Medicine often balances patient and population interests. Resource-rich areas, such as the United States, often focus on maximizing benefit for the individual patient, whereas resource-limited settings, such as sub-Saharan Africa, often focus on maximizing population benefit (1). In a data-driven analysis of adherence behavior, disease outcomes, and health care use in this issue, Nachega and colleagues (2) demonstrate that these pressures do not necessarily conflict. They provide compelling data indicating that increasing resources to enhancing HIV-infected patients’ antiretroviral adherence is associated with substantial cost savings for the entire public health system.
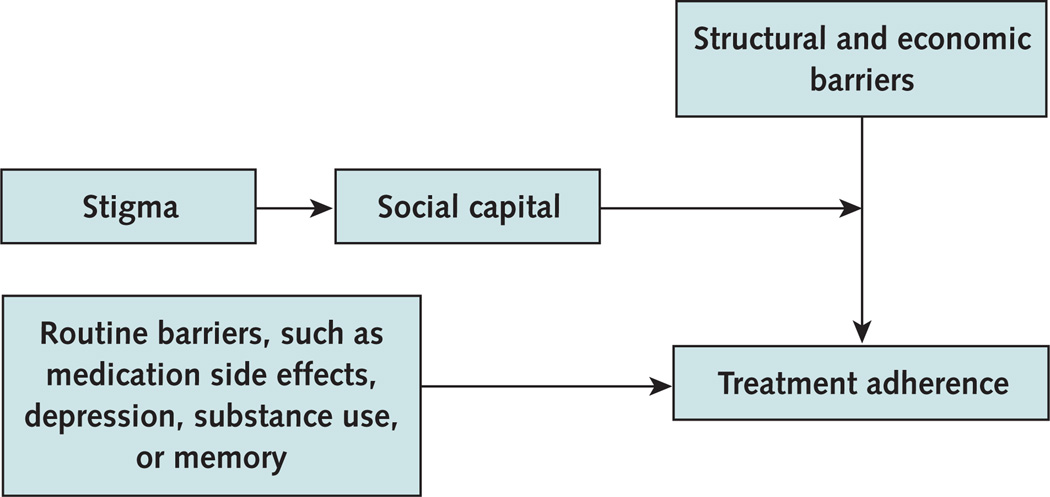
Among HIV-infected persons, antiretroviral treatment adherence strongly predicts viral suppression, drug resistance development, disease progression, and death (3, 4). Despite early concerns that providing therapy to people in extreme poverty would lead to incomplete adherence and antiretroviral resistance, adherence to antiretroviral adherence among persons in resource-limited settings is at least as good as in resource-rich regions (5). Successful adherence is particularly striking, considering the many barriers to sustained adherence in resource-limited settings. Not only do persons living in these settings experience the same barriers to adherence that affect all HIV-infected persons worldwide (such as medication side effects, substance use, depression, and difficulty remembering to take medications), they also experience severe and unique structural and economic barriers (6); the latter include transportation costs and the opportunity costs associated with time away from income or food production while seeking HIV care. Although HIV treatment in resource-limited settings is a major success story, missed doses do occur. These missed doses, which often represent treatment interruptions due to structural and economic barriers, may lead to viral rebound and drug resistance (7).
Nachega and colleagues (2) examined the relationship between incomplete adherence and medical costs among HIV-infected persons in South Africa who were enrolled in an HIV/AIDS managed care program (Aid for AIDS). Although medication costs increased with adherence, costs associated with HIV morbidity decreased as adherence increased. Nachega and colleagues estimate that improving adherence decreases the mean total cost of medical care by $85 (interquartile range, $41 to $116) per month. This underestimates the full benefit of improved adherence, because these calculations did not include the societal benefits of restoring health and economic productivity. Although the magnitude of cost savings conferred by public health programs in sub-Saharan Africa may differ from the private managed care program that Nachega and colleagues studied, the relationship between adherence and cost savings is probably similar.
The obvious next step is to determine how best to allocate resources to promote adherence and realize these benefits. We suggest 3 broadly defined approaches: community-level interventions to reduce structural, economic, and stigma-related barriers; patient-level interventions delivered to all persons receiving HIV treatment; and patient-level interventions that target persons with objectively defined suboptimal adherence. Each area has different relative costs, benefits, and rationales.
The most important community-level intervention to enhance adherence is to increase local access to free antiretroviral therapy (8). Better treatment access at districtlevel treatment facilities has reduced, but by no means eliminated, the structural and economic barriers to sustained treatment adherence. Many patients in resource-poor settings may spend 30% to 50% of their monthly income on transportation costs to access clinical care and antiretroviral therapy (9), in addition to the opportunity costs of time spent away from food or income production. Improving the availability of treatment in districts and villages will lessen these structural and economic barriers to adherence.
Another important focus of community-level intervention is stigma. Stigma is one of the most powerful determinants of adherence because it interferes with a person’s ability to leverage social capital to overcome common structural and economic barriers to care. Stigma not only leads to depression and isolation but also compromises the most important tool to achieve sustained adherence in resource-limited settings (Figure) (10).
Figure 1.
Adherence determinants in resource-limited settings.
Interventions deployed at the individual level that have improved adherence in resource-limited settings include education and counseling, telephone calls, electronic reminders, nursing home visits, and the use of community-based treatment partners (which may include directly observed therapy) (11–13). The accompagnateur model, in which a community health worker partners with and works daily with a patient, combines directly observed therapy with community-level interventions to reduce adherence barriers (14). These interventions are generally directed at all persons receiving therapy. Although intensive interventions have been successful in U.S. settings (15–17), the results have been inconsistent (18, 19), and such interventions may only be cost-effective in populations with especially low levels of adherence (20). Because many patients in resource-limited settings have good adherence without intervention, it is unclear whether targeting all patients with intensive interventions to promote adherence is the best use of resources.
