherence to modern HAART was strongly associated with increased mortality. Of note, the adherence association with mortality was modified by HAART regimen, and patients on efavirenz-based NNRTI therapies were particularly at a higher risk of mortality if nonadherent. Our results highlight the need to develop further strategies to help sustain sufficiently high levels of adherence to sustain long-term viral suppression and therefore avoid disease progression and death.
This was the first study that we know of to evaluate the longitudinal and the gradated impact of adherence and type of HAART regimen on the mortality risk in a cohort of patients exclusively on HAART and with a long follow-up. Several studies report evidence on the association between adherence and mortality among HIV-infected individuals.1,24,41–44 However, most of these studies are cross-sectional, and therefore, they can misclassify the level of adherence because patients’ adherence behavior changes over time. Few longitudinal studies have examined the role of adherence on mortality.44–49 These longitudinal studies, as ours, confirm the importance of high levels of adherence to avert mortality, and they have shown that patients change their level of adherence over time, with longer periods on treatment being associated with lower adherence levels.20,28,31–33 Different HAART regimens have also been suggested to have a different impact on the risk of mortality with boosted PI–based regimens having a superior survivorship over NNRTI-based regimens.50,51 Unfortunately, the prior studies failed to address the complex interaction of adherence and HAART therapy on mortality. We found no such difference among the highly adherent subgroup but a benefit of being on a boosted PI in those with suboptimal adherence.
Several features of this study are noteworthy. Our study represents the first attempt to assess risk of mortality by class of HAART regimen, restricted to currently recommended regimens. Of note, we did so after adjusting for time-dependent adherence, which accounts for changes in an individual’s adherence pattern over time, and several other fixed and time-dependent factors. This study was based on a large population-based sample including patients within a province-wide treatment program, in which all individuals had free access to medical attention, antiretroviral therapy, and laboratory monitoring. We are confident, therefore, that our results are not biased by access to therapy issues, a factor that has often compromised the interpretation of other population-based and cohort-based studies. Overall, in this population, the median CD4 for therapy initiation was as low as 170 cells per cubic millimeter (IQR: 80–250 cells/mm3), even though HAART and care for HIV disease in the province of British Columbia is free of charge to eligible individuals. One reason for this could have been the reluctance of some patients, particularly if asymptomatic, to embrace a lifelong commitment to take antiretroviral therapy because of reservations regarding toxicity/tolerability or just because of the complicated lifestyle of some patients. Also, this study provides a substantially longer follow-up time than most previous studies (median: 33 months; IQR: 22–48 months). Finally, delayed reporting of deaths was not likely a factor in this study because most deaths were reported within 3 months of death through active follow-up with physicians and hospitals and regular linkages to the province’s Vital Statistics Agency.
An important limitation in this study relates to the fact that, at this time, accurate information on methadone or current IDU among our population is not available. It is possible that the lack of this information may have influenced the results of our analysis for this population group. In the future, it would be worthwhile to explore whether there is a difference in duration of first regimen between active drug users receiving boosted PIs and NNRTIs to properly assess if the nondifferential mortality risk found in this study still holds.
In conclusion, our results demonstrate that individual adherence to HAART decreased significantly during the 5 years of study. Furthermore, incomplete adherence to modern HAART was strongly associated with increased mortality. Of note, the latter association was modified by HAART regimen, within the modern HAART regimens included in the study. Our findings suggest that in nonadherent individuals, boosted PIs may be preferred over NNRTIs. Of course, a contemporaneous strategy would be to develop interventions to help sustain sufficiently high levels of adherence over time. Further research is needed to elucidate whether these trends apply to other modern HAART regimens not included in our study population.
ACKNOWLEDGMENTS
We thank all the staff from the British Columbia Centre for Excellence in HIV/AIDS for their input and assistance.
Supported by the Canadian Institutes of Health Research through a Fellowship Award to Dr. V.D.L. Drs. R.S.H., S.G.M., P.R.H., R.G., and D.R.B. have received honorariums, travel grants to attend conferences, and research grants from pharmaceutical companies working in the area of HIV/AIDS.
Footnotes
V.D.L. and B.Y. declare no conflict.
The Centre’s HIV/AIDS Drug Treatment program has received ethical approval from the University of British Columbia Ethics Review Committee at its St Paul’s Hospital site. The program also conforms with the province’s Freedom of Information and Protection of Privacy Act.
Author contributions: study concept and design: V.D.L., B.Y., J.S.G.M., R.S. H., D.R.B., and P.R.H.R.G.; acquisition of data: R.S.H. and J.S.G.M.; analysis and interpretation of data: V.D.L.; drafting of the article: V.D.L.; critical revision of the article for important intellectual content: V.D.L., B.Y., J.S.G.M., R.S.H., D.R.B., and P.R.H., Gross; statistical analysis: V.D.L.; obtained funding: R.S.H., V.D.L., and J.S.G.M.; administrative, technical, or material support: R.S.H. and J.S.G.M.; study supervision: R.S.H., D.R.B., P.R.H., and J.S.G.M.
The funding sources had no role in the choice of methods, the contents or form of this work, or the decision to submit the results for publication.