Intensive interventions may be most justified if they target individuals at the highest risk for nonadherence, such as patients with active substance abuse or psychiatric disorders. An alternative strategy is to use standard measurements, such as late pharmacy pick-up or disease progression (for example, virologic failure or decreasing CD4 T-cell counts), to determine who might benefit from more intensive adherence support (21). Yet, pharmacy refill and clinical response monitoring are coarse measures of adherence and may detect suboptimal adherence only after prolonged virologic failure and the emergence of drug-resistant HIV.
Given these limitations, interest is growing in the use of novel approaches that can detect adherence problems before irreversible harm has occurred. For example, wireless devices are now available to monitor adherence in real time (22), with medication bottles that send a signal every time they are opened. A central computer can then generate warnings about missed doses. Warnings can trigger various possible interventions, including a call or visit from a health care worker. Such strategies have the potential to shift reactive responses to proactive interventions designed to prevent treatment failure. Because adherence is dynamic in resource-limited settings and declines either gradually (such as from pill fatigue) (23) or suddenly (such as from washed-out roads or family emergencies), real-time monitoring has the advantage of enabling intervention before virologic rebound. This approach may be well suited to sub-Saharan Africa because of the extensive wireless network already in place. The major barrier to using real-time wireless monitoring, however, has been its perceived cost. Nachega and colleagues provide evidence that strategies to promote adherence may actually save money.
Nachega and colleagues tell us that it makes sense to invest in improving adherence even when resources are scarce. We suggest that resources should be directed at both the community and patient levels. In addition, we believe these findings define the need for novel—albeit expensive—real-time monitoring and intervention to both improve outcomes and potentially reduce health care costs.
Acknowledgments
Grant Support: By the National Institute of Mental Health, grants RO1 54907 and K-24 87227, and The Mark and Lisa Schwartz Family Foundation (Dr. Bangsberg) and the National Institute of Allergy and Infectious Diseases, grants RO1 and K24AI069994 (Dr. Deeks).
Footnotes
Potential Conflicts of Interest: None disclosed.
Contributor Information
David R. Bangsberg, Massachusetts General Hospital, Boston, MA 02114.
Steven G. Deeks, University of California, San Francisco, San Francisco, CA 94110.
References
- 1.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [PMID: 16890837] [DOI] [PubMed] [Google Scholar]
- 2.Nachega JB, Leisegang R, Bishai D, Nguyen H, Hislop M, Cleary S, et al. Association of antiretroviral therapy adherence and health care costs. Ann Intern Med. 2010;152:18–25. doi: 10.7326/0003-4819-152-1-201001050-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [PMID: 10770537] [DOI] [PubMed] [Google Scholar]
- 4.Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [PMID: 19223785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [PMID: 16896111] [DOI] [PubMed] [Google Scholar]
- 6.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [PMID: 17121449] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [PMID: 17457090] [DOI] [PubMed] [Google Scholar]
- 8.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [PMID: 16530575] [DOI] [PubMed] [Google Scholar]
- 9.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern uganda: A qualitative study. AIDS Behav. 2009 doi: 10.1007/s10461-009-9533-2. [PMID: 19283464] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, Agbaji O, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6:e11. doi: 10.1371/journal.pmed.1000011. [PMID: 19175285] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zhou J, Huang L, Li X, Fennie KP, Williams BA. Effects of nurse-delivered home visits combined with telephone intervention on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J Clin Nurs. [Forthcoming] doi: 10.1111/j.1365-2702.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- 12.Frick PA, Lavreys L, Mandaliya K, Kreiss JK. Impact of an alarm device on medication compliance in women in Mombasa, Kenya. Int J STD AIDS. 2001;12:329–333. doi: 10.1258/0956462011923048. [PMID: 11368808] [DOI] [PubMed] [Google Scholar]
- 13.Sarna A, Luchters S, Geibel S, Chersich MF, Munyao P, Kaai S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48:611–619. doi: 10.1097/QAI.0b013e3181806bf1. [PMID: 18645509] [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H. Antiretroviral therapy in resource-poor settings. Decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S123–S126. doi: 10.1097/01.qai.0000248348.25630.74. [PMID: 17133195] [DOI] [PubMed] [Google Scholar]
- 15.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [PMID: 17712763] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macalino GE, Hogan JW, Mitty JA, Bazerman LB, Delong AK, Loewenthal H, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [PMID: 17589194] [DOI] [PubMed] [Google Scholar]
- 17.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38(Suppl 5):S409–S413. doi: 10.1086/421405. [PMID: 15156431] [DOI] [PubMed] [Google Scholar]
- 18.Wohl AR, Garland WH, Valencia R, Squires K, Witt MD, Kovacs A, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–1627. doi: 10.1086/503906. [PMID: 16652320] [DOI] [PubMed] [Google Scholar]
- 19.Gross R, Tierney C, Andrade A, Lalama C, Rosenkranz S, Eshleman SH, et al. AIDS Clinical Trials Group A5073 Study Team. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–1232. doi: 10.1001/archinternmed.2009.172. [PMID: 19597072] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings—the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [PMID: 16971720] [DOI] [PubMed] [Google Scholar]
- 21.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [PMID: 18494555] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes TL, Hunt JM, Adami A, Kaye JA. An electronic pillbox for continuous monitoring of medication adherence. Conf Proc IEEE Eng Med Biol Soc. 2006;1:6400–6403. doi: 10.1109/IEMBS.2006.260367. [PMID: 17946369] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, Musoke P, et al. Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav. 2009;13(Suppl 1):82–91. doi: 10.1007/s10461-009-9546-x. [PMID: 19301113] [DOI] [PubMed] [Google Scholar]