REFERENCES
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Wood E, Hogg RS, Harrigan PR, et al. When to initiate antiretroviral therapy in HIV-1-infected adults: a review for clinicians and patients. Lancet Infect Dis. 2005;5:407–414. doi: 10.1016/S1473-3099(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 5.Knobel H, Carmona A, Grau S, et al. Adherence and effectiveness of highly active antiretroviral therapy. Arch Intern Med. 1998;158:1953. doi: 10.1001/archinte.158.17.1953. [DOI] [PubMed] [Google Scholar]
- 6.Vanhove GF, Schapiro JM, Winters MA, et al. Patient compliance and drug failure in protease inhibitor monotherapy. JAMA. 1996;276:1955–1956. [PubMed] [Google Scholar]
- 7.Montaner JS, Reiss P, Cooper D, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 8.Wood E, Hogg RS, Yip B, et al. The impact of adherence on CD4 cell count responses among HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:261–268. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Vermund SH. Millions of life-years saved with potent antiretroviral drugs in the United States: a celebration, with challenges. J Infect Dis. 2006;194:1–5. doi: 10.1086/505154. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gross R, Yip B, Re VL, III, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 12.Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis. 2005;40:158–163. doi: 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- 13.King MS, Brun SC, Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naive, HIV-1-infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191:2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Montaner JSG, Bangsberg DR, et al. Expanding access to HIV antiretroviral therapy among marginalized populations in the developed world. AIDS. 2003;17:2419–2427. doi: 10.1097/00002030-200311210-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sethi AK, Celentano DD, Gange SJ, et al. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 16.Bangsberg DR, Charlebois ED, Grant RM, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Porco TC, Kagay C, et al. Modeling the HIV protease inhibitor adherence-resistance curve by use of empirically derived estimates. J Infect Dis. 2004;190:162–165. doi: 10.1086/420790. [DOI] [PubMed] [Google Scholar]
- 18.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53:696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 19.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Squier C, Sivek C, et al. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8:261–269. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 21.Morse EV, Simon PM, Coburn M, et al. Determinants of subject compliance within an experimental anti-HIV drug protocol. Soc Sci Med. 1991;32:1161–1167. doi: 10.1016/0277-9536(91)90093-r. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Hogg R, Yip B, et al. The impact of baseline CD4 T-cell count and adherence on rates of disease progression among HIV-infected patients initiating triple drug therapy. Abstract 182. Presented at 9th Conference on Retroviruses and Opportunistic Infections; February 24–28, 2002; Seattle, WA. 2002. [Google Scholar]
- 23.Gordillo V, del Amo J, Soriano V, et al. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 24.Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-positive individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 25.Gross R, Bilker WB, Friedman HM, et al. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15:2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 26.Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: a critical link between behavioral and biomedical sciences. J Acquir Immune Defic Syndr. 2002;31:S98–S102. doi: 10.1097/00126334-200212153-00002. [DOI] [PubMed] [Google Scholar]
- 27.Moss AR, Hahn JA, Perry S, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 28.Tesoriero J, French T, Weiss L, et al. Stability of adherence to highly active antiretroviral therapy over time among clients enrolled in the treatment adherence demonstration project. J Acquir Immune Defic Syndr. 2003;33:484–493. doi: 10.1097/00126334-200308010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Carrieri P, Cailleton V, Le Moing V, et al. The dynamic of adherence to highly active antiretroviral therapy: results from the French National APROCO cohort. J Acquir Immune Defic Syndr. 2001;28:232–239. doi: 10.1097/00042560-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Melbourne KM, Geletko SM, Brown SL, et al. Medication adherence in patients with HIV infection: a comparison of two measurement methods. AIDS Read. 1999;9:329–338. [PubMed] [Google Scholar]
- 31.Lucas GM, Gebo KA, Chaisson RE, et al. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 32.Godin G, Cote J, Naccache H, et al. Prediction of adherence to antiretroviral therapy: a one-year longitudinal study. AIDS Care. 2005;17:493–504. doi: 10.1080/09540120412331291715. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Miller LG, Hays RD, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- 34.Hammer SM, Saag MS, Schechter M, et al. International AIDS Society- USA panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 35.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. Bethesda, MD: US Department of Health and Humans Services; 2006. [Accessed November 4, 2007]. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 36.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer D, Jr, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: Wiley; 1999. [Google Scholar]
- 38.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Petersen ML, Wang Y, van der Laan MJ, et al. Assessing the effectiveness of antiretroviral adherence interventions: using marginal structural models to replicate the findings of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43:S96–S103. doi: 10.1097/01.qai.0000248344.95135.8d. [DOI] [PubMed] [Google Scholar]
- 41.Wood E, Hogg RS, Yip B, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 42.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 43.Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 44.Park WB, Choe PG, Kim SH, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med. 2007;261:268–275. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 45.Laurent C, Ngom Gueye NF, Ndour CT, et al. ANRS 1215/1290 Study Group. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–17. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds NR. Adherence to antiretroviral therapies: state of the science. Curr HIV Res. 2004;2:207–214. doi: 10.2174/1570162043351309. [DOI] [PubMed] [Google Scholar]
- 47.Kagay CR, Porco TC, Liechty CA, et al. Modeling the impact of modified directly observed antiretroviral therapy on HIV suppression and resistance, disease progression, and death. Clin Infect Dis. 2004;38:S414–S420. doi: 10.1086/421406. [DOI] [PubMed] [Google Scholar]
- 48.Roca B, Gomez CJ, Arnedo A. Adherence, side effects and efficacy of stavudine plus lamivudine plus nelfinavir in treatment-experienced HIV-infected patients. J Infect. 2000;41:50–54. doi: 10.1053/jinf.2000.0678. [DOI] [PubMed] [Google Scholar]
- 49.Nieuwkerk PT, Sprangers MA, Burger DM, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Arch Intern Med. 2001;161:1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- 50.Crane HM, Van Rompaey SE, Kitahata MM. Initiating highly active antiretroviral therapy with newer protease inhibitors is associated with better survival compared to first-generation protease inhibitors or nevirapine. AIDS Patient Care STDS. 2007;21:920–929. doi: 10.1089/apc.2007.0020. [DOI] [PubMed] [Google Scholar]
- 51.Antiretroviral Therapy Cohort Collaboration. Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194:612–622. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]